Highlights
-
•
Continuous vancomycin exposure to concentrations ≥1 mg/cm2 is significantly cytotoxic to in vitro osteoblasts and myoblasts, while concentrations ≥3 mg/cm2 is significantly cytotoxic to fibroblasts.
-
•
1-h vancomycin exposure to concentrations <12 mg/cm2 does not significantly affect cell survival or migration of in vitro osteoblasts, myoblasts, or fibroblasts.
-
•
Future in vivo studies are required to optimize the clinical dosing of vancomycin application, and study its clinical effects on infection, healing, and bony fusion.
Keywords: Cell survival, Cell migration, Myoblast, Osteoblast, Fibroblast, Vancomycin
Abstract
The purpose of this study was to examine the influence of topical vancomycin on cell migration and survival of tissue healing cells. Human osteoblasts, myoblasts and fibroblasts were exposed to vancomycin at concentrations of 1, 3, 6, or 12 mg/cm2 for either a 1-h or 48-h (continuous) duration. Continuous exposure to all vancomycin concentrations significantly reduced cell survival (<22% cells survived) and migration in osteoblasts and myoblasts (P < 0.001). 1-h vancomycin exposure reduced osteoblast and myoblast survival and migration only at 12 mg/cm2 (P < 0.001). Further in vivo studies are warranted to optimize the dosage of intrawound vancomycin.
1. Introduction
Periprosthetic joint infection (PJI) can be a devastating complication, resulting in increased morbidity for patients. PJI increases healthcare cost due to prolonged hospitalization, long-term intravenous antibiotics, and multiple returns to the operating room.1 The prevalence of deep periprosthetic joint infections following shoulder arthroplasty ranges from 1 to 5% and up to 12% in complex spine surgery.2, 3 There has been increasing interest in strategies to reduce infection rates in orthopaedic procedures including total joint arthroplasty. Strategies to reduce infection rates include but are not limited to conventional sterile practices in the operating room, preoperative intravenous antibiotics, mupirocin nasal ointments, bacitracin irrigation, dilute povidone iodine lavage, and local application of vancomycin powder.4, 5, 6, 7
The local application of antibiotics in the surgical wound is an emerging clinical practice to decrease perioperative infection rates. The intrawound application of lyophilized vancomycin in the deeper layers of wound prior to closure has been reported to significantly decrease the rates of perioperative infection in arthroplasty and spine procedures.8, 9, 10 However, there are concerns related to emergence of resistant organisms, higher cost and local and systemic effects of high doses of local antibiotics. The local effects of clinically used supra-therapeutic concentration of vancomycin on surrounding healing tissue has been a topic of continued investigation.11, 12 The purpose of this laboratory study was to examine the in vitro effect of vancomycin on cell migration and survival of cells participating in tissue repair, specifically on osteoblasts, fibroblast, and myoblasts.
2. Materials and methods
This is a controlled laboratory study (in vitro), which was performed using three primary human cell types (osteoblast, fibroblast and myoblast). The influence of short duration and continuous exposure of cells to different concentrations of vancomycin was tested under similar, controlled conditions. All experiments were performed in triplicate.
2.1. Cell culture
Primary human osteoblasts (Lonza, Walkersville, MD, CCC-2538) were cultured in Osteoblast Basal Medium (Lonza CC-3208) supplemented with 10% fetal bovine serum, ascorbic acid, and gentamicin/amphotericin-B (Lonza CC-4193). Primary human fibroblasts (Lonza CC-2511) were cultured in Fibroblast Basal Medium (Lonza CC-3131) supplemented with human fibroblast growth factor-basic, insulin, gentamicin/amphotericin-B, and 10% fetal bovine serum (Lonza CC-4134). Primary human myoblasts (DV Biologics, Yorba Linda, CA, AM002-F) were cultured in Muscle Cellutions Medium (DV Biologics M-GRO-001-500) supplemented with basal media supplement (DV Biologics M-GRO-0010-S) and 20 ng/ml of human fibroblast growth factor-basic (R&D systems, Minneapolis, MN, 233-FB-025).
Cells were initially seeded in standard sterile 75-cm2 tissue culture flasks (Corning Life Sciences, Tewksbury, Massachusetts) at a density of 10,000 cells/cm2 and grown to 80% confluency at 37° C and 5% CO2. Cells were passaged in tissue culture flasks until passage 3, at which time the cells were seeded into 24-well plates (Corning Life Sciences, Corning, NY) at a density of 10,000 cells/cm2 and cultured until 80–90% confluent.
2.2. Vancomycin exposure
Vancomycin solutions were made by dissolving lyophilized vancomycin hydrochloride (Sigma-Aldrich, St. Louis, MO) directly into each cell type’s cellular growth medium. Four different concentrations were tested (1, 3, 6, or 12 mg/cm2 of vancomycin hydrochloride) and two different exposure times were tested (one-hour [short-duration] or 48 h [continuous] exposure). Intrawound application of vancomycin has been used most extensively in spine and total joint arthroplasties. These doses (1, 3, 6, or 12 mg/cm2 of vancomycin hydrochloride) were selected based on an ongoing clinical trial, and a study by Sweet et al. where the range of serum vancomycin levels in humans was studied after 2 g of local vancomycin was applied to thoracic and lumbar instrumented spinal fusions.13, 14
We used two different exposure times of vancomycin in this study. We chose the 1 h time point to represent the local exposure to high vancomycin dosage for short duration as seen when vancomycin is used in the wound at the time of closure. We assumed that the local vancomycin effect would be high for at least the first hour; increased blood flow, presence of local hematoma and drain, if present, in the wound will dilute the concentration of vancomycin locally in tissues. We believe that continuous vancomycin exposure (48 h) represents a scenario where cells and tissues are exposed to high doses of vancomycin continuously, as seen with antibiotic loaded cement spacers, or in certain implant-tissue interfaces where vancomycin cannot be easily washed or diluted.
2.3. Cell survival assay
At 80% confluency, cells were washed with phosphate buffer saline (PBS) and exposed to cell growth medium (control), 1, 3, 6, or 12 mg/cm2 of vancomycin hydrochloride for either a one-hour or continuous exposure as per the protocol. Following a one-hour exposure to vancomycin in the tissue culture incubator, the cells were washed 3 times with PBS, the cell growth media was reapplied, and cells were returned to the tissue culture incubator at 37° C for 48 h. Cells exposed to vancomycin continuously were immediately returned to the tissue culture incubator for a 48-h incubation.
After incubation, cells were washed again with PBS, and 10% Cell Counting Kit-8 (CCK) solution (Dojindo Molecular Technologies, Washington D.C.) in cellular growth medium was applied to the cells. The CCK solution contains a highly soluble tetrazolium salt, which receives two electrons from viable cells, to generate an orange formazan dye, allowing colorimetric detection of cellular activity.15, 16 The cells were then incubated in the tissue culture incubator for 2 h with the CCK solution, after which absorbance of cellular supernatants were measured at 450 nm according to the manufacturer’s protocol. The absorbance of the experimental conditions were compared relative to control absorbance to calculate percent cell survival.15, 16
2.4. Scratch test
The validated scratch test is an objective method to measure cell migration in vitro, and has been previously used as an assay to mimic cell migration during wound healing in vivo.17 At confluency, the cell monolayer was manually scraped in a straight line in the center of the well to create a “scratch” with a p200 pipette tip. The debris was removed by washing the cells with 1 ml of sterile PBS. After the scratch defect was made, cells were exposed to cell growth medium (control), 1, 3, 6, or 12 mg/cm2 of vancomycin hydrochloride for either a one-hour or 48 h as per the protocol above.
To obtain the same field during image acquisition, reference points were made by marking the outer bottom of the dish with a fine permanent marker. The wells were placed under a phase-contrast microscope, leaving the reference mark just outside of the camera view. Images were captured of the cells before and immediately after the scratch defect was made, after which the cells were returned to the tissue culture incubator at 37° C. Subsequent images of the cells were taken every hour for the first 24 h, every 3 h until day 3, then every 24 h until day 14. Time until scratch defect closure, which was defined as the moment when the cells at the leading edge of the defect make contact with cells from the opposite side, were recorded for each experimental condition.
2.5. Statistical analysis
The data was reported as mean ± standard deviation. All cell survival results were compared relative to their respective controls using ANOVA test.
3. Results
3.1. Cell survival assay
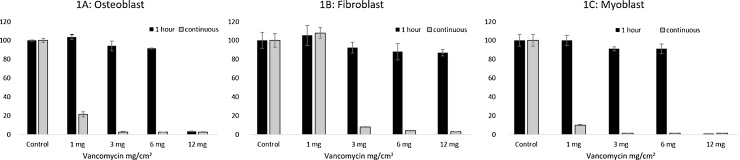
The results of the cell survival assay are demonstrated in Fig. 1. The human osteoblasts (Fig. 1A) and myoblast cells (Fig. 1C) continuously exposed (48 h) to vancomycin concentration greater than or equal to 1 mg/cm2 had significantly reduced (p < 0.001) cell survival levels relative to the control (unexposed myoblasts and osteoblasts). The cell survival of fibroblasts (Fig. 1B) was not significantly affected (p = 0.21) by continuous exposure to vancomycin of 1 mg/cm2. However, concentrations greater than or equal to 3 mg/cm2 significantly (p < 0.001) reduced fibroblast cell survival to less than 8% relative to control (Fig. 1B and Table 1).
Fig. 1.
Cell survival 48 h after being exposed to vancomycin concentrations, 1 h versus continuous (48 h) exposure. A. Osteoblasts. B. Fibroblasts. C. Myoblasts.
Table 1.
Cell survival after 1 h and 48 h exposure to different vancomycin concentrations (1, 3, 6, and 12 mg/cm2) in primary human osteoblast (A), fibroblast (B) and myoblast (C) cells.
| Duration of Vancomycin exposure | Vancomycin Concentration (mg/cm2) | Percent cell survival (±Standard deviation) | P value (vs. control) |
|---|---|---|---|
| A – Osteoblast | |||
| 1 h | Control | 100 ± 0.6 | – |
| 1 | 103 ± 2.7 | 0.1 | |
| 3 | 94 ± 5 | 0.13 | |
| 6 | 91 ± 0.6 | <0.0001 | |
| 12 | 3 ± 0.3 | <0.0001 | |
| Continuous (48 h) | Control | 100 ± 2 | – |
| 1 | 22 ± 3 | <0.0001 | |
| 3 | 3 ± 0.6 | <0.0001 | |
| 6 | 2 ± 0.01 | <0.0001 | |
| 12 | 2.64 ± 0.3 | <0.0001 | |
| B – Fibroblast | |||
| 1 h | Control | 100 ± 9 | – |
| 1 | 105 ± 10 | 0.54 | |
| 3 | 92 ± 6 | 0.27 | |
| 6 | 88 ± 9 | 0.16 | |
| 12 | 87 ± 3 | 0.07 | |
| Continuous (48 h) | Control | 100 ± 8 | – |
| 1 | 108 ± 6 | 0.2 | |
| 3 | 8 ± 0.5 | <0.0001 | |
| 6 | 4 ± 0.4 | <0.0001 | |
| 12 | 3 ± 0.2 | <0.0001 | |
| C – Myoblast | |||
| 1 h | Control. | 100 ± 6 | – |
| 1 | 100 ± 5 | 0.97 | |
| 3 | 91 ± 2 | 0.08 | |
| 6 | 91 ± 5 | 0.12 | |
| 12 | 1 ± 0.07 | <0.0001 | |
| Continuous (48 h) | Control | 100 ± 6 | – |
| 1 | 10 ± 0.4 | <0.0001 | |
| 3 | 1 ± 0.2 | <0.0001 | |
| 6 | 1 ± 0.06 | <0.0001 | |
| 12 | 1.5 ± 0.05 | <0.0001 | |
Following 1-h exposure to vancomycin concentrations of 1, 3, and 6 mg/cm2, the cell survival of osteoblasts and myoblasts was not significantly different compared to the controls (p < 0.05; Table 1 for individual P values). However, 1-h exposure to vancomycin concentrations of 12 mg/cm2 reduced osteoblast and myoblast survival to less than 3% relative to control (p < 0.001; Fig. 1A, C). Fibroblast cell survival was not affected significantly after 1-h exposure to vancomycin of any concentration (p > 0.05; Fig. 1B; Table 1 for individual p values).
3.2. Scratch test
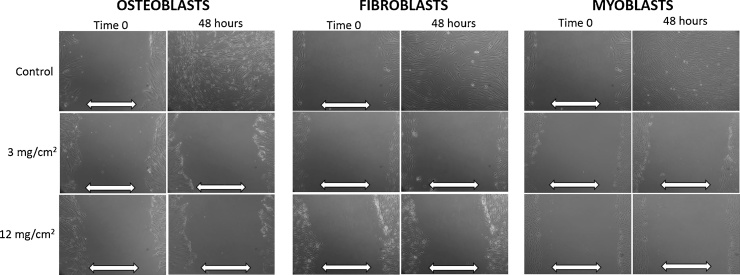
The results of the scratch test following continuous vancomycin exposure (48 h) are demonstrated in Fig. 2. The control cultures for osteoblast, myoblast and fibroblast demonstrated closure of the scratch defect within 48 h indicative of normal cell migration. Following 48 h continuous exposure to vancomycin (all concentrations), the myoblast and osteoblast cells could not close the defects in first 48 h. The scratch defects persisted even at 14 days after initiation of the defect, indicative of halted cell migration. Similar response was seen with the fibroblast cells except that continuous exposure to vancomycin in concentration of 1 mg/cm2 resulted in defect closure at 48 h (Table 2). After exposure to vancomycin concentration of 3, 6, and 12 mg/cm2, the scratch defects remain open at 48 h for all cell types.
Fig. 2.
Scratch defect results after continuous (48 h) vancomycin exposure to osteoblasts, fibroblasts, and myoblasts. White arrows denote open scratch defect.
Table 2.
Scratch defect results showing the effect of different concentrations of vancomycin on scratch defect closure within 48 h of scratch defect initiation in osteoblast, fibroblast and myoblast.
| Groups | Osteoblast |
Fibroblast |
Myoblast |
|||
|---|---|---|---|---|---|---|
| 48 h vancomycin exposure | 1 h vancomycin exposure | 48 h vancomycin exposure | 1 h vancomycin exposure | 48 h vancomycin exposure | 1 h vancomycin exposure | |
| Control | + | + | + | + | + | + |
| 1 mg/cm2 | − | + | + | + | − | + |
| 3 mg/cm2 | − | + | − | + | − | + |
| 6 mg/cm2 | − | + | − | + | − | + |
| 12 mg/cm2 | − | − | − | + | − | − |
“+” Indicates defect closure, “−” indicates open defect; control: no vancomycin exposure.
Scratch test results following 1-h vancomycin exposure are shown in Fig. 3 and Table 2. In the fibroblast cultures, all scratch defects closed within 48 h following 1-h exposure to any concentration of vancomycin. The scratch defects closed within 48 h in myoblasts and osteoblasts after 1-h exposure to 1, 3 and 6 mg/cm2 dose of vancomycin (Table 2). In contrast to fibroblasts, the scratch defects in the myoblast and osteoblast monolayer cultures persisted beyond 48 h after 1-h exposure to 12 mg/cm2, indicating halted cell migration.
Fig. 3.
Scratch defect results after 1-h vancomycin exposure to osteoblasts, fibroblasts, and myoblasts. White arrows denote open scratch defect.
4. Discussion
This study demonstrates that short duration exposure to vancomycin (1, 3, and 6 mg/cm2) in vitro does not significantly affect cell migration and survival of osteoblast, myoblast, and fibroblast in monolayer cell culture. However, continuous exposure for 48 h to vancomycin (3, 6 and 12 mg/cm2) results in significant inhibition of cell survival and cell migration.
The findings of this study have an important clinical significance. The use of perioperative antibiotics has proven to be an efficacious strategy in reducing infection rates. The use of intrawound vancomycin is an emerging practice for revision and high risk, elective non-infected surgeries and has proven to be effective in decreasing PJI and surgical site infections (SSI). Intrawound application of vancomycin has been reported to reduce PJI rates in recent studies. Khan et al. performed a meta-analysis of spinal surgical site infections and vancomycin powder, which revealed vancomycin to be a protective factor in PJI prevention (relative risk = 0.34, p = 0.021).9 Devin et al. performed a prospective multicenter observational study investigating the effects of local vancomycin treatment in patients undergoing elective spine surgery (with and without spinal fusion). A total of 2056 patients either received vancomycin in the wound prior to closure (local vancomycin group) or underwent standardized closure (no local vancomycin group). There was a significantly higher incidence (RR 2.5; p < 0.005) of surgical site infections (SSI) in the no local vancomycin group (5.1%) compared to the vancomycin group (2.2%).8 Additionally local vancomycin has proven to decrease the economic burden of rising healthcare costs. Emohare et al. performed a retrospective cost savings analysis of intrawound vancomycin in posterior spinal surgery and found that an average of $40,992 per patient is spent on return visits to the operating room in those initially treated without local vancomycin versus only $12 on the cost of vancomycin.18 Also noted in this study, no patients in the local vancomycin treatment group (n = 96) had a SSI.18 Although clinical efficacy of intrawound vancomycin in reducing infection rate has been established, there remains a concern for host toxicity, emergence of super infection and antibiotic resistance. Our study demonstrates that in a controlled laboratory environment, short duration exposure does not negatively affect cell survival and cell migration in progenitor cells participating in tissue repair but longer exposure in the same concentration is toxic.
There has been considerable debate regarding the safety profile and possible side effects of local vancomycin. A common cited side effect in spine literature is a postoperative seroma which can mimic a surgical site infection but a direct causal relationship has yet to be proven.19 There is one documented case of an anaphylactoid reaction in patient who received local vancomycin treatment but there remains debate if the local vancomycin was the true cause of the vascular collapse.20 Sweet et al. investigated the safety and efficacy of intrawound application of 2 gm of vancomycin in surgical wound during instrumented posterior thoracic and lumbar fusions. Peak intrawound vancomycin level was seen on day 0 and dropped at least eleven fold by day 3. The average concentration in surgical drain fluid was 1475 ug/ml on POD 0 and 128 ug/ml on postoperative day 3. There were no detectable serum levels of vancomycin in 80% of the patients. This information translates to similar doses used in our study, 1–12 mg/cm2 (100–12,000 ug/ml).13 Additionally the doses of 3,6 and 12 mg/cm2 have previously been used in a clinical trial to determine the most effective and safe dose of local vancomycin administration.14
In vitro studies have previously reported cytotoxic effects of vancomycin putting into question its effects on cell migration, proliferation, viability and differentiation, all of which are essential cellular processes for wound healing and bone remodeling. Previous studies have demonstrated significant cytotoxicity of vancomycin to multiple other cell types including endothelial cells, keratinocytes, human glial cells, and renal cells.21 Eder et al. showed that at a concentration of 3 mg/cm2 of vancomycin for 24 h significantly decreased cell proliferation and migration of human osteoblasts obtained from 10 patients. The authors also demonstrated significant osteoblast death when treated with vancomycin at a concentration of 6 mg/cm2 for 24 h. Osteoblast differentiation was also diminished with vancomycin concentrations of 3–12 mg/cm2, shown via an alkaline phosphatase assay,11 leading to the conclusion that intrawound vancomycin may interfere with regenerative processes and increase the risk of non-union in spinal surgeries. In contrast to the study by Eder et al. our study shows that shorter duration exposure to clinically used does of vancomycin does not affect the survival and migration of human osteoblasts. Moreover, we tested other cells that participate in tissue repair (myoblasts and fibroblast), and were unaffected by short duration exposure to vancomycin. Similar to our study, Philp et al. tested vancomycin and gentamicin irrigation in vitro on osteoblasts, specifically testing for post exposure (exposed to antibiotic for 20 min) proliferation, metabolic function and bone mineralization.22 Results showed that osteoblast proliferation transiently suffered a 15–20% reduction but returned to control values at 72 h.
Our study demonstrates the safety of short duration exposure to clinically used doses of vancomycin in tissue culture. The clinical efficacy of these dose ranges has been shown to reduce infection rates by Sweet et al. and Devin et al. Clinical translation of our results will require introduction of strategies to limit exposure of local tissue to vancomycin for a longer period of time. Kinematic studies from drug delivery experiments including antibiotic loaded bone cement have shown that majority of the drug elution occurs most rapidly in the first 4 h and decreases dramatically after the first 24 h.23 Since no carrier is used for intrawound antibiotic delivery we assume that clearance of intrawound antibiotic will occur more rapidly. Furthermore, presence of surgical drain and, increased blood supply post operatively will contribute further to dilution of antibiotic concentration. Although the cells may still be exposed to antibiotics after 1-h in vivo, the concentration of local antibiotic in the wound would be considerably lower.
There are several limitations to our study. As a limitation seen in many in vitro studies evaluating in vivo effects, the cell monolayer model used in this study is not a true representation of in vivo tissues in a surgical wound. Surgical wounds are well-vascularized environments, comprised of multiple cell types and soft-tissue layers, and may have a higher tolerance for toxic substrates than in vitro monolayer tissue cultures. Furthermore, it is unknown if cell death in the most superficial layer of the wound bed after exposure to vancomycin interferes with in vivo wound healing. Second, only two exposure durations were chosen for analysis, 1 h and 48 h. There may be variations in the intermediary time points that were not detected due to this selection. We believe the in vitro findings of these two exposure durations warrant further in vivo investigation into the optimal exposure concentrations and durations in clinical vancomycin use. Third, we did not test cell specific function or metabolic function of individual cell types. Philp et al. did test metabolic function in osteoblasts after exposure to vancomycin and no differences were detected compared to the control group 22. Finally, the in vitro doses used in this study, of 3–12 mg/cm2 are assumed therapeutic based on in vivo study performed by Sweet et al. using 2 g of local vancomycin, which has been shown to be therapeutic based on bactericidal effects in vivo and in vitro,24 13, 25. We did not test the bactericidal therapeutic efficacy of these concentrations in this study. Additional in vivo studies are required to further characterize the clinical significance of these findings.
5. Conclusions
Continuous vancomycin exposure (48 h) has a significant cytotoxic effect on proliferating osteoblasts and myoblasts at concentrations greater than or equal to 1 mg/cm2, and for fibroblasts at concentrations greater than or equal to 3 mg/cm2. Short duration exposure (1-h) to lower doses of vancomycin (1–6 mg/cm2) does not significantly reduce cell migration and survival of cells. Further in vivo studies are warranted to optimize the dosage of intrawound vancomycin and study its influence on infection rates, bony fusion, and wound healing.
Conflict of interest
None.
Financial remuneration for each author
None.
References
- 1.Kamath A.F., Ong K.L., Lau E. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492–1497. doi: 10.1016/j.arth.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Padegimas E.M., Maltenfort M., Ramsey M.L., Williams G.R., Parvizi J., Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elb Surg. 2015;24(5):741–746. doi: 10.1016/j.jse.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Schimmel J.J.P., Horsting P.P., de Kleuver M., Wonders G., van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19(10):1711–1719. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown N.M., Cipriano C.A., Moric M., Sporer S.M., Della Valle C.J. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27–30. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Chiang H.Y., Herwaldt L.A., Blevins A.E., Cho E., Schweizer M.L. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014;14(3):397–407. doi: 10.1016/j.spinee.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Owens B.D., White D.W., Wenke J.C. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Jt Surg. 2009;91(1):92–98. doi: 10.2106/JBJS.G.01566. [DOI] [PubMed] [Google Scholar]
- 7.Kalmeijer M.D., Coertjens H., van Nieuwland-Bollen P.M. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35(4):353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 8.Devin C.J., Chotai S., McGirt M.J. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery-a multicenter analysis. Spine (Phila Pa 1976) 2018;43(January (1)):65–71. doi: 10.1097/BRS.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 9.Khan N.R., Thompson C.J., DeCuypere M. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21(December):1–10. doi: 10.3171/2014.8.SPINE1445. [DOI] [PubMed] [Google Scholar]
- 10.Zebala L.P., Chuntarapas T., Kelly M.P., Talcott M., Greco S., Riew K.D. Intrawound vancomycin powder eradicates surgical wound contamination. J Bone Jt Surg. 2014;96(1):46. doi: 10.2106/JBJS.L.01257. [DOI] [PubMed] [Google Scholar]
- 11.Eder C., Schenk S., Trifinopoulos J. Does intrawound application of vancomycin influence bone healing in spinal surgery? Eur Spine J. 2016;25(4):1021–1028. doi: 10.1007/s00586-015-3943-9. [DOI] [PubMed] [Google Scholar]
- 12.Rathbone C.R., Cross J.D., Brown K.V., Murray C.K., Wenke J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29(7):1070–1074. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 13.Sweet F.A., Roh M., Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions. Spine (Phila Pa 1976) 2011;36(24):2084–2088. doi: 10.1097/BRS.0b013e3181ff2cb1. [DOI] [PubMed] [Google Scholar]
- 14.Washington University School of Medicine . 2013. Dose Escalation Trial of Intrasite Vancomycin Pharmacokinetics. [Google Scholar]
- 15.Tominaga H., Ishiyama M., Ohseto F. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;(36):47–50. [Google Scholar]
- 16.Ishiyama M., Miyazono Y., Sasamoto K., Ohkura Y., Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44(7):1299–1305. doi: 10.1016/s0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 17.Liang C., Park A.Y., Guan J. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 18.Emohare O., Ledonio C.G., Hill B.W., Davis R.A., Polly D.W., Kang M.M. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014;14(11):2710–2715. doi: 10.1016/j.spinee.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Kang D.G., Holekamp T.F., Wagner S.C., Lehman R.A. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015;15(4):762–770. doi: 10.1016/j.spinee.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Ramamani Mariappan, M.D. 1, Manninen Pirjo, M.D., F.R.C.P.C. 1, Massicotte Eric M., M.D., M.Sc., F.R.C.S.C., 2, Bhatia Anuj., M.D. FRCPC. Circulatory collapse after topical application of vancomycin powder during spine surgery. J Neurosurg Spine. 2013;19(September):381–383. doi: 10.3171/2013.6.SPINE1311. [DOI] [PubMed] [Google Scholar]
- 21.Damour O., Zhi Hua S., Lasne F., Villain M., Rousselle P., Collombel C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns. 1992;18(6):479–485. doi: 10.1016/0305-4179(92)90180-3. [DOI] [PubMed] [Google Scholar]
- 22.Philp A.M., Raja S., Philp A., Ede M.P., Jones S.W. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function and bone mineralisation Ashleigh. Spine (Phila Pa 1976) 2016 doi: 10.1097/BRS.0000000000001712. [DOI] [PubMed] [Google Scholar]
- 23.Penner M.J., Masri B.A., Duncan C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939–944. doi: 10.1016/s0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 24.Dennis H., Wei D., Darren K. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976) 2016:1. doi: 10.1097/BRS.0000000000001710. [DOI] [PubMed] [Google Scholar]
- 25.Della Valle A.G., Bostrom M., Brause B., Harney C., Salvati E.A., EA DVABMBBHCS Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand. 2001;72(3):237–240. doi: 10.1080/00016470152846547. [DOI] [PubMed] [Google Scholar]