Abstract
Objective
To develop a surgical technique for percutaneous upper extremity fracture fixation using a novel glass-based adhesive.
Methods
Three intact upper extremity cadaveric specimens with undisturbed soft tissues were obtained. Two were used to model a wrist fracture, and the third to model a proximal humerus fracture. Fractures were produced using a small osteotome in a percutaneous fashion. Banna Bone Adhesive (BBA) was delivered to the fracture site percutaneously using a 16 gauge needle under bi-planar fluoroscopic guidance. After setting of the adhesive, the specimens were dissected to qualitatively assess BBA delivery and placement.
Results
The adhesive could readily be delivered through the 16 gauge needle with an appropriate amount of pressure applied to the syringe. Using the fluoroscope, the adhesive could be seen to flow into the fracture site with minimal extravagation into the surrounding soft tissues. Successful bonding of the fracture fragments was observed.
Conclusions
Percutaneous delivery of BBA into a fracture of the distal radius and proximal humerus may be a feasible fracture fixation technique. Biomechanical testing and animal model testing are required to further develop this procedure.
Keywords: Fracture fixation, Glass polyalkanoate cement, Wrist fracture, Humerus fracture, Injectable adhesive, Bioactive glass, Surgery, Minimally invasive
1. Introduction
Despite the widespread use of pharmacologic agents for treating osteoporosis, the number of fractures that occur in the elderly population continues to rise at a steady rate1,2. In the United States, there are nearly 1.3 million fractures every year resulting in a healthcare burden of about $10 billion per year3,4. These fractures most commonly involve the upper extremities, usually at the distal radius, the proximal humerus or the spine3,5. These injuries cause significant morbidity to the patient, stress for family caregivers, and financial burden to the health care system2. Current fixation and stabilisation of these injuries is not optimised and has changed little over the years. Treatment of distal radius fracture, for example, usually consists of extended cast or sling immobilization, or surgical open reduction and internal fixation with pins, plates and screws followed by immobilization for 6 weeks5,6. Cast or sling immobilization is disruptive to the patient’s life and can threaten their independence7. Non-operative management of this type can be complicated by non-union, malunion, and stiffness5. Surgical intervention carries with it the usual operative risks which are increased in the elderly population5. Development of new fracture treatment methods using modern adhesive technology is required.
The use of surgical cement to fill traumatic or surgically induced bone voids and gaps in the skeletal system is widespread, with three main material types dominating the literature on the subject: acrylics, composites and calcium phosphates. Acrylic bone cements are employed for hip, knee and spinal augmentation. However, concerns such as (but not limited to) impaired functioning of the immune system8,9, thermal and chemical necrosis10 and lack of direct bone apposition around such implants11 has encouraged other material developments. Cortoss (Stryker, USA), a Bisphenol-A-glycidyl dimethacrylate (BIS-GMA) resin enriched with ceramic particles, is an alternative to conventional acrylics12,13. However, drawbacks are also associated with its use including; deletions in DNA sequences14 decreasing strength with respect to time15, impaired immune response14 and excessive elastic modulus15. Calcium phosphate cements (CPCs) have clinical interest due to their potential to be resorbed and replaced with new bone as part of the natural bone remodelling cycle without provoking an inflammatory response16, 17, 18. Whilst some CPCs suffer from delayed setting in the wet field environment, and viscosity contrary to injectable surgical procedures, their biocompatibility is not matched by acrylic or composite bone cements. The main deficiency associated with CPCs is their poor strength19. Glass polyalkenoate cements (GPCs) are a group of materials that have potential for skeletal cementation.
GPCs were initially developed in the early 1970′s for use in restorative dentistry20. They are formed by an acid-base reaction between a water-soluble poly(acrylic) acid (PAA) and an acid-degradable fluoro-alumino-silicate bioactive glass21. A polysalt matrix is formed in GPCs through the degradation of the glass, leading to the release of free cations which associate with the carboxylic anions from the PAA22,23. The crosslinking mechanism is a continuous process during which acrylate networks are established, leading to the increase in strength over time24. The cement used in this study has been reported in the literature with details around its chemical, physical, mechanical and biological properties25,26. This cement is called Banna Bone Adhesive (BBA).
Various techniques such as dorsal and volar plating, fragment-specific plating, screw osteo-synthesis and external fixation, have been used for distal radius fracture fixation, however it still remains a challenge, especially for the elderly27, 28, 29, 30, 31. Stabilizing distal radius fractures using CPCs has also been reported28,32.
This study aims to provide proof-of-concept evidence that percutaneous fracture fixation can be achieved using BBA. We believe that this is the first work that shows how to optimize the upper extremity fracture fixation using injectable GPCs.
2. Methods
2.1. Glass synthesis
A single glass composition was formulated for this study (Table 1). The glass was prepared by weighing out appropriate amounts of the analytical grade reagents (Fisher Scientific, Ottawa and Sigma-Aldrich, Oakville, Canada) and mixing them in a container. Platinum (Pt.) crucibles and a Lindberg/Blue M model furnace (Lindberg/Blue M, Asheville, NC USA) with a UP-550 controller were used to melt the powders (1650 °C, 1.5 h). The melt was subsequently shock quenched in water to obtain frit which was then dried in an oven (100 °C, 1 h), and ground using a ball mill (400 rounds per minute, 15 min). The resulting powder was sieved to collect the < 45 μm particles which were utilised for subsequent cement preparation. A complete characterization of the glass can be found in25.
Table 1.
Composition of glass.
| Oxide | SiO2 | ZnO | CaO | SrO | P2O5 | Ta2O5 |
|---|---|---|---|---|---|---|
| Mole Fraction | 0.48 | 0.355 | 0.06 | 0.08 | 0.02 | 0.005 |
2.2. Cement preparation
Cement samples were prepared by mixing the glass with poly(acrylic acid) (PAA, Mw = 50,000, Advanced Healthcare Ltd., Tonbridge, UK) and de-ionized (DI) water on a glass plate at a powder-liquid ratio (P:L) of 1:4, where 1 g of glass was mixed with 2 g PAA and 2 g DI water to create a paste. Complete mixing was achieved within 30 s at ambient room temperature (23 ± 1 °C). The cement was transferred, using a spatula, to a 10 ml syringe and then injected into the fracture site.
2.3. Wrist fixation
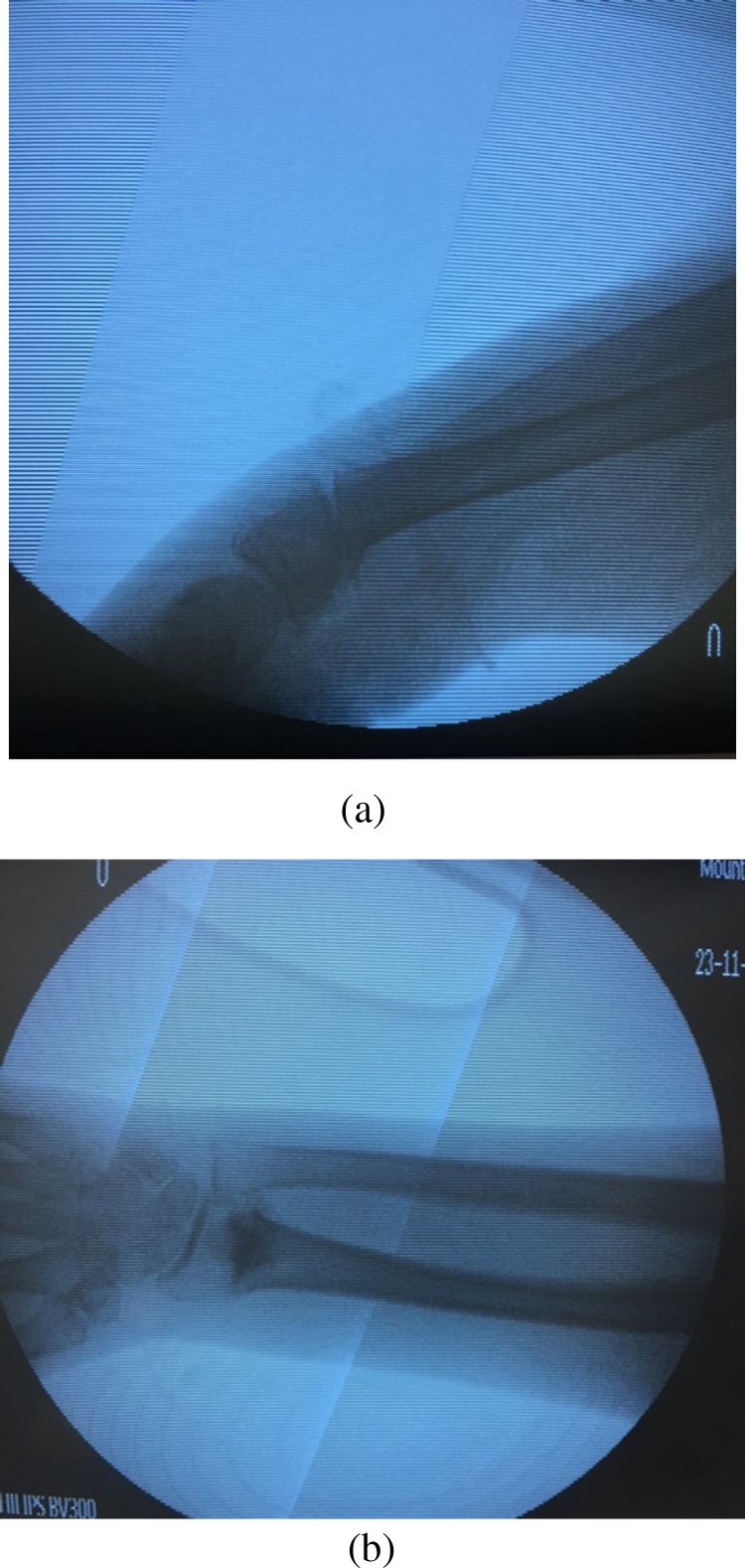
Two right-sided adult upper extremity cadaveric specimens were obtained from the Division of Anatomy, Department of Surgery, (University of Toronto, Canada). A percutaneous stab incision through the dorsum of the wrist was used to introduce a ¼ inch osteotome (Fig. 1a). A distal radius fracture was simulated and manual force was used to create complete displacement and dorsal angulation. The fracture was verified using biplanar fluoroscopy. Under fluoroscopic guidance, the fracture was reduced with in line longitudinal manual traction. Through the stab incision, 5cc of BBA was injected into the fracture site using a 16 gauge needle under fluoroscopic guidance. Traction was maintained for 5 min. The adhesive was allowed to set for 1 h. Fluoroscopy was used to verify fracture stability. Soft tissue dissection was then performed via dorsal and volar approaches to observe adhesive distribution.
Fig. 1.
Distal radial fracture and fixation using BBA; (a) Lateral view of distal radius with osteotomy, (b) AP view of distal radius after adhesive applied percutaneously.
2.4. Proximal humerus fixation
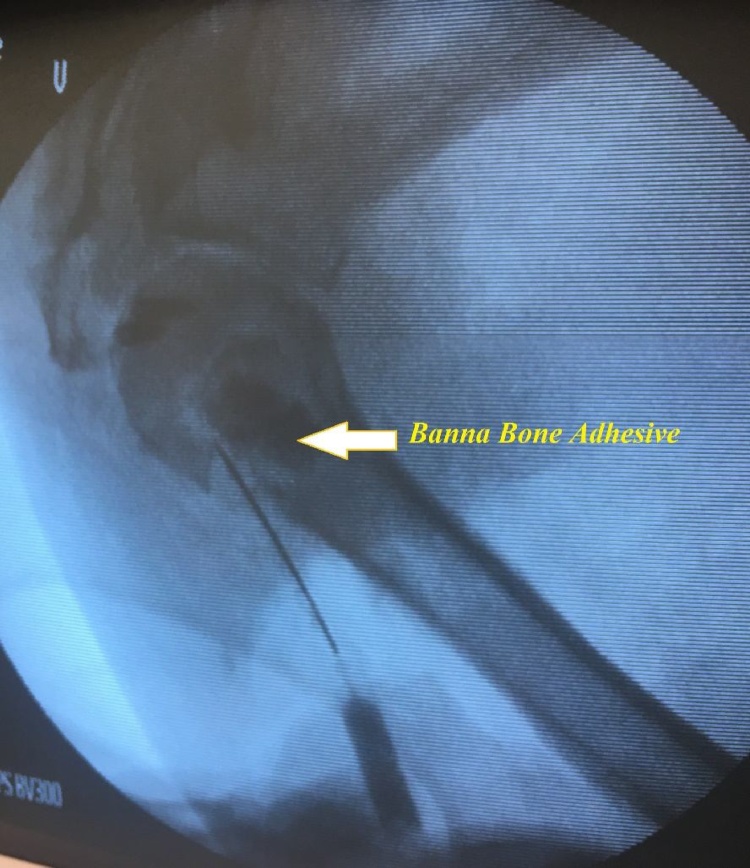
One right-sided adult upper extremity cadaveric specimen was used. A similar procedure as that described above was used to create a fracture through a stab incision on the lateral aspect of the shoulder. 10cc of BBA was injected into the fracture site (Fig. 2). Traction was maintained for 5 min then the adhesive was allowed to set for 1 h. Soft tissue dissection through an anterior approach was performed to determine if there was any extravasation of the adhesive.
Fig. 2.
Injection of BBA into proximal humerous fracture.
3. Results and discussion
Both the wrist and the proximal humerus fractures were easily accessible through the percutaneous stab incision. The BBA was readily injected through the 16 gauge needle and could be observed flowing into the fracture site using portable C-arm fluoroscopy. The wrist and shoulder were put through a range of motion and the fracture site appeared stable on fluoroscopy. Soft tissue dissection revealed that the BBA was largely contained in the fracture site with little extravasation into the soft tissues.
4. Conclusion
This technique for percutaneous upper extremity fracture fixation using BBA appears to be technically feasible in a cadaveric model. Biomechanical testing and animal models are currently underway to prepare for in vivo human pilot experiments. Biomechanical testing will aim to assess the strength and adhesion of BBA while ovine models are being employed to evaluate the safety of BBA in both non-critical and critical defects.
Conflict of interest
MT and AA are inventors of the Banna Bone Adhesive. The authors state no conflict of interest.
Acknowledgments
The authors would like to thank the financial assistance of CIHR/NSERC–Collaborative Health Research Projects (315694-DAN).
References
- 1.Cooper C., Campion G., Melton L.J. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 2.Cummings S.R., Kelsey J.L., Nevitt M.C., O’Dowd K.J. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7(1):178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- 3.Abbott T.A., Lawrence B.J., Wallach S. Osteoporosis: the need for comprehensive treatment guidelines. Clin Ther. 1996;18(1):127–149. doi: 10.1016/s0149-2918(96)80186-x. [DOI] [PubMed] [Google Scholar]
- 4.Riggs B.L., Melton L.J. The prevention and treatment of osteoporosis. N Engl J Med. 1992;327(9):620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 5.Ring D., Jupiter J.B. Treatment of osteoporotic distal radius fractures. Osteoporos Int. 2005;16(2):S80–S84. doi: 10.1007/s00198-004-1808-x. [DOI] [PubMed] [Google Scholar]
- 6.Green D.P. Pins and plaster treatment of comminuted fractures of the distal end of the radius. J Bone Jt Surg. 1975;57(3) 304 LP–310. [PubMed] [Google Scholar]
- 7.Earnshaw S.A., Aladin A., Surendran S., Moran C.G. Closed reduction of colles fractures: comparison of manual manipulation and finger-trap traction. J Bone Jt Surg. 2002;84(3) doi: 10.2106/00004623-200203000-00004. 354 LP–358. [DOI] [PubMed] [Google Scholar]
- 8.Petty W. The effect of methylmethacrylate on bacterial phagocytosis and killing by human polymorphonuclear leukocytes. JBJS. 1978;60(6) [PubMed] [Google Scholar]
- 9.Laskin R.S. 2001. Controversies in Total Knee Arthroplasty. [Google Scholar]
- 10.Lieberman I.H., Togawa D., Kayanja M.M. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5(6):S305–S316. doi: 10.1016/j.spinee.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Erbe E.M., Clineff T.D., Gualtieri G. Comparison of a new bisphenol-a-glycidyl dimethacrylate-based cortical bone void filler with polymethyl methacrylate. Eur Spine J. 2001;10(2):S147–S152. doi: 10.1007/s005860100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen M., Bae H., Maurer P. 5: 5655: clinical experience using a novel bio-ceramic for treating vertebral compression fractures with one to two year follow-up. Spine J. 2006;6(5):27S–28S. [Google Scholar]
- 13.Darmani H., Al-Hiyasat A.S. The effects of BIS-GMA and TEG-DMA on female mouse fertility. Dent Mater. 2006;22(4):353–358. doi: 10.1016/j.dental.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Boyd D., Towler M.R., Wren A., Clarkin O.M. Comparison of an experimental bone cement with surgical Simplex P, Spineplex and Cortoss. J Mater Sci Mater Med. 2008;19(4):1745–1752. doi: 10.1007/s10856-007-3363-4. [DOI] [PubMed] [Google Scholar]
- 15.Friedman C.D., Costantino P.D., Takagi S., Chow L.C. BoneSource™ hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res. 1998;43(4):428–432. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Larsson S., Bauer T.W. Use of injectable calcium phosphate cement for fracture fixation: a review. Clin Orthop Relat Res. 2002;395 doi: 10.1097/00003086-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Tsai C.-H., Lin R.-M., Ju C.-P., Chern Lin J.-H. Bioresorption behavior of tetracalcium phosphate-derived calcium phosphate cement implanted in femur of rabbits. Biomaterials. 2008;29(8):984–993. doi: 10.1016/j.biomaterials.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Ooms E.M., Wolke J.G.C., van de Heuvel M.T., Jeschke B., Jansen J.A. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials. 2003;24(6):989–1000. doi: 10.1016/s0142-9612(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson J.W. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–494. doi: 10.1016/s0142-9612(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 20.Wilson A.D., Kent B.E. The glass-ionomer cement, a new translucent dental filling material. Appl Chem Biotechnol. 1971;21:313. [Google Scholar]
- 21.Lewis G., Towler M.R., Boyd D. Evaluation of two novel aluminum-free, zinc-based glass polyalkenoate cements as alternatives to PMMA bone cement for use in vertebroplasty and balloon kyphoplasty. J Mater Sci Mater Med. 2010;21(1):59–66. doi: 10.1007/s10856-009-3845-7. [DOI] [PubMed] [Google Scholar]
- 22.Zainuddin N., Karpukhina N., Hill R.G., Law R.V. A long-term study on the setting reaction of glass ionomer cements by (27)Al MAS-NMR spectroscopy. Dent Mater. 2009;25(3):290–295. doi: 10.1016/j.dental.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Alhalawani A.M.F., Curran D.J., Boyd D., Towler M.R. The role of poly(acrylic acid) in conventional glass polyalkenoate cements: a review. J Polym Eng. 2015;0 [Google Scholar]
- 24.Wasson E.A., Nicholson J.W. New aspects of the setting of glass-ionomer cements. J Dent Res. 1993;72:481–483. doi: 10.1177/00220345930720020201. [DOI] [PubMed] [Google Scholar]
- 25.Alhalawani A.M., Towler M.R. A novel tantalum-containing bioglass: part I. Structure and solubility. Mater Sci Eng C. 2017;72:202–211. doi: 10.1016/j.msec.2016.11.066. [DOI] [PubMed] [Google Scholar]
- 26.Alhalawani A.M., Mehrvar C., Stone W., Waldman S.D., Towler M.R. A novel tantalum-containing bioglass: part II. Development of a bioadhesive for sternal fixation and repair. Mater Sci Eng C. 2017;71:401–411. doi: 10.1016/j.msec.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Fitoussi F., Ip W.Y., Chow S.P. Treatment of displaced intra-articular fractures of the distal end of the radius with plates. J Bone Jt Surg. 1997:79A. doi: 10.2106/00004623-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Jakubietz M.G., Gruenert J.G., Jakubietz R.G. The use of beta-tricalcium phosphate bone graft substitute in dorsally plated, comminuted distal radius fractures. J Orthop Surg Res. 2011;6(1):24. doi: 10.1186/1749-799X-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapoor H., Agarwal A., Dhaon B.K. Displaced intraarticular fractures of distal radius: a comparative evaluation of results following closed reduction, external fixation and open reduction with internal fixation. Injury. 2000;31 doi: 10.1016/s0020-1383(99)00207-7. [DOI] [PubMed] [Google Scholar]
- 30.Sakano H., Koshino T., Saito T. Treatment of the unstable radius fracture with external fixation and a hydroxyapatite spacer. J Hand Surg. 2001:26A. doi: 10.1053/jhsu.2001.27771. [DOI] [PubMed] [Google Scholar]
- 31.Orbay J.L., Fernandez D.L. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96–102. doi: 10.1016/j.jhsa.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Hidaka N., Yamano Y., Kadoya Y., Nishimura N. Calcium phosphate bone cement for treatment of distal radius fractures: a preliminary report. J Orthop Sci. 2002;7 doi: 10.1007/s007760200031. [DOI] [PubMed] [Google Scholar]