Abstract
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mass and deterioration of bone microarchitecture, which results in increased bone fragility and fracture risk. Casein kinase 2-interacting protein-1 (CKIP-1) is a protein that plays an important role in regulation of bone formation. The effect of CKIP-1 on bone formation is mainly mediated through negative regulation of the bone morphogenetic protein pathway. In addition, CKIP-1 has an important role in the progression of osteoporosis. This review provides a summary of the recent studies on the role of CKIP-1 in osteoporosis development and treatment.
Cite this article: X. Peng, X. Wu, J. Zhang, G. Zhang, G. Li, X. Pan. The role of CKIP-1 in osteoporosis development and treatment. Bone Joint Res 2018;7:173–178. DOI: 10.1302/2046-3758.72.BJR-2017-0172.R1.
Keywords: Osteoporosis, CKIP-1, Bone formation, BMP pathway
Article focus
An overview of the role of CKIP-1 in osteogensis and osteoclastogenesis and its relationship with osteoporosis.
Reveal the possible treatment of osteoporosis through manipulating the biological function of CKIP-1.
Key messages
The structure and function of casein kinase 2-interacting protein-1 (CKIP-1).
CKIP-1 negatively regulates bone formation through bone morphogenetic protein pathway.
Progress in research on the role of CKIP-1 in osteoporosis treatment.
Strengths and limitations
This review summarizes the mechanism of action of CKIP-1 in the pathogenesis of OP and predicts CKIP-1 siRNA therapy may be a novel treatment option for OP.
Whether CKIP-1 can interact with other signaling molecules to regulate the BMP pathway or regulate bone metabolism via other pathways remains unconfirmed.
The osteoblast-targeted treatment using CKIP-1 siRNA has only been tested in animals and similar drugs have not yet been developed for human application.
Introduction
Osteoporosis (OP) is a systemic multifactorial skeletal disorder characterized by reduced bone mass, deterioration of bone microarchitecture and increased bone fragility, resulting in a tendency of fracture and leading to possible lifelong disability or death.1 Therefore, with an expanding ageing population, OP has become an important public health problem and the understanding of the physiological and biochemical regulations of osteoblast-osteoclast balance, along with the mechanisms of bone destruction and loss, and the development of possible novel therapeutic strategies has become of real importance.
Structure and function of casein kinase 2-interacting protein-1 (CKIP-1)
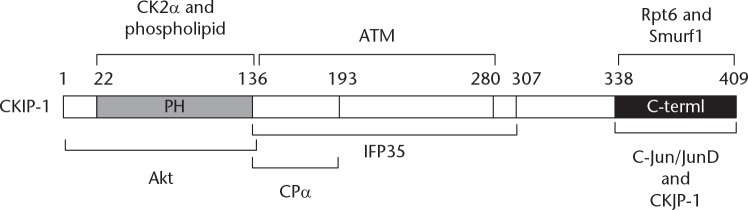
CKIP-1 is a transcription factor that was first identified in the gene expression profile in the liver of fetuses. This gene is commonly known as CKIP-1 or Pleckstrin Homology domain-containing family O member 1 (PLEKHO1).2 The full-length CKIP-1 cDNA encodes a protein of 409 amino acids, of approximately 46 kD; the N-terminus of CKIP-1 protein contains a pleckstrin homology (PH) domain, and the C-terminus contains a hypothetical leucine zipper (LZ) motif (352 amino acids (aa) to 380 aa), along with five proline-rich motifs throughout the protein.2 CKIP-1 acts as a scaffold adaptor to mediate the interactions with multiple signalling and cellular proteins, such as CK2a, CPa, PI3K/Akt, c-Jun/JunD, ATM/p53, IFP35/Nmi, Smurf1.3 CKIP-1 therefore mediates cell growth, differentiation, apoptosis, the cytoskeleton, and bone formation.4,5 CKIP-1 has also been found to be a promising target for the treatment of OP and it has been suggested that CKIP-1 might function as a potential tumour suppressor.6,7
The PH domain at the N-terminus of CKIP-1 contains a nuclear localization signal that can bind to CK2a and regulate the cellular localization of CK2.8 A fragment of the CKIP-1 C-terminus can be cleaved by caspase-3. Once released, this fragment is translocated to the cell nucleus where it binds and regulates the activity of the C-Jun/JunD transcription factor, thereby increasing cell apoptosis (Figure 1).9,10
Fig. 1.
Illustration of CKIP-1 binding regions and interacting proteins. CKIP-1, Casein kinase 2-interacting protein-1; CK2a, The a-subunit of casein kinase 2; PH, Pleckstrin homology; Akt, A serine/threonine-specific protein kinase; ATM, Ataxia-telangiectasia mutated; CPa, The a-subunit of Capping protein; IFP35, Interferon-induced 35-kDA protein; Smurf1, Smad ubiquitination regulatory factor 1; C-Jun/JunD, A protein that in humans is encoded by the JUN gene.
Cooper and Sept11 and Canton et al12 reported that CKIP-1 could regulate the actin cytoskeleton and cell morphology by enhancing actin polymerization through recruiting the capping protein to the cell membrane. In addition, Tokuda et al7 and Xi et al13 have reported that the N-terminus amino acid sequence of CKIP-1 binds to the PH domain of serine/threonine kinase Akt, and dimerization of CKIP-1 through its LZ domain at the C-terminus could inactivate Akt and inhibit tumour proliferation. Zhang et al14 showed that CKIP-1 could protect p53 and delay p53 degradation by directly binding and activating the ataxia-telangiectasia mutated (ATM) protein to enhance the level of p53 phosphorylation and by recruiting some nuclear ATM to relocalize at the plasma membrane. Interferon (IFN)-induced 35-kDA protein (IFP35) and its homologue N-myc-interactor (NMI) are two proteins that can interact with CKIP-1. IFP35 is a short-lived protein that can be easily degraded by proteasomes when it presents alone. However, IFP35 is stabilized when it forms a heterodimer with NMI. CKIP-1 can directly interact with IFP35 and NMI, and overexpression of CKIP-1 can interfere with IFP35-NMI dimerization, resulting in the instability of IFP35 with the loss of protection from NMI. Moreover, similar to IFP35 and NMI, the expression of CKIP-1 in peripheral blood mononuclear cells can be induced by IFN-γ and interleukin-2, indicating that CKIP-1 may be involved in cytokine signalling and may function as a regulator of the physiological functions of IFP35 and NMI in vivo.15
CKIP-1 induces osteoporosis through negative regulation of bone formation
In order to maintain their normal functions bone tissues undergo constant metabolic turnover. However, with ageing, the loss of bone components gradually leads to a negative balance in bone mass. There is increased activity of osteoclasts along with loss of osteogenic function which contributes to an overall reduction in bone formation.16 This imbalance 0f ‘bone loss > bone formation’ forms the cellular basis for the development of OP. Lu et al demonstrated that bone density and bone mass increased with the age of CKIP-1-knockout (KO) mice and the activity of osteoblasts in these mice was significantly higher than those of wild-type mice.17 Zhou et al have shown that in a rat mandibular distraction osteogenesis model, the small interfering RNA of CKIP (siRNA) significantly promoted bone formation both in vitro and in vivo,.18 This indicates that the physiological level of CKIP-1 is an important negative regulator of bone formation, suggesting that high levels of CKIP-1 can lead to OP.
CKIP-1 and primary osteoporosis
Primary OP is the most common type of OP and is mainly caused by ageing, decreased physiological functions of organs and reduced secretion of sex hormones.1 There are two types of primary OP, postmenopausal (Type I) and senile (Type II). Type 1 is a high-turnover OP (bone resorption > bone formation). Using an ovariectomized rat model, Zhang et al19 evaluated the efficacy of CKIP-1 siRNA delivered by a bone-targeted delivery system (DSS6-liposome) and examined bone morphometric parameters, bone mass, and bone structures. They found that therapeutic CKIP-1 siRNA intervention could significantly promote bone formation without affecting bone resorption, indicating that CKIP-1 plays a significant role in reversing bone loss during Type 1 OP.
Type II OP, is mainly found among individuals aged ⩾ 70 years with a male to female ratio of 1:2. Ling et al studied the efficacy of CKIP-1 siRNA for treating Type II OP in old male Sprague-Dawley rats, (also assessed by examining bone morphometric parameters, bone mass and bone structures), and concluded that there was inhibition of bone formation by osteoblasts.20 On the other hand, Liu et al21 reported that, with aging, expression of CKIP-1 was increased in patients with bone fractures and that there was an association between reduced bone morphogenetic protein (BMP)-Smad signalling and bone formation. Hence using genetic approaches, the loss of CKIP-1 in osteoblasts, could promote BMP-Smad signalling and thereby alleviate the reduction in bone formation in Type II OP.
CKIP-1 and secondary osteoporosis
Secondary OP induced by diseases or drugs, can be classified as: (1) congenital e.g. osteogenesis imperfecta or homocystinuria (2) endocrinological (3) nutritional deficiency; induced by vitamin D and K deficiency, long-term calcium insufficiency, or long-term insufficiency of protein or elements including magnesium, manganese, strontium, and zinc (4) blood disease-induced e.g. monoclonal gammopathy of uncertain significance, multiple myeloma, systemic mastocytosis, beta thalassemia major (5) drug-induced, e.g. long-term use of adrenal cortex hormones (6) renal, caused by chronic kidney disease (7) due to respiratory diseases and (8) weightlessness or disuse-induced, caused by long-term immobilization or from space flight.1
Long-term usage of glucocorticoids can inhibit osteoblast differentiation and mineralization, promote osteoblasts and osteocytes undergoing apoptosis and reduce trabecular bone mass. In addition, it can inhibit bone matrix protein synthesis, suppress the action of vitamin D, reduce calcium absorption, increase calcium secretion and stimulate parathyroid hormone (PTH) secretion, leading to enhanced bone resorption and loss of bone mass, in turn contributing to OP.22 Glucocorticoid-induced OP (GIOP) is the most common drug-induced OP and one of the most serious side effects of glucocorticoid use. Liu et al6 showed that high expression of CKIP-1 during extracorporeal glucocorticoid treatment could inhibit osteoblast differentiation and mineral deposition in osteoblasts by disrupting the Smad-dependent BMP signaling pathway.
CKIP-1 and mechanical stimulation on osteogenesis
Weightlessness-induced OP is a type of disuse-induced OP as gravity has an important role in normal growth and function of the human skeletal system. In a microgravity environment, reduced mechanical stimulation in the skeletal system enhances osteoclast function and attenuates osteoblast function.23 Zhang et al24 reported that during a tail suspension test, which simulated the effect of a microgravity environment, the femoral bone in CKIP-1 KO mice was not changed significantly, suggesting that loss of CKIP-1 could alleviate microgravity-induced OP by influencing the process of bone formation.
Distraction osteogenesis (DO) is also becoming an effective therapy for bone defects, by stimulating endogenous bone regeneration. Zhou et al have shown that CKIP-1 silences inhibited cell apoptosis and improves calcification through the induced expression of Wnt-3a, β-catenin and osteocalcin (OCN) in rat mandibular distraction osteogenesis.18 Also in fracture repair in animals, Wnt signalling may be involved in the bone formation of chitosan/si-CKIP-1 in DO.25
In summary, CKIP-1 can decrease osteogenic differentiation and cause primary OP through negative regulation of bone formation and can reduce bone formation by negatively regulating the BMP pathway in GIOP and Wnt-3a /β-catenin pathway in DO.
CKIP-1 regulates bone formation through bone morphogenetic protein pathway
Osteogenesis is comprised of two basic processes, bone formation and bone resorption,26 both of which involve various complex factors. These factors include numerous cytokines and signalling pathways, such as nuclear receptor-interacting protein 1 (NRIP1), interleukin-1 receptor-associated kinase 3 (IRAK3), bone morphogenetic protein 7 (BMP7), SMAD family member 1 (SMAD1), MAPK3,C-X-C chemokine receptor type 4 (CXCR4), OPG/RANKL/RANKL pathway, Wnt/β-catenin pathway, cathepsin K pathway, and BMP pathway.27,28 The BMP pathway is the most important signalling during these processes, as it can induce osteogenic differentiation, enhance bone formation without activating resorption and can promote bone repair without affecting non-skeletal tissues.29
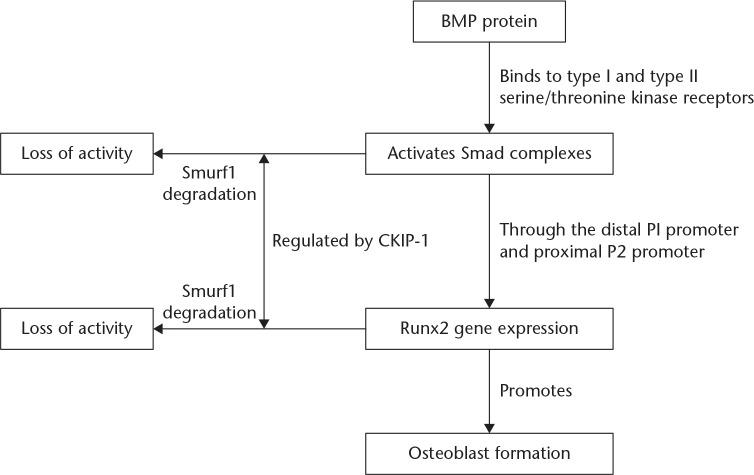
BMP’s are members of the transforming growth factor-β (TGF-β) superfamily and are significant extracellular molecules which promote bone formation and induce osteoblast differentiation.30 There are two types of transmembrane serine/threonine kinase receptors for BMPs, namely, the type I BMP receptor (BMPR-I) and the type II BMP receptor (BMPR-II).31 As one of the most widely studied and most potent osteogenic activity-inducing members of the BMP family, BMP-2, plays an important role in osteogenesis by activating Smad signal transduction and regulating osteogenic gene transcription.32 The process of BMP-2 signal transduction begins with its binding to BMPR-II. Subsequently, BMPR-II phosphorylates BMPR-I and the activated BMPR-I activates downstream Smads.33 Both in vitro and in vivo studies have confirmed that the BMP-Smad signalling could regulate various aspects of the osteoblast lifecycle, including mesenchymal stem cell (MSC) differentiation into osteoblasts, osteoprogenitor proliferation, osteoblast mineralization and osteoblast-osteoclast coupling.34 Defects in the BMP-Smad signalling pathway often result in reduced bone formation and mass, with the development of OP. In addition, the activation of BMP-Smad signalling can lead to a bone sclerosis phenotype.34
After the BMP-2 has mediated the activation of Smads (Smad1, Smad5 and Smad8), it initiates the Runt-related gene 2 (RUNX2) expression through the distal P1 and proximal P2 promoters of the proteins. RUNX2 plays a vital role in bone formation and remodelling and it has been shown that RUNX2-KO mice display non-mineralized bone formation. This complete blockage of intramembranous and endochondral ossifications in mice has demonstrated the importance of RUNX2 in bone development.35-38 Smad ubiquitination regulatory factor 1 (Smurf1) is a member of the Nedd4 family, Homologous to the E6-associated protein Carboxyl Terminus (HECT) E3 ligases. It can promote the ubiquitination and degradation of substrates such as Smad1/5, mitogen-activated protein kinase kinase kinase 2 (MEKK2), and RUNX2; moreover, it has an important function during the osteoblast differentiation.39 Smurf1 is comprised of one HECT domain and two WW domains. A highly conserved cysteine residue at the C-terminus of the HECT domain can form a thioester bond with ubiquitin. However, once this highly conserved cysteine residue is mutated to alanine or glycine, the ubiquitination and protein-specific degradation activities of Smurf1 are completely lost.40 The WW domain is another important feature of Smurf1, located between 236 aa and 311 aa, and is approximately 30 amino acids long. It consists of two highly conserved tryptophan residues and one highly conserved proline residue and is capable of binding to small proline-rich (PPxY motif) peptides. Zhang et al have shown that mutation of the PY motif in the Smad protein (proline- and tyrosine-rich domain) abrogated the interaction between Smad and Smurf1, resulting in the inhibition of Smad degradation.41
Lu et al17 reported that CKIP-1 could increase the affinity between the WW domain and its substrates by binding to the linking region between the two WW domains of Smurf1. This enhanced the ubiquitin ligase activity of Smurf1, promoting the degradation of Smad1/5 and negatively regulating the BMP signalling pathway. Moreover, CKIP-1 could bind to the proteasome subunit Rpt6 as a linker and be coupled with the Smurf1 proteasome to enhance degradation of ubiquitinated protein substrates and inhibit osteoblast differentiation. Therefore, CKIP-1 plays dual roles in the interaction between Smurf1 and its substrates and the recruitment of substrates to the 26S proteasome.42
Taken together, CKIP-1 enhances ubiquitin ligase activity by binding to Smurf1, which in turn accelerates the degradation of bone formation-related substrates and results in the inhibition of new bone formation.17,42 Therefore, CKIP-1 plays an important negative regulatory role in the process of bone formation (Figure 2).
Fig. 2.
The mechanism of action of CKIP-1 in regulating bone formation through the BMP pathway. BMP, Bone morphogenetic protein; Smurf1, Smad ubiquitination regulatory factor 1; CKIP-1, Casein kinase 2-interacting protein-1; RUNX2, Runt-related gene 2.
Progress in research on the role of CKIP-1 in osteoporosis treatment
Osteoporosis is easily misdiagnosed and can be difficult to completely cure.43,44 Ageing and oestrogen deficiency are the key pathophysiological mechanisms. Although a number of antiresorptive drugs are thought to be effective for OP prevention, they do not provide comprehensive protection,45 which may be due to postmenopausal state and age, both of which reduce the ability of recruitment of stem cells and osteogenesis.28 Currently, the antiresorptive drugs, such as selective oestrogen receptor modulators (SERMs), bisphosphonate and denosumab, inhibit bone resorption. Wang et al conducted a systematic review of ten different therapies used for postmenopausal OP and showed that PTH and zoledronic acid (ZOL) have the highest probability of treatment efficacy in preventing clinical vertebral fractures.46 However, these drugs have several drawbacks. SERMs (such as raloxifene) when used long term, can lead to breast and endometrial cancer.47 Bisphosphonates have relatively high gastrointestinal toxicity which can induce, abdominal pain, gastritis and oesophagitis, and can also lead to jaw necrosis, femoral fractures and atrial fibrillation.48 To overcome these shortcomings, new therapeutic drugs for OP are being developed with optimized efficacy, reduced side effects, and, hopefully, enhanced patient compliance.49
Regulatory imbalance between the activity of osteoblasts and osteoclasts is thought to be the main cause of OP. There have been several studies which have explored the mechanisms of regulation of bone metabolism by osteoclasts.51-52 However, osteoclast-targeted treatments for OP are still a problem, as they are only able to reduce or decelerate the rate of bone resorption and are unable to effectively promote new osteogenesis.51-52 It is known that CKIP-1 acts as a negative regulator of bone formation and its gene knockdown could significantly increase bone density and bone mass.
The siRNA delivery is a promising approach for the treatment of various diseases. Zheng et al53 reported that CKIP-1 siRNA could reduce the expression of CKIP-1 mRNA and promote osteoblast gene expression and osteoblast mineralization. Guo et al54 also found that the cross-species CKIP-1 siRNA promoted osteoblast differentiation in human, rhesus monkey, rat and mouse osteoblast-like cells in vitro, with stimulated bone formation but without elevating bone resorption in healthy rodents and osteoporotic mice in vivo.54 Zhang et al55 reported that a chitosan/si-CKIP-1-biofunctionalized titanium implant significantly improved the in vitro osteogenic differentiation of MSCs and led to dramatically enhanced osseointegration in the in vivo rat model. Moreover, it has been shown in animals that biweekly intravenous injections of 7.5 mg/kg CKIP-1 siRNA could maintain long-term low expression levels of CKIP-1 mRNA.19,56 Zheng et al developed a liposome-based bone tissue-specific delivery system to enable osteoblast-targeted delivery of CKIP-1 siRNA, which was highly effective in inhibiting CKIP-1 expression in osteoblasts, promoting osteogenic gene expression, increasing bone mass and improving bone microarchitecture.53 Considering the highly efficient and specific silencing effect of siRNA, CKIP-1 siRNA therapy this may be a novel treatment option for OP. However, this strategy has drawbacks as the introduction of external siRNA into mammalian cells can potentially induce an immune response. Also CKIP-1 plays a role in many other areas, such as in the regulation of the cytoskeleton, cell growth and apoptosis. Therefore, its clinical application will need further investigation.
Future prospects
This review summarizes the mechanism of action of CKIP-1 in the pathogenesis of OP. CKIP-1 reduces bone formation mainly through binding to Smurf1 and the subsequent specific negative regulation of the BMP pathway. However, whether CKIP-1 can interact with other signaling molecules to regulate the BMP pathway or regulate bone metabolism via other pathways remains unconfirmed. In addition, the osteoblast-targeted treatment using CKIP-1 siRNA has only been tested in animals and similar drugs have not yet been developed for human application. Furthermore, it is largely unknown whether CKIP-1 siRNA will have human specificity or will induce side effects. Therefore, further studies are required to fully understand he mechanism of CKIP-1 regulation in OP pathogenesis and its potential as a targeted treatment for OP in humans.
Footnotes
Author Contribution: X. Peng: Designing the review.
X. Wu: Reviewing the literature, Writing the manuscript.
J. Zhang: Revising the manuscript.
G. Zhang: Revising the manuscript.
G. Li: Designing the study.
X. Pan: Literature review, Writing the manuscript.
Conflict of Interest Statement: None declared
Funding Statement
The work was partially supported by grants from Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. 14119115, 14160917, 9054014 N_CityU102/15, T13-402/17-N); National Natural Science Foundation of China (81371946, 81430049 and 81772322); Hong Kong Innovation Technology Commission Funds (ITS/UIM-305) and grants from the Shenzhen Science and Technology Innovation Council of China (JCYJ20150630165236960, JSGG20160229195900623 and JCYJ20150402152005636), Sanming Project of medicine in Shenzhen (SZSM20150602).
References
- 1. Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol 2017;18:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosc DG, Graham KC, Saulnier RB, et al. Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J Biol Chem 2000;275:14295-14306. [DOI] [PubMed] [Google Scholar]
- 3. Nie J, Liu L, He F, et al. CKIP-1: a scaffold protein and potential therapeutic target integrating multiple signaling pathways and physiological functions. Ageing Res Rev 2013;12:276-281. [DOI] [PubMed] [Google Scholar]
- 4. Ozdamar B, Bose R, Barrios-Rodiles M, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 2005;307:1603-1609. [DOI] [PubMed] [Google Scholar]
- 5. Yamashita M, Ying SX, Zhang GM, et al. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 2005;121:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Lu C, Wu X, et al. Targeting osteoblastic casein kinase-2 interacting protein-1 to enhance Smad-dependent BMP signaling and reverse bone formation reduction in glucocorticoid-induced osteoporosis. Sci Rep 2017;7:41295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tokuda E, Fujita N, Oh-hara T, et al. Casein kinase 2-interacting protein-1, a novel Akt pleckstrin homology domain-interacting protein, down-regulates PI3K/Akt signaling and suppresses tumor growth in vivo. Cancer Res 2007;67:9666-9676. [DOI] [PubMed] [Google Scholar]
- 8. Glover CV., III On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol 1998;59:95-133. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Xing G, Tie Y, et al. Role for the pleckstrin homology domain-containing protein CKIP-1 in AP-1 regulation and apoptosis. EMBO J 2005;24:766-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serafini AN. Therapy of metastatic bone pain. J Nucl Med 2001;42:895-906. [PubMed] [Google Scholar]
- 11. Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol 2008;267:183-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canton DA, Olsten ME, Niederstrasser H, Cooper JA, Litchfield DW. The role of CKIP-1 in cell morphology depends on its interaction with actin-capping protein. J Biol Chem 2006;281:36347-36359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xi S, Tie Y, Lu K, et al. N-terminal PH domain and C-terminal auto-inhibitory region of CKIP-1 coordinate to determine its nucleus-plasma membrane shuttling. FEBS Lett 2010;584:1223-1230. [DOI] [PubMed] [Google Scholar]
- 14. Zhang L, Tie Y, Tian C, et al. CKIP-1 recruits nuclear ATM partially to the plasma membrane through interaction with ATM. Cell Signal 2006;18:1386-1395. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Tang Y, Tie Y, et al. The PH domain containing protein CKIP-1 binds to IFP35 and Nmi and is involved in cytokine signaling. Cell Signal 2007;19:932-944. [DOI] [PubMed] [Google Scholar]
- 16. Motyl KJ, Guntur AR, Carvalho AL, et al. Energy Metabolism of Bone. Toxicol Pathol 2017;45:887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu K, Yin X, Weng T, et al. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat Cell Biol 2008;10:994-1002. [DOI] [PubMed] [Google Scholar]
- 18. Zhou ZC, Che L, Kong L, et al. CKIP-1 silencing promotes new bone formation in rat mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123:e1-e9. [DOI] [PubMed] [Google Scholar]
- 19. Zhang G, Guo B, Wu H, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med 2012;18:307-314. [DOI] [PubMed] [Google Scholar]
- 20. Ling S, Sun Q, Li Y, et al. CKIP-1 inhibits cardiac hypertrophy by regulating class II histone deacetylase phosphorylation through recruiting PP2A. Circulation 2012;126:3028-3040. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Liang C, Guo B, et al. Increased PLEKHO1 within osteoblasts suppresses Smad-dependent BMP signaling to inhibit bone formation during aging. Aging Cell 2017;16:360-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazziotti G, Formenti A M, Adler R A, et al. Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 2016;54:603-611. [DOI] [PubMed] [Google Scholar]
- 23. Lau R Y, Guo X. A Review on Current Osteoporosis Research: With Special Focus on Disuse Bone Loss. J Osteoporos 2011;2011:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X, Wang Q, Wan Z, et al. CKIP-1 knockout offsets osteoporosis induced by simulated microgravity. Prog Biophys Mol Biol 2016;122:140-148. [DOI] [PubMed] [Google Scholar]
- 25. Xu H, Duan J, Ning D, et al. Role of Wnt signaling in fracture healing. BMB Rep 2014;47:666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell 1995;80:371-378. [DOI] [PubMed] [Google Scholar]
- 27. Li JJ, Wang BQ, Fei Q, Yang Y, Li D. Identification of candidate genes in osteoporosis by integrated microarray analysis. Bone Joint Res 2016;5:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanghani-Kerai A, Coathup M, Samazideh S, et al. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4. Bone Joint Res 2017;6:358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 1998;346:26-37. [PubMed] [Google Scholar]
- 30. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 2004;22:233-241. [DOI] [PubMed] [Google Scholar]
- 31. Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci 2001;114:4359-4369. [DOI] [PubMed] [Google Scholar]
- 32. Liu T, Gao Y, Sakamoto K, et al. BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol 2007;211:728-735. [DOI] [PubMed] [Google Scholar]
- 33. Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 2004;23:552-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene 2005;357:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997;89:765-771. [DOI] [PubMed] [Google Scholar]
- 36. Mundlos S, Otto F, Mundlos C, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 1997;89:773-779. [DOI] [PubMed] [Google Scholar]
- 37. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997;89:747-754. [DOI] [PubMed] [Google Scholar]
- 38. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89:755-764. [DOI] [PubMed] [Google Scholar]
- 39. Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene 2004;23:4232-4237. [DOI] [PubMed] [Google Scholar]
- 40. Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999;400:687-693. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci USA 2001;98:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Nie J, Wang Y, et al. CKIP-1 couples Smurf1 ubiquitin ligase with Rpt6 subunit of proteasome to promote substrate degradation. EMBO Rep 2012;13:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sale JEM, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067-2082. [DOI] [PubMed] [Google Scholar]
- 44. Shepherd AJ, Cass AR, Ray LA, Tan A, Wilkinson GS. Treatment for older men with fractures. Osteoporos Int 2012;23:1041-1051. [DOI] [PubMed] [Google Scholar]
- 45. Farrier AJ, Sanchez Franco LC, Shoaib A, et al. New anti-resorptives and antibody mediated anti-resorptive therapy. Bone Joint J 2016;98-B:160-165. [DOI] [PubMed] [Google Scholar]
- 46. Wang G, Sui L, Gai P, et al. The efficacy and safety of vertebral fracture prevention therapies in post-menopausal osteoporosis treatment: which therapies work best? a network meta-analysis. Bone Joint Res 2017;6:452-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldstein SR, Duvernoy CS, Calaf J, et al. Raloxifene use in clinical practice: efficacy and safety. Menopause 2009;16:413-421. [DOI] [PubMed] [Google Scholar]
- 48. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010;95:1555-1565. [DOI] [PubMed] [Google Scholar]
- 49. Faienza MF, Chiarito M, D’Amato G, et al. Monoclonal antibodies for treating osteoporosis. Expert Opin Biol Ther 2017;7:1-9. [DOI] [PubMed] [Google Scholar]
- 50. Ram VS, Parthiban, Sudhakar U, Mithradas N, Prabhakar R. Bone biomarkers in periodontal disease: a review article. J Clin Diagn Res 2015;9:ZE07-ZE10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature 2003;423:349-355. [DOI] [PubMed] [Google Scholar]
- 52. Du YY, Zhao YX, Liu YP, et al. Regulatory Tweak/Fn14 signaling pathway as a potent target for controlling bone loss. Biomed Pharmacother 2015;70:170-173. [DOI] [PubMed] [Google Scholar]
- 53. Zheng L, Zhang G, Tang T, et al. Identifying a cross-species siRNA targeting a newly discovered inhibitor of bone formation CKIP-I for promoting osteoblast differentiation and mineralization in vitro. Bone 2010;47:S371-S372. [Google Scholar]
- 54. Guo B, Zhang B, Zheng L, et al. Therapeutic RNA interference targeting CKIP-1 with a cross-species sequence to stimulate bone formation. Bone 2014;59:76-88. [DOI] [PubMed] [Google Scholar]
- 55. Zhang L, Wu K, Song W, et al. Chitosan/siCkip-1 biofunctionalized titanium implant for improved osseointegration in the osteoporotic condition. Sci Rep 2015;5:10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo B, Zhang G, Tang T, et al. Continuous in vivo RNA interference mediated CKIP-1 gene silencing in mouse and rat bone: Optimization for dosage and duration. Bone 2010;47:S363. [Google Scholar]