Abstract
Background
Liver biopsies have been partially replaced by noninvasive methods for assessing liver fibrosis. We explored the usefulness of four novel biomarkers, enhanced liver fibrosis (ELF), glycosylation isomer of Mac-2 binding protein (M2BPGi), galectin-3, and soluble suppression of tumorigenicity 2 (sST2), in association with liver fibrosis.
Methods
ELF, M2BPGi, galectin-3, and sST2 were assayed in 173 patients with chronic liver diseases. The results were analyzed according to fibrosis grade (F0/1, F2, and F3/4) by transient elastography (TE).
Results
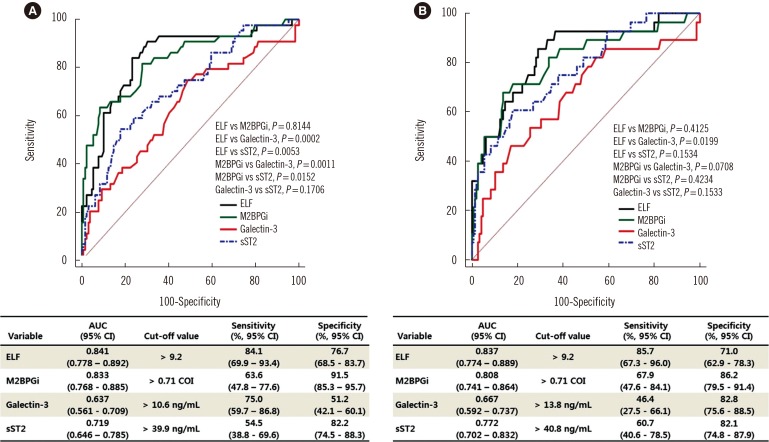
ELF, M2BPGi, galectin-3, and sST2 values differed significantly according to TE grade; ELF and M2BPGi values were higher in F2 and F3/4 than in F0/1 (P≤0.001, all), sST2 values were higher in F3/4 than in F0/1 and F2 (P<0.05), and galectin-3 values were higher in F3/4 than in F0/1 (P=0.0036). ELF and M2BPGi showed good TE fibrosis detection performance (area under the curves [AUC], 0.841 and 0.833 for ≥F2; and 0.837 and 0.808 for ≥F3). The sensitivity and specificity for predicting TE grade F≥2 were 84.1% and 76.7% for ELF and 63.6% and 91.5% for M2BPGi.
Conclusions
This is the first study to compare the liver fibrosis assessment of four novel biomarkers: ELF, M2BPGi, galectin-3, and sST2. The biomarkers varied significantly according to TE grade, and each biomarker showed a different trend. ELF and M2BPGi seem to have comparable good performance for detecting liver fibrosis.
Keywords: Liver fibrosis, Biomarker, ELF, M2BPGi, Galectin-3, sST2
INTRODUCTION
Liver fibrosis, defined as the excessive accumulation of extracellular matrix, can occur in all chronic liver diseases (CLD) [1,2]. Progressive liver fibrosis is a major cause of morbidity, ultimately resulting in hepatocellular carcinoma (HCC) and death without proper treatment [3]. Although liver fibrosis has been considered irreversible, liver fibrosis regression has been achieved by alleviating hepatic inflammation and damage with potent antiviral agents [4]. Accurate assessment and monitoring of the degree of liver fibrosis is important for the management of patients with CLD [5].
Liver biopsy (LB) has long been regarded as the reference method for assessing and grading liver fibrosis [6]. However, it is invasive and has limitations including sampling errors, inter- and intra-observer variability, and procedural complications [6,7]. It is difficult to perform repeated LB examinations to monitor fibrotic burden in clinical practice; thus, many noninvasive methods have been suggested as an alternative to LB [7]. Transient elastography (TE) measures liver elasticity using a low frequency elastic wave transmitted through the liver and is considered a highly reproducible and reliable option for grading liver fibrosis, except in some cases such as obesity [5,8,9,10].
Suggested blood biomarkers range from simple tests using routine laboratory parameters, such as the aspartate aminotransferase-to-platelet ratio index (APRI), to more complex equations such as the FibroTest (FT) or Enhanced Liver Fibrosis (ELF) Test (Siemens Diagnostics, Tarrytown, NY, USA) [11,12]. Recently, glycosylation isomer of Mac-2 binding protein (M2BPGi, Wisteria floribunda agglutinin [WFA]-positive Mac-2 binding protein, WFA+-M2BP) has emerged as a novel biomarker for estimating liver fibrosis and predicting HCC; however, this biomarker has been evaluated mainly in Japanese populations [13,14,15,16]. Galectin-3 and the soluble isoform of suppression of tumorigenicity 2 (sST2) have emerged as biomarkers of heart failure (HF), mainly reflecting cardiac fibrosis [17,18]. These proteins are involved in inflammation and fibrosis in various, non-cardiac conditions [19,20,21] and play prognostic roles [22,23]. Given their possible involvement in liver fibrosis, further investigations could shed important light on these markers [24].
This study aimed to evaluate the usefulness of four new biomarkers, ELF, M2BPGi, galectin-3, and sST2, for assessing liver fibrosis status. We examined the association of these biomarkers with fibrosis grade and determined the optimal cut-off values for their application in patients with CLD. In addition, we evaluated the association between these biomarkers and prognostic scores.
METHODS
1. Study population
This study was approved by the Institutional Review Board (KUH1200072) of the Konkuk University Medical Center, Seoul, Korea, prior to collecting the first sample from the first patient. We included a total of 173 patients (105 males and 68 females) with CLD, who visited our center from October 2016 to February 2017 and completed TE and blood sampling on the day. We excluded patients when their TE measurements failed or their residual blood samples were insufficient for other tests. To evaluate prognosis, the model for end-stage liver disease (MELD) and Child-Turcotte-Pugh (CTP) scores were calculated at enrollment [25,26]. Clinical and laboratory data, including underlying diseases, APRI, and fibrosis-4 index (FIB-4), were collected by reviewing the medical records. The characteristics of the study population are summarized in Table 1.
Table 1. Characteristics of the study population.
| Variable | Total (N = 173) |
|---|---|
| Age (year) | 53.0 (44.0–73.0) |
| Gender | |
| Male | 105 (60.7) |
| Female | 68 (39.3) |
| Underlying disease | |
| Chronic hepatitis B | 123 (71.1) |
| Chronic hepatitis C | 21 (12.1) |
| Autoimmune hepatitis | 12 (7.0) |
| Alcoholic liver disease | 9 (5.2) |
| Non-alcoholic fatty liver disease | 4 (2.3) |
| Unknown | 4 (2.3) |
| Hepatocellular carcinoma | 8 (4.6) |
| Transient elastography (kPa) | |
| F0/1 (≤7.0) | 129 (74.6) |
| F2 (≥7.1) | 16 (9.2) |
| F3 (≥10.0) | 3 (1.7) |
| F4 (>13.0) | 25 (14.5) |
| AST/ALT (N=172) | 1.1 (0.8–1.4) |
| APRI (N=149) | 0.4 (0.3–0.6) |
| FIB-4 (N=149) | 1.5 (1.0–2.3) |
| MELD score (N=83) | |
| <9 | 77 (92.8) |
| ≥9 | 6 (7.2) |
| CTP score (N=111) | |
| Class A (5–6) | 104 (93.7) |
| Class B (7–9) | 5 (4.5) |
| Class C (10–15) | 2 (1.8) |
Data are presented as median (interquartile range) or number (percentage). Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4; MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh.
TE was performed by a well-trained technician using the FibroScan device (Echosens, Paris, France), as previously described [9]. TE results with at least 10 valid measurements and a success rate of at least 60% were considered reliable. The degree of fibrosis was graded as follows: F0/1≤7.0 kilopascal (kPa), presumed no or minimal fibrosis; F2≥7.1 kPa, presumed moderate fibrosis; F3≥10.0 kPa, presumed severe fibrosis; and F4≥13 kPa, presumed cirrhosis [10].
The residual samples were collected following routine blood tests, divided into small aliquots to avoid repeated freezing and thawing, and then stored at −70℃ until use. Frozen samples were thawed at room temperature (20 to 26℃) and gently mixed immediately prior to biomarker measurement. The data were analyzed anonymously and this study required neither study-specific intervention nor any other intervention; therefore, written informed consent from enrolled patients was exempted.
2. Assays
For the ELF test, hyaluronic acid (HA), tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), and aminoterminal propeptide of procollagen type III (PIIINP) concentrations were measured using an ADVIA Centaur XP automated immunoanalyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). ELF values were calculated using the algorithm (ELF=2.278+0.851 ln[HA] +0.751 ln[PIIINP]+0.394 ln[TIMP-1]). The values were interpreted as: <7.7, none to mild; ≥7.7 −<9.8, moderate; and ≥9.8, severe, according to the manufacturer's recommendation; results above moderate (≥7.7) were considered positive [27]. M2BPGi was measured using an HISCL-5000 immunoanalyzer (Sysmex Co., Kobe, Japan) based on the 2-step sandwich chemiluminescent enzyme immunoassay [13]. The measured values of M2BPGi conjugated to WFA were indexed with obtained values using the following equation: cut-off index (COI)=(S-N)/(C-N), where S is the light intensity of the sample, C is the cutoff value, and N is the light intensity of the negative control. The positive control was supplied as a calibration solution preliminarily standardized to yield a COI value of 1.0. The sample was considered positive when the COI was >1.00 (1+, 1.00≤COI <3.00; 2+, COI≥3.00) [13]. Galectin-3 concentrations were measured using the VIDAS automated enzyme-linked fluorescent assay (bioMérieux, Marcy-l'Etoile, France). sST2 concentrations were measured using the Presage ST2 Assay (Critical Diagnostics, San Diego, CA, USA), which is based on a quantitative sandwich monoclonal ELISA.
3. Statistical analysis
Data were expressed as median and interquartile range (IQR) or number and percentage. The difference between the continuous variables was analyzed using the Mann-Whitney U test, and the difference between categorical variables was analyzed using the chi-square test, Fisher's exact test, or the Cochran-Armitage test for trend. Differences between biomarkers were assessed according to TE grade (F0/F1, F2, and F3/4), MELD score, and CTP score. Agreement between assays was determined using Cohen's Kappa statistic (agreement: <0.20, none; 0.21–0.39, minimal; 0.40–0.59, weak; 0.60–0.79, moderate; 0.80–0.90, strong; >0.90, nearly perfect) [28]. The TE grade prediction performance of each biomarker (F≥2 and F≥3) was assessed using ROC curves. The area under the curve (AUC) with 95% confidence interval (CI) was calculated; good performance was defined as AUC >0.8 [29]. The TE grade prediction sensitivity and specificity of each biomarker were also calculated. Statistical analysis was performed using MedCalc Statistical Software (version 15.8, MedCalc Software, Mariakerke, Belgium) and IBM SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA). P <0.05 was considered statistically significant.
RESULTS
1. Comparison of biomarkers according to TE grade
ELF, M2BPGi, galectin-3, and sST2 values differed significantly according to TE grade (Table 2 and See Supplemental Data Fig. S1). All four biomarkers increased in F3/4 compared with F0/1 (P <0.0001 except galectin-3, P =0.0036). ELF and M2BPGi values were higher in F2 than in F0/1 (P ≤0.001, all); however, no significant differences were observed between F2 and F3/4. In contrast, sST2 concentration increased in F3/4 compared with F2 (P =0.0192); however, no significant difference was observed between F0/1 and F2. The positivity of ELF and M2BPGi increased according to TE grade (both P <0.0001 by Cochran-Armitage test for trend).
Table 2. Comparison of biomarkers according to TE grade.
| Variable | F0/1 (N = 129) | F2 (N = 16) | F3/4 (N = 28) | P* | ||
|---|---|---|---|---|---|---|
| F0/1 vs F2 | F0/1 vs F3/4 | F2 vs F3/4 | ||||
| ELF | 8.7 (8.2–9.2) | 9.8 (9.3–10.1) | 10.2 (9.4–11.8) | 0.0001 | < 0.0001 | 0.0831 |
| None to mild (%) | 4 (3.1) | 0 (0.0) | 0 (0.0) | 0.0010 | < 0.0001 | 0.160 |
| Moderate (%) | 112 (86.8) | 9 (56.2) | 11 (39.3) | |||
| Severe (%) | 13 (10.1) | 7 (43.7) | 17 (60.7) | |||
| M2BPGi (COI) | 0.4 (0.3–0.6) | 0.8 (0.6–1.3) | 0.9 (0.6–1.6) | < 0.0001 | < 0.0001 | 0.4494 |
| Negative (%) | 126 (97.7) | 9 (56.3) | 14 (50.0) | < 0.0001 | < 0.0001 | 0.059 |
| Positive (%) | 3 (2.3) | 7 (43.7) | 14 (50.0) | |||
| Galectin-3 (ng/mL) | 10.6 (8.5–13.2) | 11.3 (8.9–13.3) | 13.1 (10.8–16.3) | 0.3618 | 0.0036 | 0.2831 |
| sST2 (ng/mL) | 31.0 (24.0–36.4) | 37.0 (27.1–41.6) | 42.9 (33.3–74.9) | 0.1331 | < 0.0001 | 0.0192 |
Data are presented as median (interquartile range) or number (percentage).
*Mann-Whitney U test (galectin-3 and sST2); chi-square test or Fisher's exact test (ELF and M2BPGi).
Abbreviations: TE, transient elastography; ELF, enhanced liver fibrosis; M2BPGi, Mac-2-binding protein glycosylation isomer; sST2, soluble suppression of tumorigenicity 2.
2. Comparison of ROC curves for prediction of TE grade
Both ELF and M2BPGi predicted TE grade F≥2 and F≥3 with good performance (AUC of 0.841 and 0.837, respectively, for ELF; AUC of 0.833 and 0.808, respectively, for M2BPGi; Fig. 1). The AUCs of ELF and M2BPGi were greater than those of galectin-3 and sST2 for detecting TE grade F≥2 (P =0.0002, 0.0053 and P =0.0011, 0.0152, respectively). TE grade F≥2 prediction sensitivity and specificity were 84.1% and 76.7% for ELF and 63.6% and 91.5% for M2BPGi. For TE grade F≥3, only ELF showed a greater AUC compared with galectin-3 (P =0.0199).
Fig. 1. Comparison of the receiver operating characteristics curves for predicting transient elastography grade F≥2 (A) and F≥3 (B).
Abbreviations: TE, transient elastography; ELF, enhanced liver fibrosis; M2BPGi, Mac-2-binding protein glycosylation isomer; sST2, soluble suppression of tumorigenicity 2.
3. Distribution and agreement of fibrosis grades according to TE, ELF, and M2BPGi
Concordance and agreement between TE grade, ELF, and M2BPGi were determined using both the manufacturer-suggested cut-off values for ELF and M2BPGi (7.7 and COI of 1.0, respectively) and their optimal cut-off values derived from ROC curve analyses (ELF>9.2; M2BPGi, COI of 0.71). Using the pre-set cut-off values for detecting TE grade F≥2, only M2BPGi showed a concordance of 85.0% with weak agreement (kappa 0.534, 95% CI=0.383–0.685; Table 3). The concordance between TE grade and ELF and between ELF and M2BPGi was very low (27.7% and 16.1%, respectively) with no agreement. When the optimal cut-off values for ELF and M2BPGi were used, the concordance between TE, ELF, and M2BPGi improved, ranging from 70.5% to 84.3%.
Table 3. Distribution and agreement of fibrosis grades according to TE, ELF, and M2BPGi.
| TE grade | ELF grade | Concordance* | kappa (95% CI)* | Concordance† | kappa (95% CI)† | ||
|---|---|---|---|---|---|---|---|
| None to mild | Moderate | Severe | |||||
| F0/1 | 4 | 112 | 13 | 27.70% | 0.016 (−0.000–0.032) | 77.50% | 0.499 (0.370–0.629) |
| F2 | 0 | 9 | 7 | ||||
| F3/4 | 0 | 11 | 17 | ||||
*The agreement between groups was calculated based on TE grade F≥2 and the suggested cut-off values for ELF and M2BPGi (7.7 and COI of 1.0, respectively); †The agreement between groups was calculated based on TE grade F≥2 and the optimal cut-off values for ELF and M2BPGi (9.2 and COI of 0.71, respectively).
Abbreviations: TE, transient elastography; ELF, enhanced liver fibrosis; M2BPGi, Mac-2-binding protein glycosylation isomer; sST2, soluble suppression of tumorigenicity 2.
4. Comparison of each biomarker according to MELD and CTP scores
The distribution of the four biomarkers did not differ between high and low MELD scores (Table 4). In terms of CTP scores, the distribution of ELF, M2BPGi, and sST2 values differed significantly between high and low CTP scores (P =0.0096, 0.0320, and 0.0258, respectively).
Table 4. Comparison of each biomarker according to MELD and CTP scores.
| MELD < 9 (N = 77) | MELD ≥ 9 (N = 6) | P* | CTP < 7 (N = 104) | CTP ≥ 7 (N = 7) | P* | |
|---|---|---|---|---|---|---|
| ELF | 9.1 (8.3–9.8) | 9.2 (8.4–9.7) | 0.9477 | 9.1 (8.5–9.8) | 10.6 (9.7–12.1) | 0.0096 |
| None to mild (%) | 1 (1.3) | 0 (0.0) | 0.7704 | 1 (1.0) | 0 (0.0) | 0.0632 |
| Moderate (%) | 56 (73.7) | 6 (85.7) | 77 (74.8) | 3 (37.5) | ||
| Severe (%) | 19 (25.0) | 1 (14.3) | 25 (24.3) | 5 (62.5) | ||
| M2BPGi (COI) | 0.4 (0.3–0.7) | 0.5 (0.2–1.2) | 0.9935 | 0.5 (0.3–0.8) | 1.1 (0.6–2.4) | 0.0320 |
| Negative (%) | 64 (84.2) | 5 (71.4) | 0.3359 | 85 (82.5) | 4 (50.0) | 0.0479 |
| Positive (%) | 12 (15.8) | 2 (28.6) | 18 (17.5) | 4 (50.0) | ||
| Galectin-3 (ng/mL) | 10.7 (8.7–12.9) | 10.4 (8.6–15.7) | 0.5443 | 10.8 (8.7–13.2) | 13.7 (11.5–15.3) | 0.0830 |
| sST2 (ng/mL) | 31.3 (24.9–39.8) | 36.0 (27.9–42.1) | 0.5443 | 31.9 (27.7–39.8) | 44.6 (35.0–72.9) | 0.0258 |
Data are presented as median (interquartile range) or number (percentage).
*Mann-Whitney U test (galectin-3 and sST2); chi-square test or Fisher's exact test (ELF and M2BPGi).
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4; MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh; TE, transient elastography; ELF, enhanced liver fibrosis; M2BPGi, Mac-2-binding protein glycosylation isomer; sST2, soluble suppression of tumorigenicity 2.
DISCUSSION
We evaluated the usefulness of the emerging biomarkers ELF, M2BPGi, galectin-3, and sST2 for assessing liver fibrosis and prognostic values in CLD. All biomarker values differed significantly according to fibrosis grade; ELF (≥7.7 score) and M2BPGi (≥1+) liver fibrosis positivity increased significantly according to TE grade (both P <0.0001) (Table 2 and See Supplemental Data Fig. S1). However, the distribution patterns of the biomarkers varied. While ELF and M2BPGi could discriminate F0/1 and F2, they could not discriminate F2 and F3/4. In contrast, sST2 could discriminate F2 and F3/4, but could not discriminate F0/1 and F2. Compared with ELF and M2BPGi, sST2 appears to be related to more advanced fibrosis grades.
ELF and M2BPGi use different targets for assessing fibrosis. ELF incorporates HA, TIMP-1, and P3NP, which are all involved in extracellular matrix synthesis and degradation [30]. During the progression of liver disease and fibrosis, the N-glycosylation of M2BP is altered, and it binds to galectin-3, β-1 integrins, collagens, and fibronectin [15]. M2BPGi assays measure the WFA-positive, glycosylation isomer of M2BP. Previous studies have shown that ELF has a better ability to discriminate moderate fibrosis (F≥2) [31], whereas M2BPGi possesses a better capacity to discriminate severe fibrosis (F≥3) [13]. However, our data did not show this trend; thus, further studies are needed to gain more insight.
ELF and M2BPGi were comparable in ROC curve analyses, exhibiting equally good performance for detecting TE grade F≥2 and F≥3 (Fig. 1). Considering the number of laboratory markers, M2BPGi, which uses a single marker, would be a more practical option than ELF, which requires three markers. However, the respective agreement between TE, ELF, and M2BPGi was weak (Table 3); the reported values of ELF and M2BPGi were interpreted semi-quantitatively using manufacturer-provided, pre-defined cut-off values. Our data indicate that the optimal cut-off values of ELF and M2BPGi for detecting liver fibrosis differed from the manufacturer-provided cut-off values (9.2 vs 7.7 and 0.71 COI vs 1.00 COI, respectively). Using the manufacturer-recommended cut-off (7.7), ELF showed too many positive results compared with TE and poor agreement with TE or M2BPGi. A higher cut-off value would be desirable to improve the performance of ELF. The recent guidelines of the National Institute for Health and Care Excellence (NICE) recommend the use of ELF for monitoring advanced liver fibrosis in non-alcoholic fatty liver disease and suggest a cut-off of 10.51 [32].
To the best of our knowledge, this is the first study to evaluate the four biomarkers, ELF, M2BPGi, galectin-3, and sST2, in association with liver fibrosis. Galectin-3, a paracrine factor mainly secreted by activated macrophages, has been identified as a critical regulator in inflammation and fibrogenesis [19], while sST2 is known to be involved in non-cardiac inflammation [21]. Both are promising markers for systemic inflammation and fibrosis process, and their evaluation in various clinical conditions would be valuable. In this study, galectin-3 and sST2 increased significantly in liver fibrosis; however, their ability to detect liver fibrosis was not superior to that of the more specific and validated liver biomarkers, ELF and M2BPGi. Galectin-3 and sST2 might reflect systemic fibrosis rather than organ-specific fibrosis. Further studies are necessary to elucidate the diagnostic and prognostic roles of galectin-3 and sST2 in relation to liver fibrosis. Regarding prognosis, although all four biomarkers were unrelated to MELD score, higher values of ELF, M2BPGi, and sST2 were associated with high CTP score (Table 4). Additional data are required to evaluate the independent roles of these biomarkers in prognosis prediction.
This study has several limitations. We could not assess the degree of liver fibrosis based on LB, which is still considered the gold standard; however, its utilization is restricted because of invasiveness and has been mostly replaced by non-invasive approaches [10]. From a practical viewpoint, it was difficult to enroll patients who had undergone LB, because our hospital uses TE to assess liver fibrosis in most routine situations. Recently, a non-invasive approach implementing both elastographic and serologic tests has been proposed. In this approach, the concordance between elastographic and serologic tests is assessed and LB is limited solely to patients with discordant results [33]. In addition, our study population was not sufficiently large for each grade of liver fibrosis, especially high-grade fibrosis. Because the performance of each biomarker may differ according to fibrosis grade, further large-scale studies would be needed to elucidate the clinical usefulness of each biomarker. Our study focused on novel, potential fibrosis markers; thus, we excluded the analysis of conventional markers, including APRI or FIB-4, because they have been extensively studied.
In conclusion, we evaluated four potential biomarkers, ELF, M2BPGi, galectin-3, and sST2, for assessing liver fibrosis in patients with CLD. The values of each biomarker differed significantly according to fibrosis grade and showed a different trend. ELF and M2BPGi exhibited equally good performance for detecting moderate and severe liver fibrosis. Optimal cut-off values should be applied for the interpretation of liver fibrosis, following validation in each population. Large-scale, prospective studies are required to elucidate the clinical usefulness of galectin-3 and sST2 for assessing liver fibrosis.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
SUPPLEMENTARY MATERIAL
Distribution of each biomarker according to TE grade. Boxes designate the interquartile range (25 to 75 percentile) and the middle line represents the median. The error bar represents minimum and maximum values.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Kim MN, Han KH, Kim SU. Clinical application of transient elastography in patients with chronic viral hepatitis receiving antiviral treatment. Liver Int. 2015;35:1103–1115. doi: 10.1111/liv.12628. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911–915. doi: 10.1016/j.gcb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 5.Kim SU, Kim BK, Han KH. Clinical application of liver stiffness measurement using transient elastography: a surgical perspective. Digestion. 2013;88:258–265. doi: 10.1159/000355948. [DOI] [PubMed] [Google Scholar]
- 6.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 7.Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 8.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–1859. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13:402–411. doi: 10.1038/nrgastro.2016.86. [DOI] [PubMed] [Google Scholar]
- 11.Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191–198. doi: 10.1016/j.jhep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776–784. doi: 10.1007/s00535-014-1007-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–1570. doi: 10.1002/hep.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Kuno A, Ikehara Y, Sugiyama M, Saito H, Aoki Y, et al. LecT-Hepa, a glyco-marker derived from multiple lectins, as a predictor of liver fibrosis in chronic hepatitis C patients. Hepatology. 2012;56:1448–1456. doi: 10.1002/hep.25815. [DOI] [PubMed] [Google Scholar]
- 17.Wettersten N. Biomarkers for heart failure: an update for practitioners of internal medicine. Am J Med. 2016;129:560–567. doi: 10.1016/j.amjmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 19.Henderson NC. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh WC, Mackinnon AC, Lu WY, Jung J, Boulter L, Henderson NC, et al. Galectin-3 regulates hepatic progenitor cell expansion during liver injury. Gut. 2015;64:312–321. doi: 10.1136/gutjnl-2013-306290. [DOI] [PubMed] [Google Scholar]
- 21.Mueller T. The Presage(®) ST2 Assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev Mol Diagn. 2013;13:13–30. doi: 10.1586/erm.12.128. [DOI] [PubMed] [Google Scholar]
- 22.Hur M, Kim H, Kim HJ, Yang HS, Magrini L, Marino R, et al. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. 2015;35:570–577. doi: 10.3343/alm.2015.35.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Hur M, Moon HW, Yun YM GREAT Network. Multimarker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. 2017;7:27. doi: 10.1186/s13613-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J Gastroenterol. 2016;22:9706–9717. doi: 10.3748/wjg.v22.i44.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamath PS, Kim WR Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 26.Murray KF, Carithers RL, Jr AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 27.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 28.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein MC, Fineberg HV. Clinical decision analysis. Philadelphia: WB Saunders; 1980. [Google Scholar]
- 30.Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18:23–31. doi: 10.1111/j.1365-2893.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim BK, Kim HS, Park JY, Kim DY, Ahn SH, Chon CY, et al. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e41964. doi: 10.1371/journal.pone.0041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. NICE guideline [NG49] 2016 Jul; [PubMed] [Google Scholar]
- 33.Tapper EB. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377:756–768. doi: 10.1056/NEJMra1610570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of each biomarker according to TE grade. Boxes designate the interquartile range (25 to 75 percentile) and the middle line represents the median. The error bar represents minimum and maximum values.