Abstract
The aim of this study was to evaluate the functional and radiological outcome of TruFit plugs. We retrospectively reviewed 10 patients who underwent treatment for a symptomatic chondral/osteochondral lesion using one or more Trufit Plugs. Full incorporation of the bony portion of the plug occurred in only 3 and partial incorporation in 7 lesions. The remaining portion of these 7 lesions looked cystic on MRI. The significance of this cystic change is not clear. Though all 10 patients showed some improvement on the IKDC scoring system but the amount of the improvement was small.
Keywords: Trufit plug, Chondral defect, Cartilage regeneration, Knee, Osteochondral graft, Mosaicplasty
1. Introduction
The treatment of chondral knee injuries remains a challenge for the orthopaedic surgeon, mainly owing to the characteristics of the cartilage tissue, which promote low potential for regeneration. Chondral lesions can be caused by metabolic stimulation, or by genetic, vascular and traumatic events, and are classified according to the size and thickness of the affected cartilage.
Despite the development and research in osteoarthritis and cartilage regeneration, currently there is no consensus as to the best treatment option for chondral damage in young patients.1 The surgical techniques used for the treatment of partial thickness defects are Debridement and Ablation. These techniques aim to improve symptoms, since they do not restore normal structure and function of the cartilage. For full-thickness defects (osteochondral lesion), available treatments are Abrasion, Drilling, Microfracture, Osteochondral Autologous and Allogeneic Transplantation, and biological techniques such as the use of Autologous Chondrocyte Transplantation and stem cells.
Mosaicplasty is an accepted technique for the treatment of localized full-thickness articular cartilage defects in the weight-bearing areas of the knee. However the size of area that can be treated is limited by graft availability and donor site morbidity.2 A synthetic osteochondral plug, if successful, has the potential to overcome some of these issues.
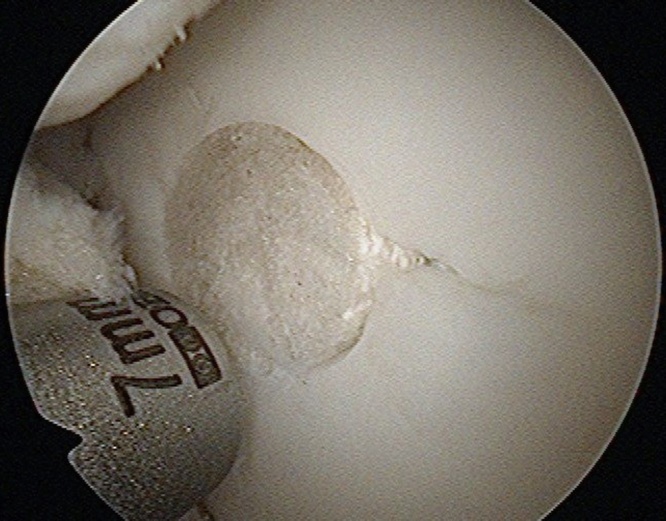
The Trufit plug, a bi-layer scaffold implant, (Smith&Nephew) (Picture 1) has been developed to treat chondral and osteochondral defects. This synthetic plug is designed to be inserted into the articular surface providing a scaffold to encourage chondrocyte regeneration to repair such defects.3 The aim of this study is to assess the 2 year MRI appearance of a series of Trufit plugs, used in the knee joint in order to evaluate their incorporation. It has previously been recognized that the plug can take up to 24 months to incorporate.3
Picture 1.
Arthroscopic view showing Trufit plug insertion into the femoral chondral defect.
2. Method
2.1. Patient selection
Between December 2007 and August 2009, 10 patients who underwent treatment of 12 symptomatic chondral or osteochondral lesions using Trufit Plugs at our institution were evaluated. These patients had not previously undergone any other surgical procedure for their osteochondral or chondral lesion. Patients with history of inflammatory arthritis, infective arthritis and any major trauma to the knee were excluded. Patients below 18 years and above 50 years of age were also excluded. Of the 10 patients, 7 were male and 3 were female with mean age of 35.2 years (range 22–49).
2.2. Details of the lesions and TruFit plugs used
There were 12 lesions treated with one or 2 plugs used for each lesion. A total of 14 Trufit plugs were used. The plug sizes used were; 3 × 5 mm, 2 × 7 mm, 5 × 9 mm and 4 × 11 mm10 lesions were in the medial femoral condyle with the remaining 2 lesions in the lateral femoral condyle. 1 or 2 plugs were used per lesion. 9 of the 12 lesions were chondral defects. The other 3 lesions were osteochondral. 1 patient also underwent ACL reconstruction and 2 patients had a concomitant meniscal repair.
2.3. Outcome measures
At a minimum of 2 years, MRI scan was performed with a 1.5 T MRI scanner with PD fat sat sagittal, coronal, axial and PD sagittal sequence. An independent Consultant Musculoskeletal Radiologist reviewed the scan for evidence of incorporation according to the descriptive modified magnetic resonance observation of cartilage repair tissue (MOCART) Scoring system.4 This system was devised to systematically record only those observations that can most accurately and reproducibly be determined and to avoid ambiguous terms. It has been shown to be reliable and to have excellent intra-observer reproducibility.4 All patients were prospectively scored with IKDC before and 2 years after the operation.
2.4. Statistical analysis
The nonparametric Wilcoxon signed rank test was used to determine differences between the data of pre-operative and follow-up IKDC scores.
3. Results
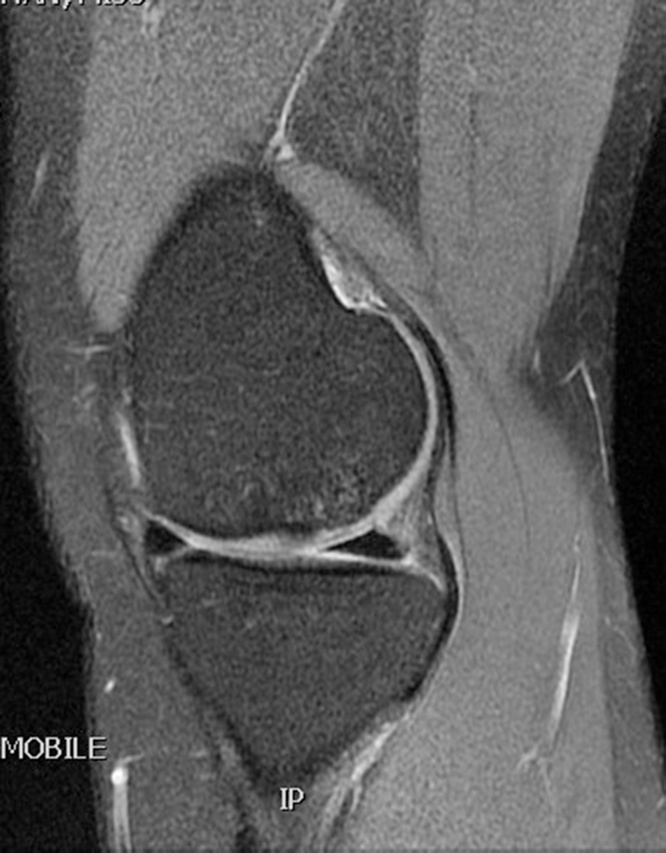
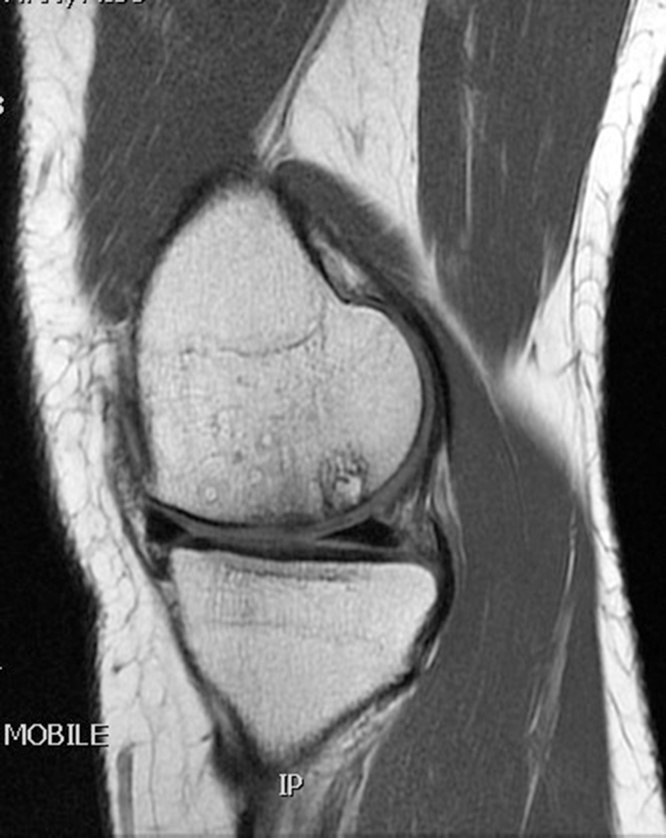
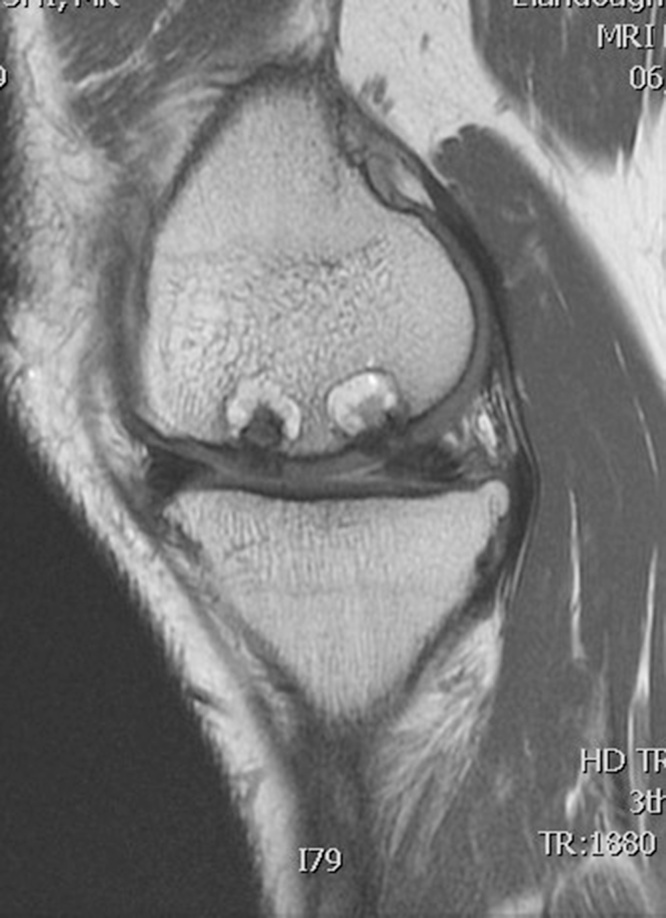
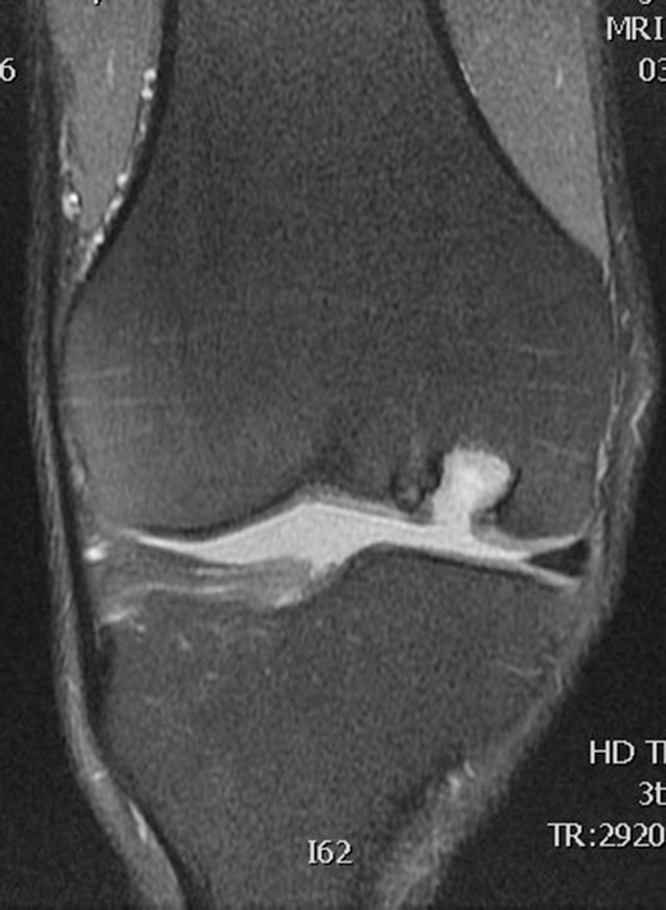
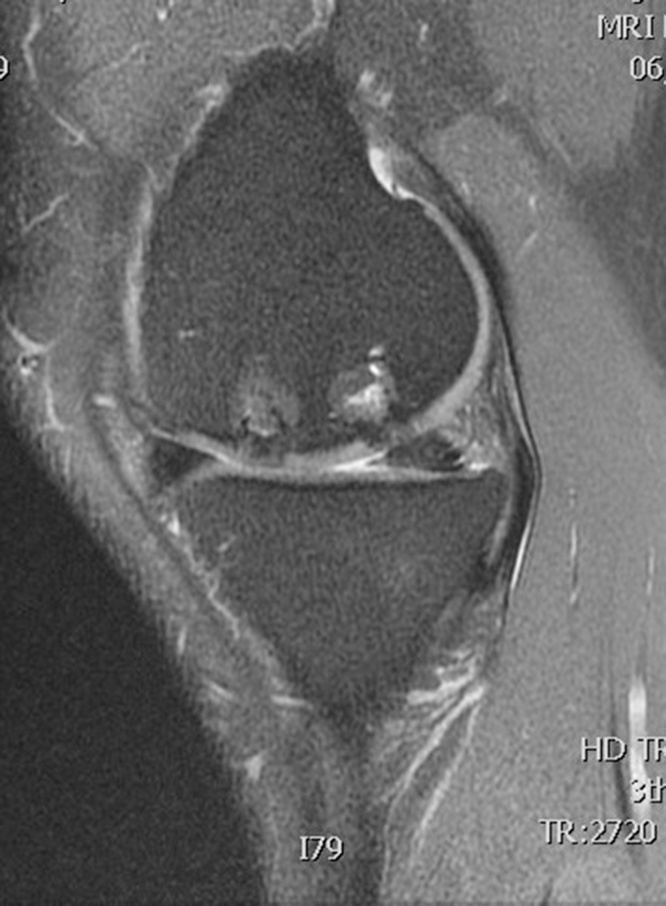
Table 1 summarizes the result of the MOCART scoring. 8 of 12 lesions showed congruent articular cartilage cover with a surface with a similar thickness and signal to the surrounding articular cartilage and reconstitution of the subchondral bone plate (Picture 2, Picture 3). 2 lesions had a thicker congruent articular surface with a similar signal to the surrounding articular cartilage without restoration of the subchondral bone plate. 2 lesions showed no graft incorporation at all and were filled with granulation tissue (Picture 6). Full incorporation of the bony portion of the plug had occurred in only 3 lesions with partial incorporation in 7 lesions. The remaining portion of these 7 lesions looked cystic on MRI (Picture 4, Picture 5).
Table 1.
MOCART scoring on MRI scan at 2years.
| Variable | No. of patients (12 lesions in 10 patients) |
|---|---|
| A − Degree of defect repair and defect filling | |
| 1- Complete (on level with adjacent cartilage) | 10 |
| 2- Hypertrophy (over the level of adjacent cartilage) | 00 |
| 3- Incomplete (under the level of adjacent cartilage) | 00 |
| 3a −>50% of adjacent cartilage | 00 |
| 3b −<50% of adjacent cartilage | 00 |
| 4- Subchondral bone exposed | 02 |
| B- Integration to border zone | |
| 1- Complete (complete integration with adjacent cartilage) | 10 |
| 2- Incomplete | 00 |
| 3- Demarcating border visible (split like) | 00 |
| 4a-<50% of the length of the repair tissue | 00 |
| 4b->50% of the length of repair tissue | 02 |
| C- Surface of the repair tissue | |
| 1- Surface intact | 10 |
| 2- Surface damaged (fibrillations, fissures and ulcerations) | 00 |
| 2a- <50% of repair tissue depth | 00 |
| 2b- >50% of repair tissue depth or total degeneration | 02 |
| D − Structure of the repair tissue | |
| 1- Homogeneous | 10 |
| 2- Inhomogeneous or cleft formation | 02 |
| E- Signal intensity of the repair tissue | |
| 1- Isointense | 10 |
| 2- Moderately hyperintense | 00 |
| 3- Markedly hyperintense | 02 |
| F- Subchondral lamina | |
| 1- Intact | 08 |
| 2- Not intact | 04 |
| G- Subchondral bone | |
| 1- Intact | 01 |
| 2- Oedema, granulation tissue, cysts, sclerosis | |
| 2a- Cyst | 07 |
| 2b- Granulation tissue | 02 |
| 2c- Oedema and sclerosis | 02 |
| H- Adhesions | |
| 1- No | 12 |
| 2- Yes | 00 |
| I- Effusion | |
| 1- No effusion | 07 |
| 2- Effusion | 05 |
Picture 2.
T2 weighted MRI scan sagittal section showing full incorporation of Trufit plug in femoral condyle defect.
Picture 3.
T1 weighted MRI scan sagittal section showing the full incorporation of Trufit plug.
Picture 6.
T1 wted MRI scan sagittal section showing partial incorporation of Trufit plugs.
Picture 4.
T2 weighted MRI scan coronal section showing no incorporation and the entire Trufit plug replaced by granulation tissue.
Picture 5.
T2 weighted MRI scan sagittal section showing partial incorporation of Trufit plugs in femoral condyle.
All 10 patients showed improvement on the IKDC scoring system. The overall mean IKDC score improved from 41.8 preoperatively to 57.4 at two years. Although this improvement was statistically significant (Wilcoxon signed rank test p = 0.028), the amount of clinical improvement was small.
4. Discussion
The Trufit plug is a biphasic construct attempting to reproduce the osteochondral unit. Each layer of this synthetic graft is made from a poly-lactide-co-glycolide copolymer. The 2 layers are designed to allow the ingrowth of bone and articular cartilage respectively. The 2 layers have differing porosities with the bone portion containing calcium sulphate and the cartilage layer containing a surfactant. The plug is designed to act as a wick bringing blood from the subchondral bone to the articular surface of the plug to stimulate healing of the defect.
As the plug is synthetic, there is no donor site morbidity, no risk of transmitted infection and no need to culture cells or for 2 surgeries. However, it is recognized that the plugs often take a long time to fill in (up to 2 years)3 and in 2/12 cases in this series there was no evidence of any healing even after 2 years. In order to insert the plug, a core of bone has to be removed, so if the plug does not heal, a larger bone and chondral defect is left which makes further treatment more difficult. Also, full incorporation was unusual with 7/12 lesions having incomplete healing of the bony portion of the plug. The significance of this cystic change is not clear. The size and frequency of this cystic area could possibly be reduced by drilling to a shallower depth and using a shorter plug but this could also reduce the stability of the plug.
The literature on the incorporation of the Trufit plug is conflicting. Barbar & Dockery reported on 9 cases using CT scan to assess the incorporation of plugs that had been used to back fill osteochondral donor sites in 9 cases.5 They found that the plugs failed to incorporate. Bedi et al. used MRI scanning and T2 mapping in 26 cases over a mean of 21 months (range 6–39 months).6 They noticed a predictable pattern of incorporation over time with 90% near or complete fill in those over 16 months. The MRI scan appearance deteriorated between 6 and 12 months before improving again with longer follow up. Dhollander et al. also felt that at 1 year the incorporation was incomplete, still ongoing and that longer follow up was required.7 Verhaegen et al. performed a systematic review of the literature on Trufit plugs.8 They did not find any evidence of osteoconductive bone ingrowth in any of the 5 studies that were included in the systematic review. Our findings were also similar to these.
The clinical outcome using the Trufit plug has also been mixed. Sgaglione & Florence reported 2 cases where the plug had failed to incorporate at 9 and 20 months respectively.9 Carmont et al. had a series of 11 patients who showed improvement in Lysholm, Tegner IKDC scores over the first 12 months.3 Joshi et al. reported on a series of 10 cases where the plugs had been used for patellofemoral cartilage damage.10 They showed improvement in KOOS, VAS and SF36 in 8/10 at 1 year with a poor result in 2/10. By 18 months all but 1 patient had pain and knee swelling and their reoperation rate was 70%. Dhollander et al. reported on 20 cases.7 At 1 year, 3 of the 15 they reviewed had persistent symptoms that required revision surgery with bone grafting. All 3 improved following this. Overall the KOOS & Tegner scores had improved at 1 year. Our cohort had encouraging MRI findings at 2 years but only small improvement in symptoms as measured by the IKDC score at 2 years.
4.1. Limitations
Our study has a few of limitations. This is a retrospective study. The number of patients is small and it has no control group for comparison but it opens up the doors for more research in this area to further assess the suitability of this option in the management of the full thickness chondral defects.
5. Conclusion
The MRI appearances of the Trufit Plug at 2 years are encouraging with the majority showing good restoration of the articular surface with tissue of similar thickness, congruity and signal as the surrounding articular cartilage. Full incorporation of the Trufit Plug is rare though and cystic change in the bone portion is common. The significance of this cystic change is not clear. The clinical results in our cohort have been disappointing because amount of clinical improvement was small. Further research is required in this area to know the suitability of this option in the management of full thickness chondral defects.
Author contribution
Azam A and Forster M contributed equally to this work. Azam A collected and analyzed the data, and drafted the manuscript. Forster M provided analytical oversight and supervised the study. Robertson A helped collecting the IKDC scores. All authors have read and approved the final version to be published.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest statement
The authors have none to declare.
References
- 1.McCoy B., Miniaci A. Osteochondral autograft transplantation/mosaicplasty. J Knee Surg. 2012;25(2):99–108. doi: 10.1055/s-0032-1322508. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A., Feeley B.T., Williams R.J., 3rd Management of articular cartilage defects of the knee. JBJS Am. 2010;92(4):994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 3.Carmont M.R., Carey-Smith R., Saithna A., Dhillon M., Thompson P., Spalding T. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy. 2009;25(7):810–814. doi: 10.1016/j.arthro.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Marlovits S., Singer P., Zeller P., Mandl I., Haller J., Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation:determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Barber F.A., Dockery W.D. A computer tomography scan assessment of synthetic multiphase polymer scaffolds used for osteochondral defect repair. Arthroscopy. 2011;27(1):60–64. doi: 10.1016/j.arthro.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A., Foo L.F., Williams R.J., Potter H.G. The maturation of synthetic scaffolds for osteochondral donor sites of the knee: an MRI and T2 mapping analysis. Cartilage. 2010;1(1):20–28. doi: 10.1177/1947603509355970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhollander A.A., Liekens K., Almqvist K.F. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy. 2012;28(2):225–233. doi: 10.1016/j.arthro.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Verhaegen J., Clockaerts S., Van Osch G.J.V.M., Somville J., Verdonk P., Mertens P. TruFit plug for repair of osteochondral defects –where is the evidence? Systematic review of literature. Cartilage. 2015;6(1):12–19. doi: 10.1177/1947603514548890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sgaglione N.A., Florence A.S. Bone graft substitute plug failure with giant cell reaction in the treatment of osteochondral lesions of the distal femur: a report of 2 cases with operative revision. Arthroscopy. 2009;25(7):815–819. doi: 10.1016/j.arthro.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 10.Joshi N., Reverte-Vinaixa M., Diaz Ferreiro E.W., Dominguez-Oronoz R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients. Am J Sports Med. 2012;40(6):1289–1295. doi: 10.1177/0363546512441585. [DOI] [PubMed] [Google Scholar]