Abstract
ATP:citrate lyase (ACL) catalyzes the conversion of citrate to acetyl-coenzyme A (CoA) and oxaloacetate and is a key enzyme for lipid accumulation in mammals and oleaginous yeasts and fungi. To investigate whether heterologous ACL genes can be targeted and imported into the plastids of plants, a gene encoding a fusion protein of the rat liver ACL with the transit peptide for the small subunit of ribulose bisphosphate carboxylase was constructed and introduced into the genome of tobacco. This was sufficient to provide import of the heterologous protein into the plastids. In vitro assays of ACL in isolated plastids showed that the enzyme was active and synthesized acetyl-CoA. Overexpression of the rat ACL gene led to up to a 4-fold increase in the total ACL activity; this increased the amount of fatty acids by 16% but did not cause any major change in the fatty acid profile. Therefore, increasing the availability of acetyl-CoA as a substrate for acetyl-CoA carboxylase and subsequent reactions of fatty acid synthetase has a slightly beneficial effect on the overall rate of lipid synthesis in plants.
Acetyl-CoA is the basic building block of fatty acid chains, and enters the metabolic pathways both as a substrate and as a primer for fatty acid biosynthesis. Since acetyl-CoA does not cross through the membranes of subcellular compartments (Kuhn et al., 1981), it must be synthesized inside plastids, where de novo fatty acid synthesis occurs. In plants, two enzymes have previously been suggested as possible sources of acetyl-CoA. First, acetyl-CoA can be formed from free acetate by acetyl-CoA synthetase (Springer and Heise, 1989). Second, pyruvate, derived either as a product of ribulose bisphosphate carboxylase (Andrews and Kane, 1991) or supplied from glycolysis (Liedvogel and Bauerle, 1986), can be decarboxylated to acetyl-CoA by plastidial pyruvate dehydrogenase. However, it is still unclear whether there is sufficient activity of these enzymes in the plastids to produce all of the acetyl-CoA needed for acetyl-CoA carboxylase (ACC) and the subsequent reaction of fatty acid synthetase (Ohlrogge et al., 1993). In addition, the concentration of acetyl-CoA in plastids has been estimated to be only 30 to 50 μm, which is sufficient to meet the demand of acetyl-CoA for fatty acid synthesis for only a few seconds (Post-Beittenmiller et al., 1992).
While the origin of plastid acetyl-CoA has been the subject of much speculation in plant fatty acid biosynthesis, in animals and eukaryotic microorganisms (principally yeasts and fungi), an alternative acetyl-CoA-forming mechanism occurs in which citrate, generated in the mitochondria, is exported into the cytosol via a tricarboxylate transporter (Evans et al., 1983) and is converted into acetyl-CoA by ATP:citrate lyase (ACL; EC 4.1.3.8) (Evans and Ratledge, 1985a; Elshourbagy et al., 1990; Ratledge, 1997). The possession of this enzyme is also considered to be a biochemical marker for the classification of yeasts and fungi as “oleaginous” (Ratledge and Evans, 1989).
In plants, ACL has received little attention as an enzyme that could influence fatty acid biosynthesis. Some evidence of ACL activity in plant cells (Fritsch and Beevers, 1979; Kaethner and ap Rees, 1985; Ratledge et al., 1997) has led to the hypothesis that ACL activity might regulate the rate of plant fatty acid synthesis by controlling the rate at which acetyl-CoA is provided for ACC, similar to what occurs with oleaginous yeasts (Boulton and Ratledge, 1981). Recent work in our laboratory has shown that ACL is associated in part with the plastids of different plant species rather than being solely a cytosolic enzyme, as it is in yeasts and animals (Rangasamy and Ratledge, 2000). In addition, the activity of ACL increases during seed development in oilseed rape and closely correlates with lipid accumulation therein (Ratledge et al., 1997). However, the role of ACL in plants has never been studied at the molecular level primarily because of difficulties associated with its purification and of identifying the corresponding gene. ACL has so far been cloned and sequenced only from rat (Elshourbagy et al., 1990) and human (Elshourbagy et al., 1992), which share at least 95% homology at the nucleotide level. Rat and human ACL are both composed of four homomeric subunits, each with 110-kD polypeptides (Elshourbagy et al., 1990, 1992).
To elucidate the enzymic steps involved in metabolic pathways, the overexpression of specific enzymes in transgenic plants could help to delineate the flux of carbon into lipids. Our interest in ACL has led us to ask the question of whether this enzyme could be the controlling step for acetyl-CoA synthesis prior to fatty acid biosynthesis commencing? And, if overexpressed, would it lead to increased lipid production? However, attempts to overexpress some enzymes of fatty acid biosynthesis in plants has sometimes led to co-suppression of genes with the result that the target gene product is not enhanced but, rather, is decreased (Verwoert et al., 1994). Thus, to avoid this potential problem of co-suppression, an alternative approach to express the cytosolic rat ACL gene was attempted. Since de novo fatty acid synthesis in plants occurs in the plastids, a transit peptide has to be added to the cytosolic rat ACL to accomplish the appropriate subcellular localization. The protein would not only have to be imported efficiently, but would also have to fold correctly into an active structure and then function in a non-native, subcellular location. In the present study, we describe efforts to target the cytosolic gene product of rat ACL to the plastids of transgenic plants and examine the effects of plastidial expression of ACL gene on the fatty acid composition of lipids.
MATERIALS AND METHODS
Plant Materials
Tobacco (Nicotiana tobacum L. cv White Burley) plants were maintained under sterile conditions as previously described (Mathis and Hinchee, 1994).
DNA Construction
Routine DNA manipulations were performed according to the method of Sambrook et al. (1989). For studies involving expression of ACL in plastids, the 3.8-kb PstI and BamHI fragment containing the complete sequence of cDNA produced from the rat liver ACL gene (Elshourbagy et al., 1990) was first inserted into the PstI-BamHI sites of pJIT117 (Guerineau et al., 1988). This places ACL between the transit peptide sequence of a pea Rubisco small subunit (SSU) gene (Anderson and Smith, 1986) and the cauliflower mosaic virus (CaMV) 35S polyadenylation signals, and is then under the transcriptional regulation of a doubled CaMV 35S promoter. The nucleotide sequence of recombinant SSU/ACL, including the 5′ and 3′ junctions, was confirmed by sequence determination. The resulting chimeric gene was re-isolated as a SstI/XhoI fragment and cloned into the SacI/SalI sites of binary vector pBin19 (Frisch et al., 1995). The construct was then introduced into Agrobacterium tumefaciens LBA4404 by electroporation. The positive clones from transformed LBA4404 were further confirmed by Southern hybridization of total DNA of A. tumefaciens using a 1.6-kb internal HindIII fragment of the ACL as a probe.
Transformation of Tobacco
Leaf discs from tobacco plants were transformed with A. tumefaciens LBA4404 harboring the construct of interest (p35S-SSU/ACL) as described by Mathis and Hinchee (1994) with some modifications. The leaf discs were initially grown for 1 week on the shooting medium containing 50 μg kanamycin/mL to select the transformants and with 500 μg carbenicillin/mL to counterselect the A. tumefaciens. Explants were then transferred to fresh shooting medium (Mathis and Hinchee, 1994) that contained 100 μg kanamycin/mL and 400 μg carbenicillin/mL and were incubated in the culture vials for about 3 to 4 weeks in a ventilation apparatus (Armstrong et al., 1996) to stimulate faster growth of plants. (This device not only provides a sustained oxygen supply, but also removes excess and inhibitory volatile substances, such as ethylene and polyphenolics, around tissues.) The regenerated shoots were excised and transferred to the rooting medium as described previously (Mathis and Hinchee, 1994). The kanamycin-resistance seedlings were transferred to compost substrate and grown in the greenhouse.
PCR Screening
PCR was used to screen the transformants for the presence of ACL sequences in their genome. A forward primer (5′-TCGTGGAAAAGCCGTTCCACC-3′) (7287–7311 of 35S promoter) and reverse primer (5′-TACCAAGCTCTGGC- TAAGCAG CAA-3′) (138–162 of ACL) were used to amplify a fragment at the junction of the 35S-SSU/ACL gene. The PCR reaction mixture and assay conditions were performed as described previously (McGarvey and Kaper, 1991).
Plant DNA Extraction and Analysis
Total DNA was extracted from plants and digested with PstI and electrophoresed in 0.7% (w/v) agarose gels. DNA was alkaline blotted by the standard Southern procedure (Guerineau et al., 1988) to Hybond-N+ nylon membranes (Amersham, Buckinghamshire, UK) and probed with the ACL coding region cDNA labeled with 32P-dCTP by a random priming procedure as described by the manufacturer. After hybridization for 18 h at 65°C in Expresshyb solution (CLONTECH Laboratories, Palo Alto, CA), filters were washed sequentially in 40 mm Na2HPO4, 0.1% (w/v) SDS at 65°C for 20 min (three times), and then exposed to x-ray film at −70°C for 2 d.
Plastid Isolation
The mature leaves were homogenized in ice-cold buffer (0.25 m Suc, 0.25 m sorbitol, 10 mm HEPES [pH 7.6], 0.2% [w/v] polyvinylpyrrolidone, 0.05% [w/v] bovine serum albumin [BSA], and 5 mm EDTA) at a ratio of 1 g fresh weight/mL using 10-s bursts of a Polytron homogenizer at its highest setting. The crude homogenate was then filtered through two pre-wetted layers of Miracloth (Calbiochem, San Diego) and centrifuged for 2 min at 3,000g. The supernatant was decanted and the plastid-enriched pellet was washed again with the above buffer. In some experiments, 10 μg of thermolysin (Sigma, St. Louis) was added to 100-μL plastid fractions and incubated on ice for 20 min. After inactivation of the thermolysin by adding EDTA to give 10 mm, the plastid fractions were separated by centrifuging in a 10% to 80% (v/v) Percoll density gradient as described by Robinson and Barnett (1988). The purified plastids were suspended in 50 mm Tris/HCl, pH 7.8, 1 mm EDTA, 1 mm MgCl2, 2 mm dithiothreitol (DTT), and 1 mm p-aminobenzamidine, and freeze-thawed once before being used. Portions of the crude homogenate and 3,000g supernatant and plastid fractions were kept for further analysis.
Immunoblot Analysis of Proteins
For quantitative immunoblots, equal amounts of leaf proteins were separated by SDS-PAGE using 6% (w/v) gels and transferred to nitrocellulose membranes following a standard procedure (Sambrook et al., 1989). ACL was detected with rat anti-ACL antibody as described previously (McGarvey and Kaper, 1991) and visualized with alkaline phosphatase-conjugated secondary antibody. Relative amounts of ACL proteins were estimated by densitometer scanning of the immunoblots.
Enzyme Activity
Determination of ACL activities was performed using dialyzed homogenates of young leaves as detailed previously (Rangasamy et al., 1997). Protein concentrations were estimated by the method of Bradford (1976) using a microprotein kit assay (Sigma) with γ-globulin as the control. The purity of plastid and cytosol fractions were determined by assaying glyceraldehyde-3-P dehydrogenase (Entwistle and ap Rees, 1988) and PEP carboxylase (Wedding and Kline, 1994) as plastidic and cytosolic markers, respectively.
Lipid and Fatty Acid Analysis
Leaf lipids were extracted according to the method of Browse et al. (1993). Fatty acid composition of individual lipids (three independent analyses per plant) was determined by gas chromotography as previously described (Horiguchi et al., 1996).
RESULTS
Construction of Chimeric SSU/ACL Gene
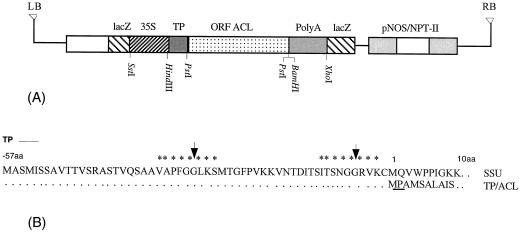
As plant fatty acid synthesis primarily occurs in the plastids, the ACL construct was designed to target the product of the rat liver cytosolic ACL gene (Elshourbagy et al., 1990) to the plastids of tobacco. The transit peptide of Rubisco SSU was selected for plastid targeting and import because it is known to import heterologous proteins efficiently. Our previous report on transient expression of ACL in pea (Rangasamy et al., 1997) suggested that the N-terminal extension of the Rubisco SSU alone has sufficient functions to import heterologous ACL proteins efficiently and to ensure proper cleavage of the transit peptide. The SSU/ACL construct is basically composed of a full-length cDNA containing the entire coding region of rat liver ACL, which is cloned in between the SSU transit peptide and poly(A+) tail sequences under the control of duplex CaMV 35S promoter (Fig. 1). These constructs were then inserted between the T-DNA borders of pBin19 containing a selectable neomycin phosphotransferase gene (NPT II).
Figure 1.
Map of plasmid SSU/ACL constructs. A, The open reading frame of rat ATP:citrate lyase was inserted between the transit peptide of SSU and polyadenylation signals under the control of the CaMV 35S promoter. Sites of action of the restriction endonucleases BamHI, HindIII, PstI, SstI, and XhoI are indicated. B, Amino acid sequences at the junction between the transit peptide of SSU (TP) and the N-terminal region of the rat ACL polypeptides. Sequences are aligned with the corresponding region of authentic Rubisco small subunit (SSU) precursor. Asterisks indicate the conserved amino acid residues for stromal protease recognition. Arrows show the cleavage sites between the TP and the mature ACL protein. The underlined sequence is the PstI site at which the ACL fragment was joined.
The chimeric SSU/ACL construct was introduced into the tobacco genome by co-cultivation of a leaf disc with transformed A. tumefaciens LBA 4404 transformation. Independently transformed plants were selected by their ability to survive in media containing kanamycin and screened for nopaline synthesis. Transformation frequencies based on the percentage of explants producing kanamycin-resistance shoots was between 8% and 10%. All of the transformants appeared to be phenotypically normal and produced seed in a good yield. Five of the SSU/ACL transformants and a control (transformed with vector alone) were selected for further analysis.
Expression of the Chimeric SSU/ACL Gene in Transgenic Plants
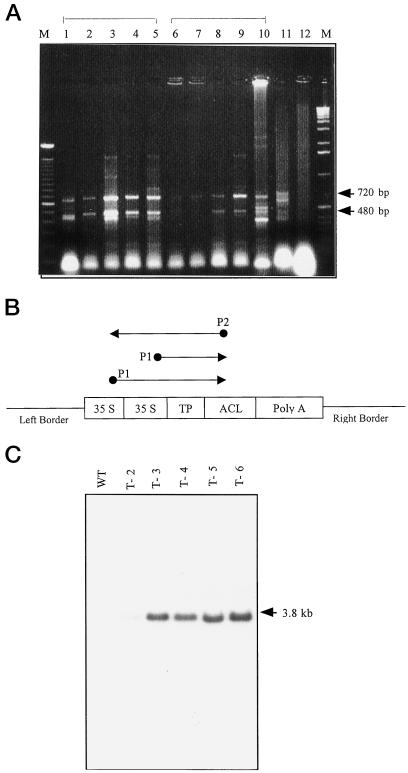
Successful transfer of SSU/ACL constructs in the transformed plants was confirmed by PCR of the total DNA isolated from young leaves of mature plants using two sets of reactions with varying annealing temperatures (Fig. 2A). As SSU/ACL constructs contained a duplicated sequence of CaMV 35S promoter, equivalent to position 7,040 to 7,433 (transcription initiator) and 7,139 to 7,379 (enhancer) of CaMV, the primer P1 recognized two binding sites corresponding to the position at 7,287 to 7,311 of the CaMV 35S duplex (Fig. 2B), thereby giving rise to two major products with sizes of 720 and 480 bp correlating with the region between the CaMV 35S promoter and the ACL gene (Fig. 2A). In addition, the Southern-blot analysis of total DNA isolated from young leaves of mature plants, using the rat ACL gene as a probe, revealed a 3.8-kb fragment corresponding to the full-length rat ACL gene (Fig. 2C).
Figure 2.
Analysis of five primary transformants by PCR and Southern blotting. A, Five nanograms of genomic DNA was used as a template for the PCR reaction as per McGarvey and Kaper (1991) but with an annealing temperature of 48°C (lanes 1–5 for transformants T2–T6) or 55°C (lanes 6–10 for same transformants). The plasmid SSU/ACL construct was used as a positive control (lane 11) and an untransformed plant (lane 12) as a negative control. B, Panel showing the location of primer sequences, P1 (CaMV primer) and P2 (ACL primer) in SSU/ACL constructs. The primer, P1, was expected to bind at two sites in the duplicated CaMV 35S promoters, giving rise to two sequences of DNA of 720 and 480 bp, which are seen in A. C, Southern analysis of PstI-digested genomic DNA (5 μg in each lane) of SSU/ACL transformants that were positive on PCR screening, probed with the rat ACL cDNA. Lane 1, Untransformed plant; lanes 2 to 6, SSU/ACL transformants; a 3.8-kb band corresponding to a fragment of the rat ACL can be observed.
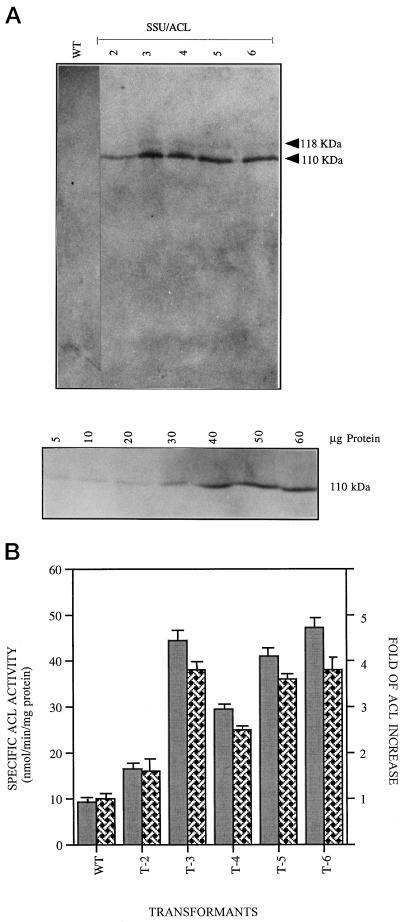
The anti-ACL immunoblots were also used to assess the presence or absence of the ACL gene products in the transformed plants. As the rat ACL antibody has no immuno-cross-reactivity with tobacco endogenous ACL proteins, and thus serves as a control (Rangasamy and Ratledge, 2000), the antibody was expected to detect only introduced ACL proteins in the transformants. The results in Figure 3A indicated that the SSU/ACL gene constructs were correctly expressed in the transformants, were presumptively expressed in the plastids, and yielded a full-length polypeptide of the appropriate size, with its immunoresponse being directly proportional to the amount of protein. The immunoblot of transformants revealed two immunoreactive bands at 110 and 118 kD. The latter band was unexpectedly slightly larger than the mature ACL polypeptides at 110 kD. It seems likely that the removal of the Rubisco SSU transit peptide from the fusion proteins SSU/ACL was, therefore, incomplete and could thus account for the presence of the larger polypeptide. Other similar recombinant junctions have been previously reported to be defective for complete removal of the transit peptide after import into plastids (Wu et al., 1993).
Figure 3.
Expression of ACL in tobacco leaves. A, Protein extracts, 50 μg, of crude plastid fractions were loaded on 6% (w/v) SDS-PAGE, blotted on membranes, and probed with rat ACL antibody. Lane 1, Untransformed cells (wild type, WT); lanes 2 to 6, SSU/ACL transformants T2 to T6, respectively. The 110-kD band corresponds to the mature ACL protein. Bottom panel, Plastids isolated from an SSU/ACL transformant (T6) were treated with thermolysin and different amounts of proteins (5–60 μg) were loaded in each lane and probed with anti-ACL antibody. An increased intensity of signal was observed with an increase in the amount of proteins electrophoresed in individual lanes. B, Comparison of ACL activity (gray bars) and relative increase of ACL activity (hatched bars) among the SSU/ACL transformants T2 to T6 compared with the activity in the original, untransformed wild type (WT) cells. Bar represents the mean of three separate assays.
Analysis of ACL Protein and Activity
To determine whether the transgene product was active, the ACL activity was determined with young leaves of both control and SSU/ACL plants (Fig. 3B). Newly emerged leaf samples were collected randomly from each of transformants, pooled together to obtain a mean value of activity in each plant, and analyzed for ACL activity and quantified for the amount of enzyme via its cross-reaction with rat-ACL antibody. A range of values from 1.4- to up to 4-fold higher than the control was observed among the SSU/ACL transformed plants, whereas in the control plants, the activity was barely detectable, indicating that the SSU/ACL gene construct was yielding correctly assembled and active enzyme.
To assess the quantitative differences of ACL between the plants, a portion of the proteins was immunoblotted and the signal intensity of immunoreactive bands was quantified by densitometry using a standard curve obtained from a graduated series of known protein concentrations. The average of two such determinations from separate western blots was used to generate the value, and is presented (see Fig. 3B) as the increase in activity of the ACL proteins compared with that of the control. A simple regression comparison of the amount of ACL protein to ACL activity showed a positive correlation (r = 0.82; n = 6; n is control plus five independent transformants), supporting the conclusion that the amount of ACL had increased among the transformed plants.
Localization of SSU/ACL Gene Product
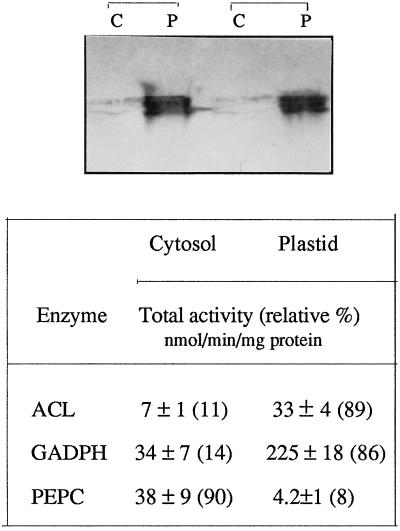
To study the subcellular localization of the SSU/ACL gene product, the plastids were isolated from young leaves of transformed plants by Percoll-density gradient centrifugation. The purity of organelle fractions was checked by assay of cytosolic and plastidic markers. Plastid-enriched fractions were free of cytosolic contamination, as indicated by the presence of NADH-glyceraldehyde-3-P dehydrogenase (at 90% of the total activity) and a meager presence (7% of total activity) of the cytosolic marker PEP carboxylase. ACL activity was estimated in both isolated fractions of cytosol and plastids to determine whether the transgenic products were active. By comparing the total ACL activity, the majority of activity was found to be localized in the plastid-enriched fractions of SSU/ACL plants. These results were confirmed by subjecting a portion of each protein extract to SDS-PAGE, followed by immunoblotting with rat-ACL antibody (Fig. 4). The western-blot signal was highly enriched in plastidic fractions, which could be easily detected within 5 min of exposure of the blots, whereas in the cytosol the signal could be observed only after overnight exposure (this experiment was repeated with identical results; see Fig. 4.). It is likely that the presence of SSU/ACL proteins in the cytosol might have arisen by incomplete transportation of fusion proteins. However, based on densitometry, the amounts of ACL protein were at least 4- to 5-fold more abundant in all plastidic fractions than the cytosolic fractions of the SSU/ACL plants, but the visual examination of the bands (see Fig. 4) would suggest that there was 10 times more ACL protein in the plastids than in the cytosol. This discrepancy between the activity and amount of ACL may be due to partial inactivation of ACL (which is well-known to be unstable), giving a higher response to protein per se in the western blot than activity in the enzyme assay. These results indicate that the SSU/ACL construct achieved the targeting of the cytosolic ACL into the plastids.
Figure 4.
Localization of ACL protein in plastids. About 30 μg of protein extracts of both plastid (P) and cytosol (C) fractions were separated on SDS-PAGE and the resulting gel was immunoblotted with rat ACL antibody. The majority of ACL proteins localized in the plastidic fraction can be observed. This experiment was repeated with identical results (duplicate is shown in top panel). Bottom panel, Purity of plastid and cytosol fractions based on the activities of NADPH-glyceraldehyde-3-P dehydrogenase (as a plastidic marker) and PEP carboxylase (as a cytosolic marker). The relative percentage of activity is shown in parentheses.
To confirm that the protein was inside the plastids rather than associated with the plastid envelope, the plastid-enriched fractions were either treated with the protease thermolysin or left untreated as a control. Treated plastids were then subjected to immunoblot analysis with rat ACL antibodies. Densitometry analysis indicated that the same level of SSU/ACL proteins was detected in protease-treated and untreated plastids (data not shown).
Fatty Acid Analysis of Transgenic Plants
The influence of the probable increased flux of acetyl-CoA produced by the cloned ACL on fatty acid synthesis was investigated in the SSU/ACL plants. The newly emerged and uppermost leaf was collected and used for fatty acid analysis to avoid any variations of fatty acids due to the effect of the developmental stage of leaf tissues. The amount of total fatty acids was increased by 16% in the transformed plants compared with the controls (Table I), and there was a slight change in the fatty acid profiles between the control and transformed plants: only oleic acid (18:1) and palmitic acid (16:0), which increased by approximately 18% and 11%, respectively, in the transgenic plants, were altered to any extent (Table I).
Table I.
Fatty acid content and composition of fatty acids in transformed tobacco (SSU/ACL) leaves compared with those in control leaves
| Plant | Total Fatty Acid | Fatty Acid
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:2 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | 22:1 | ||
| mg/g fresh wt | relative % (w/w) | |||||||||
| Control | ||||||||||
| 1 | 25.2 | 24.7 | 9.6 | 3.6 | 4.3 | 3.2 | 28.5 | 3.1 | 14.9 | 8.1 |
| 2 | 24.3 | 23.4 | 10.1 | 3.7 | 4.5 | 3.8 | 27.8 | 3.6 | 14.7 | 8.3 |
| SSU/ACL | ||||||||||
| T-2a | 24.8 | 24.1 | 8.9 | 3.5 | 3.7 | 3.6 | 29.5 | 3.8 | 14.2 | 8.7 |
| T-3 | 27.2 | 25.3 | 7.4 | 3.7 | 4.4 | 3.8 | 31.7 | 3.7 | 14.7 | 5.3 |
| T-4 | 27.4 | 25.9 | 7.5 | 3.8 | 4.4 | 3.2 | 31.6 | 3.1 | 15.6 | 4.9 |
| T-5 | 29.3 | 26.3 | 7.4 | 3.7 | 4.1 | 2.8 | 35.3 | 2.7 | 13.5 | 4.2 |
| T-6 | 28.3 | 29.4 | 5.5 | 2.8 | 2.7 | 2.2 | 35.1 | 2.3 | 15.8 | 4.2 |
| Control mean | 24.7 | 24.0 | 9.8 | 3.7 | 4.4 | 3.5 | 28.2 | 3.4 | 14.8 | 8.2 |
| SSU/ACL meanb | 28.7 | 26.7 | 6.9 | 3.5 | 3.9 | 3.0 | 33.4 | 3.0 | 14.9 | 4.7 |
Each analysis of the transformed tobacco was carried out in triplicate (on three separate leaves) with less than 3% variation in the proportions of individual fatty acids; the controls were assayed twice using six different leaves per assay, also with less than 3% variation.
This transformant showed only a meager increase in ACL activity over the control (see Fig. 3B), and its fatty acid profile is correspondingly closer to the control plants than the transformants.
Excluding T-2.
Available information about fatty acid composition in leaf lipids suggests that the relative amounts of 16:0 and 18:1 remain fairly constant during leaf growth (Browse et al., 1993). In addition, the changes in fatty acid composition in relation to developmental stage of tobacco cells are insignificant because the leaves are composed of mosaic cells of different ages. Studies on Arabidopsis and wheat leaf lipids (Browse et al., 1993; Horiguchi et al., 1996) suggest that only the content of triene fatty acids such as 16:3 and 18:3 would fluctuate with the developmental stage of the chloroplasts. However, in the present study, the proportions of triene fatty acids of both control and transgenic plants showed no marked differences. Thus, the effect of chloroplast development on fatty acid composition could be ruled out, suggesting that the slight increases observed in 16:0 and 18:1 in the SSU/ACL plants could be due to the action of ACL in the transformed plants.
DISCUSSION
An understanding of the factors that influence lipid accumulation in plant cells is of considerable practical value for oilseed crops. In lipid-accumulating yeasts and molds, ACL has been regarded as a potential rate-limiting reaction in lipid biosynthesis (Boulton and Ratledge, 1981; Evans and Ratledge, 1985a, 1985b; Ratledge and Evans, 1989) because: (a) its activity closely parallels the rate of fatty acid biosynthesis, and (b) the substrate for ACL, i.e. citrate, physically accumulates in the cytosol and then in the culture media during lipogenesis. In addition, the enhancement of ACC activity by citrate ensures that acetyl-CoA generated in the ACL reaction is directly utilized for lipid synthesis by this “feed-forward” activation (Evans and Ratledge, 1985a).
In plants, acetyl-CoA may be derived from several alternative pathways, including pyruvate dehydrogenase and acetyl-CoA synthetase. Recent studies on the occurrence of enzymes in the plastids of different species (Rangasamy and Ratledge, 2000) and comparison of the activity of ACC and ACL in relation to the rate of lipid accumulation during oilseed development (Ratledge et al., 1997) revealed that ACL might also play a key role in the regulation of fatty acid biosynthesis in plants. Although the sources of citrate for plant ACL are not yet clear, the citrate-malate shuttle system (Watson and Lowenstein, 1970) provides convincing evidence that citrate generated in the mitochondria can be exported into the cytosol, and thus would be able to enter the plastids. This citrate would then be cleaved in the plastids by ACL to give acetyl-CoA and oxaloacetate; the acetyl-CoA would then be utilized directly for de novo fatty acid synthesis (Fritsch and Beevers, 1979; Ratledge et al., 1997), while the oxaloacetate would be converted to malate by plastidial malate dehydrogenase and then exported back to the mitochondria to replenish the tricarboxylic acid cycle. The permeability of citrate and malate across the chloroplast membrane does not appear to be a problem in many plant species (Masterson et al., 1990; Smith et al., 1992). Alternatively, the malate may stay within the plastid and, via the possible action of malic enzyme (see Colombo et al., 1997), be used specifically to provide NADPH, which is also essential for fatty acid biosynthesis. Such a situation occurs in the oleaginous microorganism Mucor circinelloides (Wynn and Ratledge, 1997; Wynn et al., 1999).
The results of the present study demonstrate that the heterologous rat ACL gene, when linked to a transit peptide sequence of a pea Rubisco, could be targeted and imported into the plastids of tobacco, where the gene product was active and able to interact with existing metabolic pathways, as shown by western blot and analysis of ACL activity. Up-regulation of ACL by up to 4-fold, however, only led to a 16% increase in the production of fatty acids. Slight though this increase is, it is three times greater than the 5% increase in fatty acids achieved after the cloning of the Arabidopsis homomeric ACC into the plastids of rape seeds (Roesler et al., 1997). However, there is a considerable difference between the two studies in that Roesler et al. (1997) used a commercial oil crop and targeted the ACC gene directly into the oilseed tissue, whereas we have used the more easily manipulatable tobacco leaf system for a demonstration of “proof of principle” that ACL could also be a candidate for the rate-limiting step of fatty acid biosynthesis. Nevertheless a 16% increase of fatty acids in the transgenic plants (see Table I) can only be regarded as a marginal increase.
Although ACC has been a prime (but not sole) candidate as the rate-limiting step of plastidial fatty acid biosynthesis (Ohlrogge et al., 1993), it is clear from the work of Roesler et al. (1997) and that of Kang et al. (1994), who failed to find a correlation of ACC activity and lipid synthesis throughout the development of oilseed rape, that this proposition cannot be reasonably up-held. Indeed, the entire concept of there being a “rate-limiting” step in a complex pathway such as lipid biosynthesis may be erroneous (Fell, 1997). Even the present work has shown that only a small increase in lipid biosynthesis occurs by attempting to increase the flux of acetyl-CoA in the plastid by providing additional activity of ACL. As with most other systems that have evolved over long periods of time, metabolic pathways have become closely integrated so that any increase in the activity of a single enzyme or even a group of enzymes, merely identifies the next bottleneck that becomes the new rate-limiting reaction. The only solution is to clone into the cells genetic information coding for every single enzyme activity in an entire pathway which, for lipid biosynthesis, is scarcely feasible.
We would therefore suggest that it is probably no longer sensible to search for a rate-limiting step for fatty acid biosynthesis. Instead, however, it could be instructive to ask why different plant seeds have markedly different properties for the accumulation of oil. What are the biochemical reasons for these differences? Can they be related to identifiable enzyme activities that may not be the immediate enzymes of fatty acid biosynthesis, and have largely been ignored, but could be peripheral activities involved perhaps in the supply of essential cofactors?
ACKNOWLEDGMENT
The authors are indebted to Dr. Nebil Elshourbagy (SmithKline Beecham Pharmaceuticals, Philadelphia) for providing the cDNA of the rat liver ACL gene.
Footnotes
D.R. received financial suppport from the Commonwealth Scholarship Committee, UK.
LITERATURE CITED
- Anderson S, Smith SM. Synthesis of the small subunit of ribulose bisphosphate carboxylase from gene cloned into plasmids containing SP3 promoter. Biochem J. 1986;240:709–715. doi: 10.1042/bj2400709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Kane HJ. Pyruvate is a by-product of catalysis by ribulose bisphosphate carboxylase/oxygenase. J Biol Chem. 1991;266:9447–9450. [PubMed] [Google Scholar]
- Armstrong J, Lemos EEP, Zobayed SMA, Justin FW, Armstrong W. A humidity induced convective throughflow ventilation system benefits Annona squamosa L. explants and coconut calloid. Anal Bot. 1996;79:31–40. [Google Scholar]
- Boulton CA, Ratledge C. ATP:citrate lyase: the regulatory enzyme for lipid biosynthesis in Lipomyces starkey? J Gen Microbiol. 1981;127:423–426. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browse J, McConn M, James D, Miquel D. Mutant of Arabidopsis deficient in the synthesis of α-linolenate. J Biol Chem. 1993;268:16345–16357. [PubMed] [Google Scholar]
- Colombo SL, Andreo CS, Podesta FE. Carbon metabolism in germinating Ricinus communis cotyledons: purification, characterization and development of NADP-dependent malic enzyme. Physiol Plant. 1997;101:821–826. [Google Scholar]
- Elshourbagy NA, Near JC, Kmetz PJ, Sathe GM, Southern C, Stickler JE, Gross M, Young FJ, Well TN, Groot HE. Rat ATP:citrate lyase: molecular cloning and sequence analysis of a full length cDNA and mRNA abundance as a function of diet, organ and age. J Biol Chem. 1990;204:491–499. [PubMed] [Google Scholar]
- Elshourbagy NA, Near CJ, Kmetz PJ, Wells TNC, Groot PHE, Saxty BA, Hughes SA, Franklin M, Gloger IS. Cloning and expression of a human ATP:citrate lyase cDNA. Eur J Biochem. 1992;204:491–499. doi: 10.1111/j.1432-1033.1992.tb16659.x. [DOI] [PubMed] [Google Scholar]
- Entwistle G, ap Rees T. Enzymic capacities of amyloplasts from wheat endosperm. Biochem J. 1988;255:391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CT, Ratledge C. Possible regulatory roles of ATP:citrate lyase, malic enzyme and AMP deaminase in lipid accumulation by Rhodosporidium toruloides CBS 14. Can J Microbiol. 1985a;31:1000–1005. [Google Scholar]
- Evans CT, Ratledge C. Physiological significance of citric acid in the control of metabolism in lipid-accumulating yeasts. Biotech Genet Eng Rev. 1985b;3:349–375. [Google Scholar]
- Evans CT, Scragg AH, Ratledge C. A comparative study of citrate efflux from mitochondria of oleaginous and non-oleaginous yeasts. Eur J Biochem. 1983;130:195–204. doi: 10.1111/j.1432-1033.1983.tb07136.x. [DOI] [PubMed] [Google Scholar]
- Fell D. Understanding the Control of Metabolism. London: Portland Press; 1997. [Google Scholar]
- Frisch DA, Harris-Haller LW, Yokubaitis NT, Thomas TL, Hardin SH, Hall CT. Complete sequences of the binary vector bin 19. Plant Mol Biol. 1995;27:405–409. doi: 10.1007/BF00020193. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Beevers H. ATP:Citrate lyase from germinating castor bean endosperm. Plant Physiol. 1979;63:687–691. doi: 10.1104/pp.63.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau F, Woolston S, Brooks L, Mullineaux P. An expression cassette for chloroplast targeting foreign protein into chloroplasts. Nucleic Acids Res. 1988;16:11380. doi: 10.1093/nar/16.23.11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Iwakawa H, Kodama H, Kawakami N, Nishimura M, Iba K. Expression of gene for plastid ω-3 fatty desaturase and changes in lipid and fatty acid compositions in light and dark-grown wheat leaves. Physiol Plant. 1996;96:275–283. [Google Scholar]
- Kaethner TM, ap Rees T. Intracellular location of ATP:citrate lyase in leaves of Pisum sativum. Planta. 1985;163:290–294. doi: 10.1007/BF00393520. [DOI] [PubMed] [Google Scholar]
- Kang F, Ridout CJ, Morgan CL, Rawsthorne S. The activity of acetyl-CoA carboxylase is not correlated with the rate of lipid synthesis during development of oil seed rape embryos. Planta. 1994;193:320–325. [Google Scholar]
- Kuhn DN, Knauf MJ, Stumpf PK. Subcellular locations of acetyl-CoA synthetase in leaf protoplasts of Spinacia oleracea. Arch Biochem Biophys. 1981;209:441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- Liedvogel B, Bauerle R. Fatty acid synthesis in chloroplasts from mustard cotyledons: formation of acetyl-CoA by intraplastid glycolytic enzymes and a pyruvate dehydrogenase complex. Planta. 1986;169:481–489. doi: 10.1007/BF00392096. [DOI] [PubMed] [Google Scholar]
- Masterson C, Wood C, Thomas DR. l-Acetyl carnitine, a substrate for chloroplasts fatty acid synthesis. Plant Cell Environ. 1990;13:755–765. [Google Scholar]
- Mathis NL, Hinchee MW. Agrobacterium inoculation techniques for plant tissues. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual B6. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–9. [Google Scholar]
- McGarvey R, Kaper JM. A simple and rapid method for screening transgenic plants using the PCR. Biotechnology. 1991;11:428–432. [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG, Post-Beittenmiller D. De novo fatty acid biosynthesis. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 3–32. [Google Scholar]
- Post-Beittenmiller MH, Roughan G, Ohlrogge JB. Regulation of plant fatty acid synthesis: analysis of acetyl-CoA and acyl-ACP substrate pools in chloroplasts from spinach and pea. Plant Physiol. 1992;100:923–930. doi: 10.1104/pp.100.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Ratledge C. Compartmentation of ATP:citrate lyase in plants. Plant Physiol. 2000;122:1255–1230. doi: 10.1104/pp.122.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Ratledge C, Woolston CJ. Plastid targeting and transient expression of rat liver ATP:citrate lyase in pea protoplasts. Plant Cell Rep. 1997;16:700–704. doi: 10.1007/s002990050305. [DOI] [PubMed] [Google Scholar]
- Ratledge C. Microbial lipids. In: Rehm HJ, Reed G, Puhler A, Stadler P, editors. Biotechnology. Ed 2. Vol. 7. Weinheim, Germany: VCH; 1997. pp. 133–197. [Google Scholar]
- Ratledge C, Bowater MDV, Taylor PN. Correlation of ATP: citrate lyase activity with lipid accumulation in developing seeds of Brassica napus L. Lipids. 1997;32:7–12. doi: 10.1007/s11745-997-0002-7. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Evans CT. Lipids and their metabolism. In: Rose AH, Harrison JS, editors. The Yeasts. Ed 2. Vol. 3. London: Academic Press; 1989. pp. 368–446. [Google Scholar]
- Robinson C, Barnett LK. Isolation and analysis of chloroplasts. In: Shaw CH, editor. Plant Molecular Biology. Oxford: IRL Press; 1988. pp. 67–78. [Google Scholar]
- Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J. Targeting of the Arabidopsis homomeric acetyl-CoA carboxylase to plastids of rapeseeds. Plant Physiol. 1997;113:75–81. doi: 10.1104/pp.113.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Smith RG, Gauthier DA, Dennis DT, Turpin DH. Malate and pyruvate dehydrogenase-dependent fatty acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 1992;98:1233–1238. doi: 10.1104/pp.98.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer J, Heise KP. Comparison of acetate and pyruvate-dependent fatty acid synthesis by spinach chloroplasts. Planta. 1989;177:417–421. doi: 10.1007/BF00403601. [DOI] [PubMed] [Google Scholar]
- Verwoert IGS, Vanderlinder KH, Nijkamp HJ, Stuitje AR. Developmental specific expression and organelle targeting of the Escherichia coli FabD gene, encoding malonyl coenzyme A-acyl carrier protein translocase in transgenic rape and tobacco seeds. Plant Mol Biol. 1994;26:189–202. doi: 10.1007/BF00039531. [DOI] [PubMed] [Google Scholar]
- Watson JA, Lowenstein JM. Citrate and conversion of carbohydrate into fat: fatty acid synthesis by a combination of cytoplasm and mitochondria. J Biol Chem. 1970;22:5993–6002. [PubMed] [Google Scholar]
- Wedding TR, Kline K. Comparative studies of coupled assays for phosphoenolpyruvate carboxylase. Physiol Plant. 1994;92:197–200. [Google Scholar]
- Wu HB, Feist LG, Hemmingston SH. A modified Escherichia coli chaperonin (gro EL) polypeptides synthesized in tobacco and targeted to the chloroplasts. Plant Mol Biol. 1993;22:1087–1100. doi: 10.1007/BF00028979. [DOI] [PubMed] [Google Scholar]
- Wynn JP, Hamid AA, Ratledge C. The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiology. 1999;145:1911–1917. doi: 10.1099/13500872-145-8-1911. [DOI] [PubMed] [Google Scholar]
- Wynn JP, Ratledge C. Malic enzyme is a major source of NADPH for lipid accumulation by Aspergillus nidulans. Microbiology. 1997;143:253–257. doi: 10.1099/00221287-143-1-253. [DOI] [PubMed] [Google Scholar]