Abstract
Objectives
As one of the heat-stable enterotoxins, Staphylococcal enterotoxin C2 (SEC2) is synthesized by Staphylococcus aureus, which has been proved to inhibit the growth of tumour cells, and is used as an antitumour agent in cancer immunotherapy. Although SEC2 has been reported to promote osteogenic differentiation of human mesenchymal stem cells (MSCs), the in vivo function of SCE2 in animal model remains elusive. The aim of this study was to further elucidate the in vivo effect of SCE2 on fracture healing.
Materials and Methods
Rat MSCs were used to test the effects of SEC2 on their proliferation and osteogenic differentiation potentials. A rat femoral fracture model was used to examine the effect of local administration of SEC2 on fracture healing using radiographic analyses, micro-CT analyses, biomechanical testing, and histological analyses.
Results
While SEC2 was found to have no effect on rat MSCs proliferation, it promoted the osteoblast differentiation of rat MSCs. In the rat femoral fracture model, the local administration of SEC2 accelerated fracture healing by increasing fracture callus volumes, bone volume over total volume (BV/TV), and biomechanical recovery. The SEC2 treatment group has superior histological appearance compared with the control group.
Conclusion
These data suggest that local administration of SEC2 may be a novel therapeutic approach to enhancing bone repair such as fracture healing.
Cite this article: T. Wu, J. Zhang, B. Wang, Y. Sun, Y. Liu, G. Li. Staphylococcal enterotoxin C2 promotes osteogenesis of mesenchymal stem cells and accelerates fracture healing. Bone Joint Res 2018;7:179–186. DOI: 10.1302/2046-3758.72.BJR-2017-0229.R1.
Keywords: Fracture healing, BMSC, Staphylococcal enterotoxin C2
Article focus
The aim of this study was to investigate the in vitro effect of Staphylococcal enterotoxin C2 (SEC2) on osteogenesis differentiation of human mesenchymal stem cells, and to investigate the in vivo effect of SEC2 on fracture healing.
Key messages
Staphylococcal enterotoxin C2 (SEC2) promotes the osteoblast differentiation of rat mesenchymal stem cells.
Local administration of SEC2 accelerates fracture healing in rats.
Strengths and limitations
This study confirms previous findings that SEC2 promotes osteogenic differentiation of mesenchymal stem cells (MSCs).
More research is required to understand the mechanics of how SEC2 works in promoting osteogenesis and fracture healing.
Further clinical studies are needed to validate these results in a human model.
Introduction
Fracture is a common orthopaedic condition that places a serious burden on both individuals and society. The recovery of long bone fracture generally needs over three months, but approximately 10% to 20% of patients still suffer from delayed union or nonunion, which suggests that a better therapeutic strategy is needed to help fracture union.1-2 During the last two decades, some cytokines and growth factors, such as bone morphogenetic protein (BMP), differentiation factor-5 (df-5), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), have been reported to be beneficial for fracture healing.3-7 However, most studies are still in their preclinical stage and their efficacies are controversial.8
Staphylococcal enterotoxin C2 (SEC2) is a heat-stable enterotoxin that is produced by Staphylococcus aureus. As a member of superantigens, SEC2 strongly stimulates T-cells’ activation by facilitating their crosslinking with major histocompatibility class II molecules.6,9 SEC2 have been proven to inhibit the growth of tumour cells and have been used as an antitumour agent in cancer immunotherapy.10 In China, it has been approved to use SEC2 as an antitumour drug in clinical settings due to its capabilities to induce the secretion of inflammatory cytokines such as interferon gamma (IFN-γ), interleukin 1 (IL-1), interleukin 6 (IL-6), and tumour necrosis factor alpha (TNF-α).11
In our previous study,12 SEC2 was found to promote the in vitro osteogenesis and suppress osteoclastogenesis of human mesenchymal stem cell (MSCs). These findings revealed the de novo function of SEC2 on osteogenic differentiation of human MSCs in vitro. To further elucidate the in vivo effect on fracture healing in this study, SEC2 was locally applied in a rat femoral fracture model. The quality of bone formation was evaluated by radiography, micro-CT scanning, mechanical properties testing, and histology examinations. The results demonstrated that SEC2 could promote fracture healing and accelerate the healing process, suggesting that SEC2 would be a potential new therapeutic strategy for bone fracture.
Materials and Methods
BMSCs culture and osteogenic induction
The bone marrow-derived mesenchymal stem cells (BMSCs) were first isolated from the bone marrow aspirated from four-week-old male Sprague Dawley rats, and then BMSCs were cultured and characterized using flow cytometry to confirm the MSCs identity. Isolated BMSCs were cultured in Minimum Essential Medium Alpha (α-MEM) supplemented with 10% fetal bovine serum (FBS, GIBCO, Thermo Fisher Scientific, Green Island, New York), 2 mM L-glutamine (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin (P/S, Thermo Fisher Scientific). To induce osteogenic differentiation of BMSCs, a mixture of 10 nM dexamethasone (Sigma; St. Louis, Missouri), 50 μg/ml ascorbic acid 2-phosphate (Sigma), and 10 mM glycerol 2-phosphate (Sigma) was added into the culture medium. The differentiation medium was changed every three days.
Cell viability assays
Cell viability was analyzed by using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) assays as described previously. Briefly, 5 × 103 cells were seeded per well into a 96-well plate. After being cultured with different SEC2 concentrations (1 pg/ml to 500 pg/ml) for one day, three days, and seven days, the cell viabilities were determined with MTT assays by using a Benchmark Plus microplate spectrometer (Bio-Rad Laboratories, Hercules, California).
Alizarin Red S staining
The Alizarin Red S staining was used to evaluate the calcium deposits formation. Cells were washed with phosphate buffered saline (PBS) and fixed with 75% ethanol for ten minutes, then 1% Alizarin Red S was added and maintained for 15 minutes. The result was measured at 550 nm using an automated plate reader (Thermo-Labsystems Inc., Leuven, Belgium) after elution with 1 ml of 10% cetylpyridinium chloride in 10 mM sodium phosphate.
Real-time quantitative polymerase chain reaction (qRT-PCR)
After ten days’ osteoinduction of BMSCs, total cellular RNAs were extracted using the RNeasy mini kit (Qiagen, Dusseldorf, Germany). The cDNAs were then reversely transcribed from the extracted RNAs by PrimeScript RT Master Mix (Takara, Kusatsu, Shiga, Japan). The Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) was applied in the quantitative RT-PCR to detect the target mRNAs by using ABI 7300 Fast Real-Time PCR Systems (Applied Biosystems, Foster City, California). The primers of osteogenic genes, such as alkaline phosphatase, runt-related transcription factor 2 (Runx2) osteocalcin, and osteopontin, are listed in Table I. The relative fold changes of candidate genes were analyzed by using the 2−ΔΔCt method.
Table I.
Primers used in this study
| Gene | Primer nucleotide sequence |
|---|---|
| GAPDH | 5’-GGTCGGTGTGAACGGATTTGG-3’ (Forward) |
| 5’-GCCGTGGGTAGAGTCATACTGGAAC-3’ (Reverse) | |
| Alkaline phosphatase | 5’-CCAGCAGGCTTACCAAGAA-3’ (Forward) |
| 5’-TTTATCGCACAAAGGGAACA-3’ (Reverse) | |
| Runx2 | 5’-TCCAGACCAGCAGCACTCC-3’ (Forward) |
| 5’-TCAGCGTCAACACCATCATTC-3’ (Reverse) | |
| Osteocalcin | 5’-AACGGTGGTGCCATAGATGC-3’ (Forward) |
| 5’-AGGACCCTCTCTCTGCTCAC-3’ (Reverse) | |
| Osteopontin | 5’-AATGAAGGGCCCTGAGC-3’ (Forward) |
| 5’-GCCAGTTCTGCAAGGAAGC-3’ (Reverse) |
GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; Runx2, Runt-related transcription factor 2
Rat femoral fracture model
This animal experiment was approved by the Animal Research Ethics Committee. The rat femoral fracture model was performed as previously reported.13,14 A total of 20 Sprague Dawley male rats (all 12 weeks old) were divided into two groups: treatment group (n = 10) and control group (n = 10). After intraperitoneally anaesthetizing the rats with ketamine and xylazine, a mid-femoral transverse osteotomy was made with a sagittal saw. The incision was washed and closed in two layers with absorbable suture. After seven days of the operation, 0.82 ng (in 41 ul PBS) SEC2 was locally injected into the fracture site every three days (until termination) under x-ray assays. For the control group, the same volume of PBS was injected.
Radiography examination
The fracture was monitored weekly using a digital radiographic machine (Faxitron MX-20 with DC-2 option, Faxitron, Tucson, Arizona). The fractures were scored as described previously.15 Fracture union was assessed according to the mineralized callus that bridges the fracture line (right side, one point; left side, one point; anterior side, one point; posterior side, one point). The score was estimated by two independent investigators blinded to the treatments. All rats were terminated at week four and the collected fractured femora were subjected to micro-CT examination as described previously.16 The femora were scanned by vivaCT 40 (SCANCO Medical, Bruttisellen, Switzerland) with a resolution of 10.5 μm. The scan range included 3 mm proximal and 3 mm distal to the fracture line. All samples were analyzed by using the same parameters according to our established evaluation protocol (sigma = 1.2, two voxel widths, low attenuation = 124; high attenuation = 256, in per mille of maximal image gray value). The low-density tissues represented the newly formed calluses and the high-density tissues represented the newly formed highly mineralized calluses. The following morphometric analysis parameters were accessed: TV (total volume of tissue), BVlow (volume of low radio-opacity bone), BVhigh (volume of high radio-opacity bone), and total connected density. These parameters were applied to calculate the percentage of the total tissue volume.
Biomechanical testing
The femora from each group (n = 6) were harvested for four-point bending mechanical test after micro-CT examination. A four-point bending device (H25KS; Hounsield Test Equipment Ltd. UK) with a constant displacement rate of 5 mm/min was used in accordance with our previous report.17 The femora were loaded in the anterior-posterior direction with the inner span blades set as 8 mm and the outer span blades set as 20 mm. The long axis of the femur was placed perpendicular to the blade during the test. The load-displacement curves of femora were generated using a built-in computer software (QMAT Professional; Tinius Olsen, Inc., Horsham, Pennsylvania) and ultimate load (UL), energy to failure, and modulus of elasticity (E-modulus) were analyzed.
Histological analyses
The harvested fractured femora samples were fixed in 10% formalin for 24 hours, and then decalcified with 5% ethylenediaminetetraacetic acid (EDTA) buffer for one month. They were dehydrated in successive alcohol concentrations before being embedded in paraffin. Specimens were cut into 5 μm sections by a Rotary Microtome (HM355S). Sections from each sample were stained with haematoxylin and eosin, Safranin O, or immunohistochemical staining of osteocalcin for histology examinations.
Statistical analysis
Differences between groups were examined for statistical significance using analysis of variance (ANOVA), and the results were expressed as means ± sd. All experiments were performed in triplicate. The significance level was set at p < 0.05 (two-tailed).
Results
Effects of SEC2 on cell viability of BMSCs
BMSCs were treated with SEC2 and the effects of SEC2 on cell viability were examined. As showed in Figure 1a, no obvious effect on cell proliferation was observed with SEC2 treatment at different concentration from 1 pg/ml to 500 pg/ml.
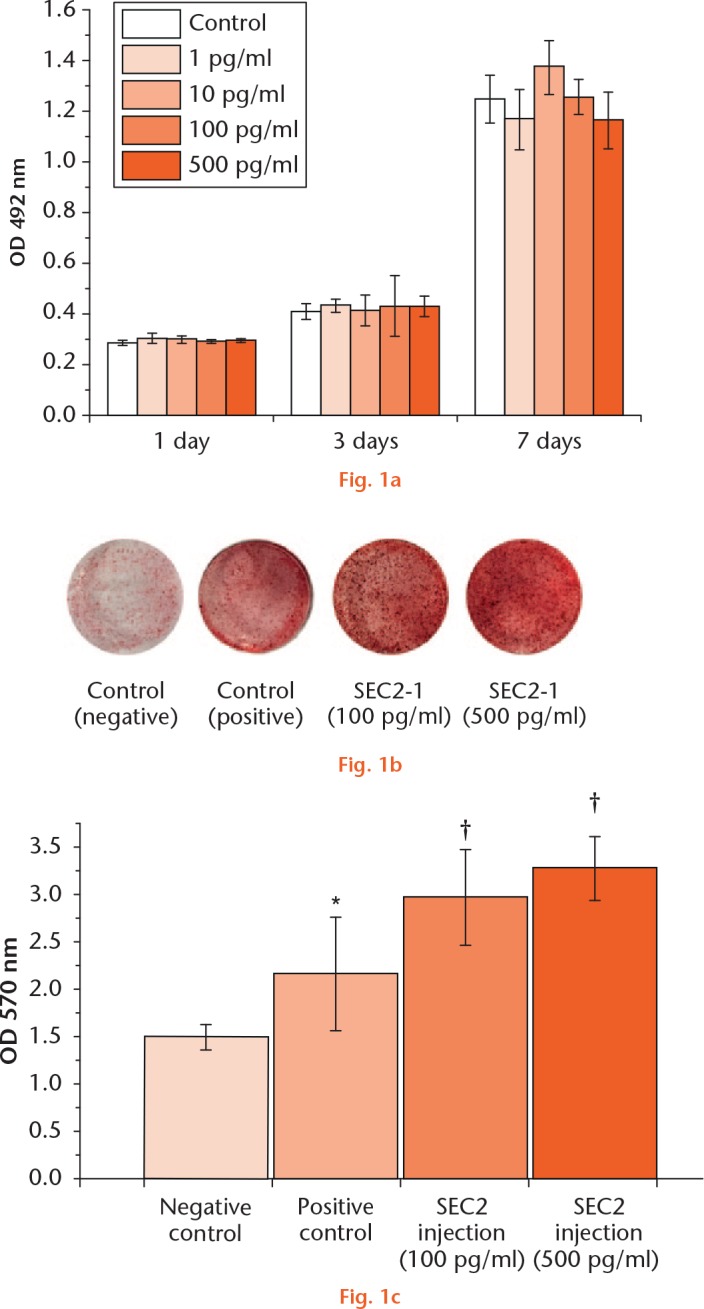
Staphylococcal enterotoxin C2 (SEC2) promoted the osteoblast differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). a) Different dose of SEC2 has no obvious effect on cell viability of BMSCs. b) and c) Calcium nodule formation was evaluated by Alizarin Red S staining and quantified by colorimetric assays. *p < 0.05 versus negative control; †p < 0.01 versus negative control. OD, osteogenic differentiation.
SEC2 promoted osteogenic differentiation of BMSCs
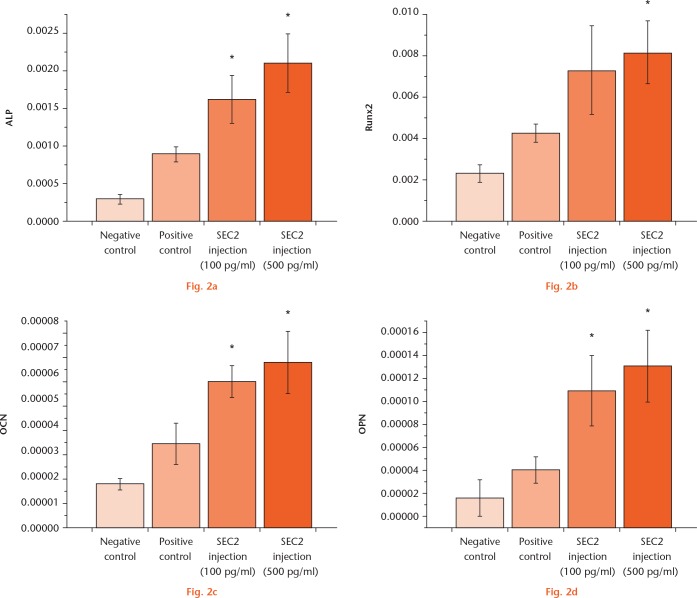
The rat BMSCs were cultured in an osteoinduction medium with either 100 pg/ml or 500 pg/ml SEC2, and the calcium nodule formation was evaluated by Alizarin Red S staining. The results showed that SEC2 significantly enhanced the calcium nodule formation (Fig. 1b), and the increased mineralization was further quantified by colorimetric assays (Fig. 1c). We further examined the expression of osteogenic markers, such as alkaline phosphatase, Runx2, osteocalcin, and osteopontin; the results showed that these markers were significantly upregulated by both 100 pg/ml and 500 pg/ml SEC2 (Fig. 2). These results suggest that SEC2 could stimulate osteogenic differentiation of BMSCs.
Staphylococcal enterotoxin C2 (SEC2) promoted the expression of the osteogenic markers during the osteogenesis of bone marrow-derived mesenchymal stem cells (BMSCs). The osteogenic marker genes, including a) alkaline phosphatase, b) runt-related transcription factor 2, c) osteocalcin, and d) osteopontin, were examined by real-time quantitative polymerase chain reaction (qRT-PCR) assays. *p < 0.05 versus negative control.
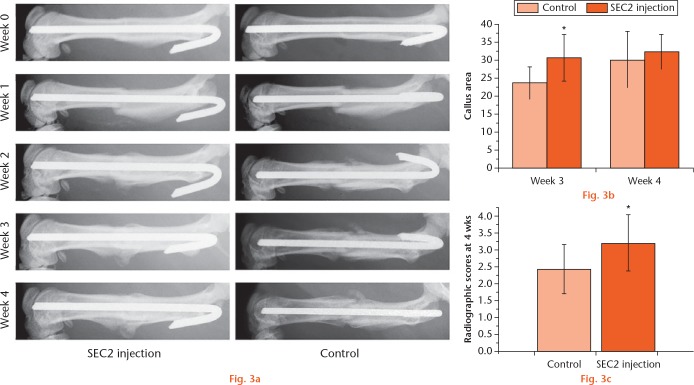
Radiographic analyses of bone fracture in the SEC2-treated group
To examine the in vivo effect of SEC2 on fracture, a rat femoral fracture model was applied in this study. SEC2 was locally injected every three days and x-ray assays were examined weekly. Although it caused a slight fever in rats, no other obvious side effects were found.18 As shown in Figure 3a, there was no difference between groups at week one; however, the size of callus (callus width and callus area) was increased in the SEC2-treated group from week two. We also found that fracture lines remained clear in the control group at week three and four; whereas it nearly disappeared and a larger size of callus bridging the fracture gap was observed in the SEC2-treated group (Figs 3a and 3b). At week four, the mean scores of fractures union were evaluated, and the results showed that the SEC2-treated group had a significantly higher score (Fig. 3c).
a) Radiographic analyses of fracture healing with Staphylococcal enterotoxin C2 (SEC2) treatment. Bone formation in fracture sites was detected by x-ray assays. b) Graph showing that larger callus areas were found in the SEC2-treated group. c) Graph showing that higher radiographic scores were observed in the SEC2-treated group. *p < 0.05 versus control.
Micro-CT analyses of femora from the SEC2 and control groups
To evaluate the osteoinduction ability of SEC2, micro-CT analysis was employed to quantify the newly formed bone tissues. As shown in Figures 4a and 4b, more newly mineralized calluses were observed in the the SEC2 group. Although the rats treated with SEC2 displayed no obvious increase in Bone Volume Density (low) (BVlow/TV) (Fig. 4c), a significant increase was observed in Bone Volume Density (high) (BVhigh/TV) (Fig. 4d), total bone volume density (BVtotal/TV) (Fig. 4e), and connected density (Fig. 4e). Therefore, local administration of SEC2 could enhance the healing of bone fractures.
Micro-CT analyses of femora from Staphylococcal enterotoxin C2 (SEC2) and control groups. a) and b) The representative image of micro-CT examination at four weeks. Graphs showing quantitative analyses of c) BVlow/TV value; d) BVhigh/TV; e) BVtotal/TV; and f) connected density. *p < 0.05 versus control. BV, bone volume; TV, total volume.
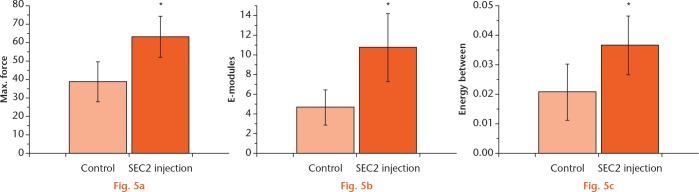
Biomechanical testing of repaired bone with SEC2 treatment
The mechanical properties of the repaired bones could be examined by the mechanical testing. The modulus of elasticity (E-modulus), ultimate loading, and energy to failure were examined; these results indicated that they were all significantly improved in the SCE2 treated group (Figs 5a to 5c), suggesting a better biomechanical recovery of the treated bone.
Graphs showing the mechanical testing of the repaired bones with control and Staphylococcal enterotoxin C2 (SEC2) treatment. Fractured femora were collected for mechanical testing. Significant increases were found in a) maximum force of failure, b) modulus of elasticity, and c) energy to failure in SEC2 group. *p < 0.05 versus control.
Histological analyses of regenerated bone tissues
Haematoxylin and eosin staining and Safranin O staining were performed to evaluate the newly mineralized-bone tissue. As shown in Figs 6a and 6d, better callus formation was exhibited and more chondroid tissues were observed in the SEC2-injected group, suggesting that this group has greater endochondral ossification (Figs 6b and 6e). The increased osteocalcin expression was also observed in the SEC2-treated group (Figs 6c and 6f). Moreover, there was a higher percentage of chondrocytes in the uncalcified callus (Fig. 6h) and more periosteal woven bones were formed in the SEC2-treated group (Fig. 6g).
Histological analyses of regenerated bone tissues. The calluses were examined by a) and d) haematoxylin and eosin staining (HE), and b) and e) Safranin O staining. c) and f) The expression of osteocalcin (OCN) was examined by immunohistochemical staining. g) Graphs showing that a higher percentage of bones and h) more chondrocytes were found in Staphylococcal enterotoxin C2 (SEC2) group. *p < 0.05 versus control.
Discussion
BMSCs are multipotent cells that can be easily expanded and differentiate into multiple cell types.19,20 It has been shown that SEC2 inhibited tumour cell growth by 60% at the concentration of 10 ug/ml.10 In our previous study,12 SEC2 with higher concentrations (20 ug/ml to 100 ug/ml) slightly suppressed the cell proliferation of human BMSCs. However, MTT results showed that SEC2 at lower concentrations had no effect on cell proliferation, suggesting that SEC2 has no toxicity to BMSCs.
To further confirm the effect of SEC2 on osteogenic differentiation of rat BMSCs, we examined the formation of calcium deposits and the expression of osteogenesis-related genes. As an important functional indicator of osteogenesis,21 SEC2 could enhance the formation of calcium nodule. Upregulation of osteogenesis-related genes induced by SEC2, such as alkaline phosphatase, osteocalcin, osteopontin, and Runx2, have further demonstrated the promoting effects of SEC2 on osteogenic differentiation of BMSCs. Among these osteogenic genes, alkaline phosphatase is considered as an early osteoblast marker and osteocalcin acts as a late-stage marker of osteogenic differentiation and mineralization.22,23 A low dose of SEC2 treatment could enhance the expression of osteopontin, a gene for prominent bone matrix protein.12 Runx2 is a pivotal transcriptional regulator that inhibits adipogenic differentiation through blocking PPARγ2 activity.24,25 Previously, we reported that 20 μg/ml SEC2 protein significantly increased osteogenic differentiation and inhibited osteoclast differentiation.12 In the present study, even a lower dose of SEC2 protein (100 pg/ml) could also demonstrate a similar pro-osteogenic effect on rat BMSCs.
In addition, we further investigated the effect of SEC2 on fracture healing. Transverse fracture of long bone diaphysis is one of the most common types of fractures.26 Therefore, an open transverse femoral (osteotomy) fracture was created in the present study and fixed using a intramedullary needle fixation. Compared with the closed fracture model, the open fracture model allows for a better controlled and more uniform fracture pattern. The results showed that local administration of SEC2 increased callus formation and improved mechanical properties.
During fracture healing, the newly formed callus consists of a less mineralized bone with a larger amount of haematoma, collagen fibers, and cartilage. The micro-CT examination was employed to analyze the bone quality. The rate of radio-opacity bone volume (BV) to total volume (TV) and the connectivity density was examined to indicate the callus formation and the mineralization. The total volume includes prefracture cortical bone, newly formed bone, and unmineralized tissues. Both BVlow and BVhigh are used for monitoring the newly formed bone, while BVhigh only represents highly mineralized callus bone. No difference was observed in BVlow/TV between the two groups, which suggests their newly formed calluses remain similar. Moreover, SEC2 group exhibited more callus mineralization and remodelling, with a significant increase in BVhigh/TV and BVtotal/TV values. These findings revealed that SEC2 accelerated ossification during fracture healing. Furthermore, biomechanical testing also showed a better biomechanical recovery of the defected bone in SEC2 group.
On the other hand, the regenerated bone tissues induced by SEC2 were examined by histological analyses. By haematoxylin and eosin staining and Safranin O staining, the larger callus was observed in SEC2 group, indicating that the treatment group had a higher percentage of cartilage and bone tissues. These findings also suggest that SEC2 treatment could accelerate endochondral ossification. The promoted endochondral ossification was also reported with other anabolic bioactive factors such as VEGF and insulin-like growth factor-I.27,28 Osteocalcin is mainly expressed in osteoblasts and hypertrophic chondrocytes during fracture healing.29,30 In this study, we found an increased osteocalcin expression in the SEC2 group. Therefore, SEC2 may accelerate endochondral bone formation and the bone remodelling process. As for the molecular mechanism, although several signalling pathways, such as PI3K/mTOR signaling, NF-ĸB signalling, and Ca2+/calcineurin (CaN)/nuclear factor of activated T cells (NFAT) signalling, were demonstrated to be involved in SEC2-induced immune activation,31,32 the pathway of SEC2 on osteogenesis differentiation remains elusive. As a coactivator of Runx2/Cbfa1, the Interferon (IFN)-inducible gene IFI16 was activated during the osteogenic differentiation in our previous study.12 Furthermore, SEC2 may activate Runx2-dependent gene transcription and promote osteoblast differentiation, whereas its binding to PPARγ represses PPARγ-dependent gene transcription and impairs adipocyte differentiation.33,34
In conclusion, we have demonstrated that SEC2 could promote osteogenic differentiation of BMSCs and the local administration of SEC2 could accelerate fracture healing in a rat model. Therefore, SEC2 may be a potential new therapeutic agent to facilitate bone repair. However, more research is required to understand the mechanics of how SEC2 works in promoting osteogenesis and fracture healing, and further clinical studies are needed to validate these results in a human model.
Footnotes
Author Contributions: T. Wu: Designing and conducting the experiments, Analyzing the data, Preparing and approving the manuscript.
J. Zhang: Designing, conducting, and supervising the experiments, Analyzing the data, Preparing and approving the manuscript.
B. Wang: Designing and conducting the experiments, Reviewing and approving the manuscript.
Y. Sun: Designing and conducting the experiments, Reviewing and approving the manuscript.
Y. Liu: Designing and conducting the experiments, Reviewing and approving the manuscript.
G. Li: Designing, supervising, and conducting the experiments, Analyzing the data, Preparing, reviewing, and approving the manuscript.
Conflict of Interest Statement: None declared
Follow us @BoneJointRes
Funding Statement
The work was partially supported by grants from: Hong Kong Government Research Grant Council, General Research Fund (14119115, 14160917, N_CityU102/15 and T13-402/17-N); National Natural Science Foundation of China (81371946, 81430049, 81772322 and 81772404); and Hong Kong Innovation Technology Commission Funds (ITS/UIM-305). This study was also supported in part by the SMART programme, Lui Che Woo Institute of Innovative Medicine, The Chinese University of Hong Kong. The research was made possible by resources donated by Lui Che Woo Foundation Limited.
References
- 1. Marsh DR, Li G. The biology of fracture healing: optimising outcome. Br Med Bull 1999;55:856-869. [DOI] [PubMed] [Google Scholar]
- 2. Axelrad TW, Kakar S, Einhorn TA. New technologies for the enhancement of skeletal repair. Injury 2007;38(suppl 1):S49-S62. [DOI] [PubMed] [Google Scholar]
- 3. Kleinschmidt K, Wagner-Ecker M, Bartek B, Holschbach J, Richter W. Superior angiogenic potential of GDF-5 and GDF-5(V453/V456) compared with BMP-2 in a rabbit long-bone defect model. J Bone Joint Surg [Am] 2014;96-A:1699-1707. [DOI] [PubMed] [Google Scholar]
- 4. Coleman CM, Scheremeta BH, Boyce AT, Mauck RL, Tuan RS. Delayed fracture healing in growth differentiation factor 5-deficient mice: a pilot study. Clin Orthop Relat Res 2011;469:2915-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hreha J, Wey A, Cunningham C, et al. Local manganese chloride treatment accelerates fracture healing in a rat model. J Orthop Res 2015;33:122-130. [DOI] [PubMed] [Google Scholar]
- 6. Groothuis A, Duda GN, Wilson CJ, et al. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: involvement of VEGF mechano-regulation. Bone 2010;47:438-444. [DOI] [PubMed] [Google Scholar]
- 7. Mifuji K, Ishikawa M, Kamei N, et al. Angiogenic conditioning of peripheral blood mononuclear cells promotes fracture healing. Bone Joint Res 2017;6:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng LH, Ko CH, Siu SW, et al. In vitro & in vivo assessment of a herbal formula used topically for bone fracture treatment. J Ethnopharmacol 2010;131:282-289. [DOI] [PubMed] [Google Scholar]
- 9. Dohlsten M, Lando PA, Björk P, et al. Immunotherapy of human colon cancer by antibody-targeted superantigens. Cancer Immunol Immunother 1995;41:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Xu M, Zhang H, et al. Enhancement of superantigen activity and antitumor response of staphylococcal enterotoxin C2 by site-directed mutagenesis. Cancer Immunol Immunother 2009;58:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue M, Plautz GE, Shu S. Treatment of intracranial tumors by systemic transfer of superantigen-activated tumor-draining lymph node T cells. Cancer Res 1996;56:4702-4708. [PubMed] [Google Scholar]
- 12. Fu WM, Zhu X, Wang H, et al. Staphylococcal enterotoxin C2 promotes osteogenesis and suppresses osteoclastogenesis of human mesenchymal stem cells. Exp Cell Res 2014;322:202-207. [DOI] [PubMed] [Google Scholar]
- 13. Shiels SM, Cobb RR, Bedigrew KM, et al. Antibiotic-loaded bone void filler accelerates healing in a femoral condylar rat model. Bone Joint J 2016;98-B:1126-1131. [DOI] [PubMed] [Google Scholar]
- 14. Rajfer RA, Kilic A, Neviaser AS, et al. Enhancement of fracture healing in the rat, modulated by compounds that stimulate inducible nitric oxide synthase: acceleration of fracture healing via inducible nitric oxide synthase. Bone Joint Res 2017;6:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyazaki M, Toyoda M, Yoshiiwa T, et al. Enhancement of the effects of exfoliated carbon nanofibers by bone morphogenetic protein in a rat femoral fracture model. J Orthop Res 2015;33:185-192. [DOI] [PubMed] [Google Scholar]
- 16. Hao YJ, Zhang G, Wang YS, et al. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 2007;41:631-638. [DOI] [PubMed] [Google Scholar]
- 17. Shi HF, Cheung WH, Qin L, Leung AH, Leung KS. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 2010;46:1299-1305. [DOI] [PubMed] [Google Scholar]
- 18. Cheng X, Cao P, Ji X, et al. Antitumour response of a double mutant of staphylococcal enterotoxin C2 with the decreased affinity for MHC class II molecule. Scand J Immunol 2010;71:169-175. [DOI] [PubMed] [Google Scholar]
- 19. Bertram H, Mayer H, Schliephake H. Effect of donor characteristics, technique of harvesting and in vitro processing on culturing of human marrow stroma cells for tissue engineered growth of bone. Clin Oral Implants Res 2005;16:524-531. [DOI] [PubMed] [Google Scholar]
- 20. Niemeyer P, Fechner K, Milz S, et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 2010;31:3572-3579. [DOI] [PubMed] [Google Scholar]
- 21. Yang HW, Lin MH, Xu YZ, et al. Osteogenesis of bone marrow mesenchymal stem cells on strontium-substituted nano-hydroxyapatite coated roughened titanium surfaces. Int J Clin Exp Med 2015;8:257-264. [PMC free article] [PubMed] [Google Scholar]
- 22. Siller AF, Whyte MP. Alkaline phosphatase: discovery and naming of our favorite enzyme. J Bone Miner Res 2017. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23. Granéli C, Thorfve A, Ruetschi U, et al. Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res (Amst) 2014;12:153-165. [DOI] [PubMed] [Google Scholar]
- 24. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997;89:747-754. [DOI] [PubMed] [Google Scholar]
- 25. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev 2000;14:1293-1307. [PubMed] [Google Scholar]
- 26. Courtney PM, Bernstein J, Ahn J. In brief: closed tibial shaft fractures. Clin Orthop Relat Res 2011;469:3518-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 2007;86:937-950. [DOI] [PubMed] [Google Scholar]
- 28. Maor G, Segev Y, Phillip M. Testosterone stimulates insulin-like growth factor-I and insulin-like growth factor-I-receptor gene expression in the mandibular condyle-a model of endochondral ossification. Endocrinology 1999;140:1901-1910. [DOI] [PubMed] [Google Scholar]
- 29. Nakase T, Sugimoto M, Sato M, et al. Switch of osteonectin and osteopontin mRNA expression in the process of cartilage-to-bone transition during fracture repair. Acta Histochem 1998;100:287-295. [DOI] [PubMed] [Google Scholar]
- 30. Nakase T, Takaoka K, Hirakawa K, et al. Alterations in the expression of osteonectin, osteopontin and osteocalcin mRNAs during the development of skeletal tissues in vivo. Bone Miner 1994;26:109-122. [DOI] [PubMed] [Google Scholar]
- 31. Fu X, Xu M, Yao S, et al. Staphylococcal enterotoxin C2 mutant drives T lymphocyte activation through PI3K/mTOR and NF-kB signaling pathways. Toxicol Appl Pharmacol 2017;333:51-59. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Xu M, Zhang H, et al. SEC2-induced superantigen and antitumor activity is regulated through calcineurin. Appl Microbiol Biotechnol 2013;97:9695-9703. [DOI] [PubMed] [Google Scholar]
- 33. Xu J, Wu T, Sun Y, et al. Staphylococcal enterotoxin C2 expedites bone consolidation in distraction osteogenesis. J Orthop Res 2017;35:1215-1225. [DOI] [PubMed] [Google Scholar]
- 34. Banke E, Rödström K, Ekelund M, et al. Superantigen activates the gp130 receptor on adipocytes resulting in altered adipocyte metabolism. Metabolism 2014;63:831-840. [DOI] [PubMed] [Google Scholar]