Abstract
Background
Aortic valve stenosis (AS) is very common in the elderly patients above 80 years. Transcatheter aortic valve replacement (TAVR) in such patients is being increasingly performed. This study sought to assess in-hospital outcome differences between octogenarians and nonagenarians and predictors of mortality in nonagenarians undergoing TAVR with severe AS.
Method
The study population was derived from the National Inpatient Sample (NIS) for the years 2012–2014 using ICD-9 CM procedure codes 35.05 and 35.06 for TAVR. Hospitalizations below 80 years of age were excluded. After performing propensity score matching (1: 2), in-hospital outcomes were compared in matched cohorts. Then, multivariate model was developed to analyze predictors of in-hospital mortality in nonagenarians.
Results
There were 11,630 hospitalizations in the octogenarian and 5815 hospitalizations in the nonagenarian group. Primary outcome of in-hospital mortality (6% vs. 4.1%, P ≤ 0.001) was higher in nonagenarians compared to octogenarians. Secondary outcomes including stroke (3.4% vs. 2.8%, P ≤ 0.001), renal failure (18.9% vs. 17.3%, P ≤ 0.001), blood transfusion (35% vs. 32.6%, P ≤ 0.001), vascular complications (4.5% vs. 3.5%, P ≤ 0.001), and pacemaker implantation (27.8% vs. 24.8%, P ≤ 0.001) were higher in nonagenarians. There was no difference in their length of stay. Median cost (70,374$ vs. 65,381$, P ≤ 0.001) was slightly higher with nonagenarian.
Conclusions
Although in-hospital mortality is slightly higher in nonagenarians, it is acceptable. This difference in mortality is at least partly explained by higher complications in nonagenarians. Efforts should be made to decrease the complications which can further narrow the difference in in-hospital mortality between the groups.
Keywords: Nonagenarian, Octogenarian, Quality of Life, Transcatheter aortic valve replacement
1. Introduction
The prevalence of aortic valve disease increases with age and it is the most common native valve disease.[1] The progression of aortic valve stenosis (AS) is gradual and has a long latency period.[2] However, after the initial appearance of symptoms, the disease progresses rapidly,[3]–[6] causing high rate of mortality. Mortality rate is near 50% in the first two years after initial symptoms appearance if patient is not treated appropriately.[1],[7],[8] Generally, elderly patients above 80 years of age with AS are frailer and have more comorbidities compared to younger age group and are considered poor candidates for surgery.[9] Also, functionality and quality of life are markedly decreased in such patients with severe AS.[10] Although, surgical repair was the only option for such patients until few years ago, transcatheter aortic valve repair (TAVR) is now an alternative treatment option for severe AS in high-risk patients who are poor surgical candidates.[2],[11] With increase in life expectancy, population of elderly age group is constantly increasing in United States. Between 1980 and 2010, nonagenarian population in the United States more than doubled from 720,000 to 19,000,000.[12] Also, nearly 300,000 patients with AS are TAVR candidate every year.[13] This is one of the major reasons why TAVR quickly gained popularity in such high surgical risk patients after its first introduction in 2002,[14],[15] and later became the established therapy.[2],[11],[16]–[21] TAVR has also shown promising results in intermediate-risk patients, which is demonstrated by SURTAVI trial.[17],[18]
TAVR was first introduced in the United States in January 2005 through the approval of feasibility trial of the Cribier-Edwards percutaneous aortic heart valve by the US Food and Drug Administration (FDA).[22] After the conclusions of various clinical trials, FDA approved Edwards SAPIEN device for TAVR in patients with inoperable status in November 2011.[23],[24] Later, in September 2012, FDA expanded TAVR indication to include patients with high surgical risk.[23],[25] In late 2016, TAVR has also been approved for intermediate-risk patients by the FDA.[26] However, the effect of TAVR in nonagenarians is largely unknown, as only a small fraction of patients were enrolled in the pivotal clinical trials.[27]–[29] As incidence of AS increases with increasing age, more studies are required to establish safety and efficacy in elderly population especially nonagenarians.
In this study, we compared the in-hospital outcomes between octogenarian and nonagenarian from a large nationwide inpatient database. To better understand what causes high mortality after TAVR in hospitalizations above 90 years of age, we also estimated the predictors of in-hospital mortality in this age group.
2. Methods
We did not require Institutional Review Board (IRB) approval for our study as each individual hospitalization in Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS) is de-identified.
2.1. Data source
Our study population was obtained from National Inpatient Sample (NIS).[30] NIS is the largest, publicly available, all-payers, inpatient care database of the United States. This database is developed for the Healthcare Cost and Utilization Project (HCUP) and is sponsored by Agency for Healthcare Research and Quality (AHRQ). HCUP NIS has 20% stratified sample of discharged records from all HCUP participating hospitals. This database represents more than ninety five percent of the United States population. Each entry in HCUP NIS contains one primary discharge diagnosis, up to thirty secondary diagnosis (up to twenty-five in 2012 and 2013), one primary procedure, up to fifteen secondary procedures, demographic information, insurance status, comorbidities, length of stay, discharge disposition, teaching status of hospital, hospital region, median household income. We performed a retrospective, observational cohort study.
2.2. Study design
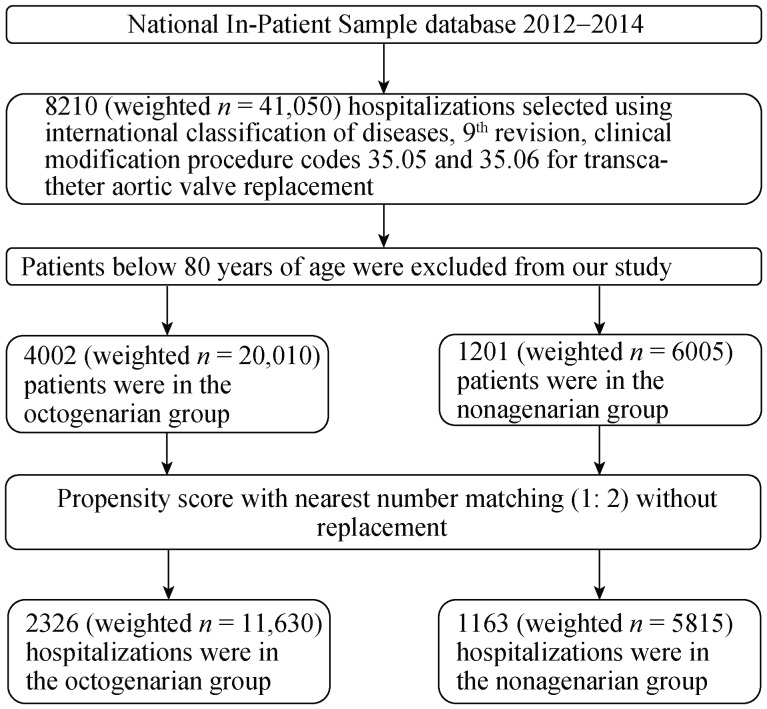
We have identified hospitalizations undergoing TAVR between 2012 and 2014. To identify the patient undergoing TAVR, we have used International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM) procedure code 35.05 (Endovascular Replacement of Aortic Valve) and 35.06 (Transapical Replacement of Aortic Valve). Hospitalizations below 80 years of age were excluded. Hospitalizations between 80 and 90 years of age were included in the octogenarian group and above 90 years of age were included in the nonagenarian group (Figure 1). We used Elixhauser comorbidities in our study.[31] ICD 9 CM codes for other comorbidities and in-hospital outcomes are showed in Table 1S. We defined the severity of comorbid conditions using Deyo Modification of Charlson's Comorbidity Index (CCI).[32] This index contains seventeen weighted comorbid conditions. The score ranges from 0–33. Higher score corresponds to a greater burden of comorbid diseases prior to the procedure. Discharge trend weights provided by the HCUP NIS were used to generate national estimate.
Figure 1. Flow chart for cohort selection.
A total of 5203 hospitalizations from HCUP NIS database between 2012 and 2014 were included in our study. After performing propensity score matching analysis, 1163 hospitalizations in the nonagenarians and 2326 hospitalizations in the octogenarian groups were included and compared.
Table 1S. International classification of diseases, ninth revision, clinical modification codes.
| Variable name | ICD 9 CM Code Used |
| Smoking | v15.82, 305.1 |
| Family history of coronary artery disease | v17.3 |
| Prior myocardial infarction | 412 |
| Ischemic cardiomyopathy | 414.8 |
| Mitral stenosis | 396.0 |
| Mitral insufficiency | 396.2, 396.8, 394.2, 424.0 |
| Chronic kidney disease | 585.x |
| Dialysis (procedural code) | Haemodialysis: 39.95Peritoneal dialysis: 54.98 |
| IABP (procedural code) | 37.61 |
| pLVAD (procedural code) | 37.68 |
| ECMO (procedural code) | 39.65 |
| Trans-femoral approach for TAVR (procedural code) | 35.05 |
| Transapical approach for TAVR (procedural code) | 35.06 |
| Composite of stroke (ischemic and hemorrhagic combined) | 430, 431, 432, 997.02, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, or 436 |
| Acute renal failure | 584 |
| Cardiac arrest | 427.5 |
| Blood transfusion | 998.11, 998.12, 285.1, 99.0 |
| Iatrogenic cardiac complication | 997.1 |
| Vascular injury requiring surgery (procedure code) | 39.31, 39.41, 39.49, 39.52, 39.53, 39.56, 39.57, 39.58, 39.59, 39.79 |
| Perioperative stroke | 997.02 |
| Perioperative infection | 998.5, 998.59, 998.62 |
| Postoperative shock | 998.0 |
| Permanent pacemaker implantation (procedural code) | 37.70 to 37.79, 37.80 to 37.89, 00.50, 00.52, 00.53 |
| Atrial fibrillation | 427.31 |
IABP: intra-aortic balloon pump; ICD 9 CM: international classification of diseases, ninth revision, clinical modification codes; ECMO: extra-corporeal membrane oxygenation; pLVAD: percutaneous left ventricle assist device; TAVR: transcatheter aortic valve replacement.
The primary end-point of our study was all-cause in-hospital mortality. Secondary in-hospital outcomes included individual end-points of stroke (ischemic and hemorrhagic), acute renal failure, blood loss requiring transfusion, vascular complication, cardiac arrest, permanent pacemaker placement, atrial fibrillation and composite of all complications. Other outcomes included in our study were length of stay and median cost of hospitalization stay. To estimate the cost, HCUP NIS data were merged with cost-to-charge ratio files available from HCUP. We estimated the final cost by multiplying total hospital charge with cost-to-charge ratio.
2.3. Statistical analysis
SAS 9.4 (SAS Institute Inc., Cary NC) was used for statistical analyses. Differences between categorical and continuous baseline variables were tested using Chi-square test and Student's t-test, respectively. Wherever Chi-square test was considered an inappropriate test due to very low frequency, we substituted it with Fischer's Exact Test. Level of significance was set at P value of less than 0.05. We used propensity score matched model to establish matched cohorts for baseline and procedural characteristics imbalances between two groups. The propensity score has been developed using a logistic regression model according to a non-parsimonious approach, and all baseline (gender, comorbidities) as well as procedural (any mechanical circulatory support, trans-femoral access) characteristics were included in the analysis. In the end, hospitalizations with similar propensity score in both groups were matched using two-to-one scheme without replacement using the matching algorithm.[33] To calculate in-hospital outcomes in matched cohorts, we used McNemar's test and Wilcoxon-rank-sum test. To analyze predictors of in-hospital mortality in nonagenarians, we developed multivariate logistic regression model. In this model, we included age, gender, admission type, primary payer, median household income, teaching status of the hospital, all Elixhauser comorbidities, approach for TAVR and all mechanical circulatory devices.
3. Results
3.1. Baseline characteristics
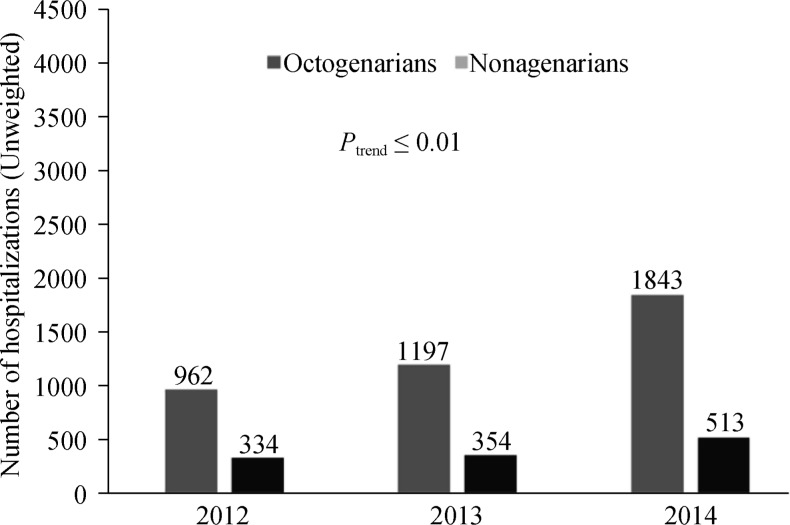
In the unadjusted cohorts, a total of 26,015 hospitalizations with severe AS underwent TAVR above 80 years of age. On an average, TAVR procedures increased from year 2012 through 2014, with highest procedure performance in 2014 (Figure 1S). Table 1 shows baseline characteristics (unadjusted) in the two groups. Nonagenarian hospitalizations were more likely to be females (52.1%) while octogenarian hospitalizations were more likely to be males (50.9%). The hospitalizations were predominantly white (88%) in both groups. In both the groups, TAVR procedures were more often performed electively (76.4%). Most common primary payment method was Medicare (94.5%). Most hospitalizations had a significant baseline burden of comorbidities with CCI score ≥ 3 (53.6% in octogenarians and 47.3% in nonagenarians). There was a significant geographical variability in utilization of TAVR procedure, with highest being in the Southern US. Most procedures were performed at urban teaching hospital (88.7%). Interestingly, higher comorbidities like uncomplicated and complicated diabetes mellitus, obesity, peripheral vascular disease, chronic pulmonary disease, ischemic cardiomyopathy and composite of hemo and peritoneal dialysis were noted in octogenarian group before matching. Only coagulopathies were significantly higher in nonagenarians before matching (Table 2S).
Figure 1S. Trends in hospitalization: stratified by octogenarians and nonagenarians.
Table 1. Baseline characteristics of hospitalizations with transcatheter aortic valve replacement (unmatched).
| Variable name | Octogenarians (n = 20010) | Nonagenarians (n = 6005) | P value |
| Unweighted numbers | 4002 | 1201 | N/A |
| Age, yrs | 85.1 | 90.5 | < 0.001 |
| *Race | |||
| White | 88.5% | 87.5% | 0.18 |
| Black | 2.9% | 3.1% | |
| Hispanic | 3.6% | 3% | |
| Asian/Pacific Islander | 1% | 0.7% | |
| Native American | 0.2% | 0.3% | |
| Other | 3.8% | 5.2% | |
| Gender | |||
| Male | 50.9% | 47.9% | 0.08 |
| Female | 49.1% | 52.1% | |
| $Admission type | |||
| Elective | 76.1% | 76.7% | 0.73 |
| Non-elective | 23.9% | 23.3% | |
| **Charlson's/Deyo comorbidity index | |||
| 0 | 6.1% | 8.1% | < 0.001 |
| 1 | 19% | 24.9% | |
| 2 | 21.3% | 19.7% | |
| ≥ 3 | 53.6% | 47.3% | |
| Primary payer | |||
| Medicare | 94.7% | 94.3% | 0.30 |
| Medicaid | 0.3% | 0.1% | |
| Private insurance (Including HMOs and PPOs) | 3.6% | 3.4% | |
| Self-pay | 0.4% | 0.1% | |
| Other | 1% | 2.1% | |
| #Median household income for patient's ZIP code | |||
| 0–25 percentile | 20.4% | 17.2% | < 0.001 |
| 26–50 percentile | 25.1% | 21.1% | |
| 51–75 percentile | 25% | 27.2% | |
| 76–100 percentile | 29.5% | 34.5% | |
| Teaching status of hospital | |||
| Rural | 0.8% | 0.4% | 0.009 |
| Urban (non-teaching) | 9.6% | 11.8% | |
| Urban (teaching) | 89.6% | 87.8% | |
| Hospital region | |||
| Northeast | 27% | 28% | < 0.001 |
| Midwest | 21.2% | 22% | |
| South | 33.7% | 27.6% | |
| West | 18.1% | 22.5% | |
*345 missing patients; **Charlson's/Deyo comorbidity index was calculated as per Deyo classification; $3 missing patients; #a quartile classification of the estimated median household income of residents in the patient's ZIP Code. These values are derived from ZIP Code-demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually; the value ranges vary by year. Http://www.hcupus.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp. Frequency is in % or in mean. HMOs: health maintenance organizations; N/A: not applicable; PPOs: preferred provider organizations.
Table 2S. Comorbidities in hospitalizations with transcatheter aortic valve replacement (unmatched).
| Variable name | Octogenarians (n = 4002) | Nonagenarians (n = 1201) | P value |
| Weighted numbers | 20010 | 6005 | N/A |
| Diabetes mellitus (uncomplicated) | 26.2% | 15.8% | < 0.001 |
| Diabetes mellitus (with chronic complications) | 4.7% | 2% | < 0.001 |
| Hypertension | 80.6% | 78.9% | 0.24 |
| Liver disease | 1% | 0.6% | 0.15 |
| Fluid and electrolytes disorder | 26.5% | 25.6% | 0.53 |
| Other neurological disorder | 6.8% | 6.1% | 0.36 |
| Obesity | 9.5% | 2.7% | <0.001 |
| Peripheral vascular disease | 30.2% | 25.7% | 0.002 |
| Smoking | 1.7% | 1% | 0.08 |
| Alcohol | 0.5% | 0.4% | 0.71 |
| Prior MI | 12.2% | 11.5% | 0.53 |
| Family history of CAD | 1.7% | 1% | 0.08 |
| Coagulopathy | 23.9% | 27.7% | 0.009 |
| Chronic pulmonary disease | 31% | 20.6% | <0.001 |
| Congestive heart failure | 12.5% | 11% | 0.21 |
| AIDS | 0% | 0% | N/A |
| Ischemic cardiomyopathy | 7% | 4.5% | 0.001 |
| Mitral stenosis | 1.1% | 1.3% | 0.58 |
| Mitral insufficiency | 18.2% | 18.7% | 0.68 |
| Chronic kidney disease | 37.9% | 38.5% | 0.66 |
| Composite of hemo and peritoneal dialysis | 3.4% | 2.1% | 0.014 |
HMOs: health maintenance organizations; PPOs: Preferred Provider Organizations, MI: Myocardial Infarction, CAD: Coronary Artery Disease, N/A: Not Applicable
3.2. In-hospital outcomes after propensity score matching
After performing propensity score matched analysis, 11,630 hospitalizations were in the octogenarian group and 5815 hospitalizations were in the nonagenarians group. Nonagenarian hospitalizations had higher in-hospital mortality (6% vs. 4.1%, P ≤ 0.001). Secondary in-hospital outcomes of stroke (3.4% vs. 2.8%, P ≤ 0.01), renal failure (18.9% vs. 17.3%, P ≤ 0.01), blood transfusion (35% vs. 32.6%, P ≤ 0.01), vascular complications (4.5% vs. 3.5%, P ≤ 0.01), and pacemaker implantation (27.8% vs. 24.8%, P ≤ 0.01) were higher in nonagenarians as well. Cardiac arrest and rates of atrial fibrillation were comparable in both groups. Higher composite of all complications was noted with nonagenarians (79.6% vs. 78.4%, P ≤ 0.001). No difference in the length of hospital stay was noted between the groups (7.9 vs. 7.7 days, P = 0.45). Median cost of hospitalization was higher with admission of nonagenarians (70,374$ vs. 65,381$, P = 0.014) (Table 2).
Table 2. Baseline characteristics outcomes after performing propensity score matched analysis (1: 2).
| Variables | Octogenarians (n = 11630) | Nonagenarians (n = 5815) | P value |
| Unweighted numbers | 2326 | 1163 | N/A |
| Age, yrs | 85.1 | 90.5 | < 0.001 |
| Male | 49.3% | 47.1% | 0.24 |
| Female | 50.7% | 52.9% | |
| Comorbidities | |||
| Diabetes mellitus (uncomplicated) | 16.7% | 16.3% | 0.78 |
| Diabetes mellitus (with chronic complications) | 2% | 2.1% | 0.93 |
| Hypertension | 79.4% | 78.6% | 0.58 |
| Liver disease | 1% | 0.5% | 0.11 |
| Fluid and electrolytes Disorder | 26.1% | 25.5% | 0.07 |
| Neurological disorder | 7.1% | 6.1% | 0.24 |
| Obesity | 2.8% | 2.7% | 0.94 |
| Peripheral vascular disease | 26.4% | 26.6% | 0.93 |
| Smoking | 1.5% | 0.9% | 0.20 |
| Alcohol | 0.6% | 0.3% | 0.39 |
| Prior MI | 11.6% | 11.5% | 0.97 |
| Family history of CAD | 1.5% | 0.9% | 0.20 |
| Coagulopathy | 24.3% | 25.4% | 0.48 |
| Chronic pulmonary disease | 22% | 21.3% | 0.68 |
| Congestive heart failure | 12.2% | 10.8% | 0.29 |
| AIDS | 0 | 0 | N/A |
| Ischemic cardiomyopathy | 4.1% | 4.6% | 0.44 |
| Mitral stenosis | 1.1% | 1.3% | 0.58 |
| Mitral insufficiency | 18.1% | 18.6% | 0.74 |
| Chronic kidney disease | 36.9% | 36.5% | 0.81 |
| Composite of hemo and peritoneal dialysis | 1.7% | 2.1% | 0.36 |
| Procedural details | |||
| IABP | 1.7% | 1.5% | 0.67 |
| pLVAD | 0.3% | 0.2% | 0.82 |
| ECMO | 0.3% | 0.2 | 0.62 |
| Trans-femoral approach | 84.5% | 83.9 | 0.65 |
| In-hospital outcome | |||
| In-hospital mortality | 4.1% | 6% | < 0.001 |
| Stroke (ischemic and hemorrhagic) | 2.8% | 3.4% | < 0.001 |
| Acute renal failure | 17.3% | 18.9% | < 0.001 |
| Blood loss requiring transfusion | 32.6% | 35% | < 0.001 |
| Vascular complication | 3.5% | 4.5% | < 0.001 |
| Cardiac arrest | 5% | 5% | 1.00 |
| Iatrogenic cardiac complication | 0% | 0% | N/A |
| Permanent pacemaker | 24.8% | 27.8% | < 0.001 |
| Atrial fibrillation | 47.3% | 48% | 0.21 |
| Composite of all complications | 78.4% | 79.6% | < 0.001 |
| Median length of stay (IQR), days | 6 (1–11) | 6 (1–11) | 0.45 |
| Cost (median) | $65,381 | $70,374 | 0.014 |
AIDS: acquired immuno deficiency syndrome; CAD: coronary artery disease; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; IQR: interquartile range; MI: myocardial infarction; N/A: not applicable; pLVAD: percutaneous left ventricular assist device.
Multivariate predictor model for in-hospital mortality was generated to assess predictors in nonagenarian hospitalizations undergoing TAVR with severe AS. Increasing age and female gender were associated with greater risk of in-hospital mortality. Interestingly, hospitalizations with median household income between 75 and 100 percentiles were associated with higher in-hospital mortality. Comorbidities like fluid and electrolyte disorders, peripheral vascular disease, coagulopathies, chronic pulmonary disease, mitral stenosis, mitral insufficiency, chronic kidney disease and hospitalizations on dialysis were associated with a greater risk of in-hospital mortality. Also, hospitalizations which required mechanical circulatory support such as intra-aortic balloon pump, percutaneous left ventricular assist device (Impella™, Tandem Heart™, etc.) and extracorporeal membrane oxygenation were associated with greater risk of in-hospital mortality. Transapical approach was not a predictor of higher in-hospital mortality in nonagenarians. In contrast, hospitalizations with uncomplicated diabetes mellitus, hypertension and ischemic cardiomyopathy were predictive of a lower incidence of in-hospital mortality (Table 3).
Table 3. Multi-variate predictors of in-hospital mortality for nonagenarian hospitalizations with transcatheter aortic valve replacement (n = 6005).
| Variable name | Odds ratio (95% CI) | P |
| *Age | 1.08 (1.05–1.10) | < 0.01 |
| Female vs. Male | 1.58 (1.38–1.81) | < 0.01 |
| Admission type | ||
| Non-elective vs. elective | 1.02 (0.89–1.18) | 0.75 |
| Primary payer | ||
| Medicare | Referent | |
| Medicaid | 1.31 (0.48–3.55) | 0.59 |
| Private insurance (Including HMOs and PPOs) | 1.23 (0.86–1.76) | 0.26 |
| Self-pay/other | 0.48 (0.19–1.23) | 0.13 |
| Median household income for patient's ZIP code | ||
| 0–25 Percentile | Referent | |
| 26–50 Percentile | 1.02 (0.83–1.25) | 0.86 |
| 51–75 Percentile | 0.98 (0.80–1.19) | 0.81 |
| 76–100 Percentile | 1.33 (1.10–1.59) | < 0.01 |
| Teaching status of hospital | ||
| Rural | Referent | |
| Urban (Non-teaching) | 0.67 (0.34–1.32) | 0.25 |
| Urban (Teaching) | 0.54 (0.28–1.05) | 0.07 |
| **Comorbidities | ||
| Diabetes mellitus (uncomplicated) | 0.67 (0.56–0.80) | < 0.01 |
| Diabetes mellitus (with chronic complications) | 1.07 (0.79–1.44) | 0.67 |
| Hypertension | 0.45 (0.39–0.52) | < 0.01 |
| Liver disease | 1.45 (0.86–2.44) | 0.17 |
| Fluid and electrolytes disorder | 1.98 (1.73–2.27) | < 0.01 |
| Neurological disorder | 0.88 (0.67–1.14) | 0.33 |
| Obesity | 0.87 (0.66–1.15) | 0.32 |
| Peripheral vascular disease | 1.25 (1.09–1.44) | < 0.01 |
| Smoking | 0.77 (0.44–1.36) | 0.37 |
| Alcohol | 1.14 (0.46–2.84) | 0.78 |
| Prior MI | 0.94 (0.75–1.16) | 0.55 |
| Coagulopathy | 1.28 (1.11–1.48) | < 0.01 |
| Chronic pulmonary disease | 1.41 (1.22–1.62) | < 0.01 |
| Congestive heart failure | 0.95 (0.79–1.16) | 0.63 |
| Ischemic cardiomyopathy | 0.55 (0.40–0.77) | < 0.01 |
| Mitral stenosis | 2.80 (1.91–4.11) | < 0.01 |
| Mitral insufficiency | 1.34 (1.15–1.56) | < 0.01 |
| Chronic kidney disease | 1.19 (1.04–1.37) | 0.01 |
| Composite of hemo and peritoneal dialysis | 4.85 (3.88–6.04) | < 0.01 |
| Procedural details | ||
| **IABP | 11.78 (9.25–15.00) | < 0.01 |
| pLVAD or ECMO | 21.34 (14.0–32.55) | < 0.01 |
| #Trans-apical approach | 0.97 (0.83–1.14) | 0.76 |
*Age is a continuous variable; **Reference group was not available; #Transfemoral approach was taken as a reference. ECMO: extracorporeal membrane oxygenation; HMOs: health maintenance organizations; IABP: intra-aortic balloon pump; MI: myocardial infarction; pLVAD: percutaneous left ventricular assist device; PPOs: preferred provider organizations.
4. Discussion
Our study compared octogenarians with nonagenarians in high surgical risk hospitalizations between 2012 and 2014 from the national inpatient sample database who underwent TAVR. We found higher all-cause in-hospital mortality in nonagenarians compared to octogenarians. Also, secondary in-hospital outcomes including stroke (ischemic or hemorrhagic), acute renal failure, blood loss requiring transfusion, vascular complication, and permanent pacemaker placement were higher in nonagenarians. Additionally, we demonstrated positive predictors of in-hospital mortality in nonagenarian hospitalizations which included higher age, female gender, high income, fluid and electrolyte disorder, peripheral vascular disease, coagulopathy, chronic pulmonary disease, mitral stenosis, mitral insufficiency, chronic kidney disease and hospitalizations on dialysis.
PARTNER-1 trial demonstrated that TAVR can safely be performed in nonagenarians with acceptable short and mid-terms outcomes and it improves quality of life.[29] Our study demonstrated clinically acceptable outcomes in nonagenarians who underwent TAVR. The study by Tamburino, et al.[34] demonstrated that the patients who presented with comorbidities like diabetes mellitus, pre-procedural mitral regurgitation, prior acute pulmonary edema, and systolic pulmonary artery pressure > 60 mmHg are more likely to have increased early mortality after the TAVR procedure. Arsalan, et al.[35] analyzed TVT registry and compared nonagenarians with those below 90 years of age. They demonstrated higher stroke, major access site complications, bleeding, and in-hospital mortality with nonagenarians. Our study supported these results and showed higher in-hospital mortality, composite of stroke, vascular complications and blood transfusion with nonagenarians. As elderly patients in nonagenarian group generally have higher comorbidities and frailty, higher in-hospital complications are likely with any percutaneous intervention. Mack, et al.[9] also mentioned about the higher frailty in nonagenarians by using frailty test. This higher composite of complications including acute kidney injury may in turn explain short-term in-hospital mortality in our study.[36] Stroke is an important secondary outcome after aortic valve replacement. Few studies have mentioned the association of post-procedural neurological events with the older age.[37] Higher stroke rates in nonagenarians were also showed by few studies in the past.[35] Our study supported the prior findings of higher stroke rates in nonagenarians and this may additionally explain higher mortality rates in this age group. Another study found that nonagenarians requires more permanent pacemaker implantation post TAVR.[38] This is much higher compared with what is showed in PARTNER-1 trial (7.6%). Prevalence of various conduction disorders increase with age which may explain higher permanent pacemaker in nonagenarians.[39] However, implantation of permanent pacemaker varies widely.[40]
Females are always subject to higher in-hospital mortality when undergoing percutaneous approach. This is showed by multiple previous studies,[41],[42] however the reason is not clear yet. Our study showed that chronic pulmonary disease was one of the predictor for in-hospital mortality. One study by Suri, et al.[43] showed that moderate to severe chronic pulmonary disease increases risk of mortality.[43] As shown by Tamburino, et al.,[34] chronic kidney disease, through multiple mechanisms, leads to mortality in TAVR patients. In our study, chronic kidney disease was associated with higher mortality in nonagenarians. Also, patients on any type of dialysis (hemo or peritoneal dialysis) are associated with worse outcomes. The similar finding of increased early mortality in patients with dialysis dependent renal failure is demonstrated in the review article by Tjang, et al.[44] Intraoperative procedures like intra-aortic balloon pump, percutaneous left ventricular assist device and extracorporeal membrane oxygenation are associated with the increased in-hospital mortality as they are usually utilized in high-risk patients with unstable hemodynamics. Patients with multiple comorbidity and poor cardiac function requires mechanical circulatory supports which makes them more prone to adverse events. A study from Peura, et al.[45] reported that in-hospital mortality in high risk patients requiring MCS is 10 times higher as compared to that seen in patients with low risk. Another study from Singh, et al.[46] states that MCS, when utilized during TAVR in extremely high risk patients, leads to higher in-hospital and long term mortality.
PARTNER trial[29] and SURTAVI trial[17],[18] demonstrated that TAVR is non-inferior to SAVR in high and intermediate risk patients, respectively. Similarly, a separate clinical trial is required to see if those results with high and intermediate surgical risk exist in octogenarians and nonagenarians age group. We explained results from observational study from the NIS database which needs to be confirmed with prospective clinical trials. In PARTNER-IIB trial, Edward Sapien XT transcatheter aortic valve were used. Newer generation valves may show further improvement in outcomes.[47] Studies with long term follow up are needed to see if such result persists at 30 days and at 1 year.
4.1. Limitations
Our study has several limitations inherent to the large administrative database such as potential coding errors and misinterpretations of diagnosis or procedures. Further, such database could have under reporting of comorbid conditions and missing data may also introduce bias. Secondly, medications data and other important procedural data (valve type, defect size, concomitant procedure performed) were not given which may have improved our analysis. Thirdly, long term complications have not been described because of the nature of the database. Also, we were not able to calculate risk scores such as STS score because of the nature of our database. We did not have information on Kansas City Cardiomyopathy Questionnaire (KCCQ-12) which may accurately predict the quality of life post-TAVR.[48] We did not have follow up information because of the inpatient nature of the database. Finally, we have hospitalizations between 2012 and 2014; database after 2014 has not been publically available yet. Also, this database is different from TVT Registry patients. However, HCUP NIS database has been widely used in the research in the past as it represents nationally representative “real-world” sample without any selection bias which we may see with any clinical trial.
4.2. Conclusions
Our study is the largest to compare the in-hospital outcomes between octogenarians and nonagenarians. Although in-hospital outcomes are slightly worse in nonagenarians, TAVR is a feasible option in patients above 90 years of age with appropriate patient selection. Patients who are older (especially above 90 years of age), females, those with chronic pulmonary disease, on dialysis or in those with need for mechanical circulatory device should be followed more closely. TAVR is an evolving technology, and naturally there is a learning curve, and we can expect lower in-hospital mortality and morbidity with more experienced interventional cardiologist in coming years.
Acknowledgments
A part of this article was presented as an abstract at American Heart Association scientific meetings, 2017 at Anaheim, California.
References
- 1.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Peter M, Hoffmann A, Parker C, et al. Progression of aortic stenosis. Role of age and concomitant coronary artery disease. Chest. 1993;103:1715–1719. doi: 10.1378/chest.103.6.1715. [DOI] [PubMed] [Google Scholar]
- 4.Davies SW, Gershlick AH, Balcon R. Progression of valvar aortic stenosis: a long-term retrospective study. Eur Heart J. 1991;12:10–14. doi: 10.1093/oxfordjournals.eurheartj.a059815. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol. 1989;13:545–550. doi: 10.1016/0735-1097(89)90590-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheitlin MD, Gertz EW, Brundage BH, et al. Rate of progression of severity of valvular aortic stenosis in the adult. Am Heart J. 1979;98:689–700. doi: 10.1016/0002-8703(79)90465-4. [DOI] [PubMed] [Google Scholar]
- 7.Kelly TA, Rothbart RM, Cooper CM, et al. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. Am J Cardiol. 1988;61:123–130. doi: 10.1016/0002-9149(88)91317-3. [DOI] [PubMed] [Google Scholar]
- 8.Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J. 1987;8:471–483. doi: 10.1093/oxfordjournals.eurheartj.a062307. [DOI] [PubMed] [Google Scholar]
- 9.Mack MC, Szerlip M, Herbert MA, et al. Outcomes of treatment of nonagenarians with severe aortic stenosis. Ann Thorac Surg. 2015;100:74–80. doi: 10.1016/j.athoracsur.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 10.van Geldorp MW, Heuvelman HJ, Kappetein AP, et al. Quality of life among patients with severe aortic stenosis. Neth Heart J. 2013;21:21–27. doi: 10.1007/s12471-012-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 12.He W, Muenchrath MN. 90+ in the United States: 2006–2008. US Census Bureau; 2011. [Google Scholar]
- 13.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43:698–703. doi: 10.1016/j.jacc.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 16.Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 18.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 19.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 20.Reinohl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373:2438–2447. doi: 10.1056/NEJMoa1500893. [DOI] [PubMed] [Google Scholar]
- 21.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 22.Dvir D, Barbash IM, Ben-Dor I, et al. The development of transcatheter aortic valve replacement in the USA. Arch Cardiovasc Dis. 2012;105:160–164. doi: 10.1016/j.acvd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 24.Department of Health and Human Services. Correspondence regarding premarket approval application for Edwards Sapien transcatheter heart valve. 2011. [Accessed April 12, 2017]. Http://www.accessdata.fda.gov/cdrh_docs/pdf10/p100041a.pdf.
- 25.US Food and Drug Administration. FDA expands approved use of Sapien artificial heart valve. 2012. [Accessed April 12, 2017]. Http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm323478.htm.
- 26.US Food and Drug Administration. FDA approves expanded indication for two Transcatheter heart valves for patients at intermediate risk for death or complications associated with open-heart surgery. 2016. [Accessed April 12, 2017]. Https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517281.htm?source=govdelivery.
- 27.Kayatta MO, Thourani VH, Jensen HA, et al. Outcomes for Transcatheter aortic valve replacement in nonagenarians. Ann Thorac Surg. 2015;100:1261–1267. doi: 10.1016/j.athoracsur.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 28.Biancari F, D'Errigo P, Rosato S, et al. Transcatheter aortic valve replacement in nonagenarians: early and intermediate outcome from the OBSERVANT study and meta-analysis of the literature. Heart Vessels. 2017;32:157–165. doi: 10.1007/s00380-016-0857-3. [DOI] [PubMed] [Google Scholar]
- 29.Thourani VH, Jensen HA, Babaliaros V, et al. Outcomes in nonagenarians undergoing transcatheter aortic valve replacement in the PARTNER-I Trial. Ann Thorac Surg. 2015;100:785–792. doi: 10.1016/j.athoracsur.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NIS database documentation archive. 2016. [Accessed October 6, 2017]. Https://www.hcup-us.ahrq.gov/db/nation/nis/nisarchive.jsp.
- 31.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Austin SR, Wong YN, Uzzo RG, et al. Why Summary comorbidity measures such as the charlson comorbidity index and elixhauser score work. Med Care. 2015;53:e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassen JA, Shelat AA, Myers J, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):S69–S80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 34.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 35.Arsalan M, Szerlip M, Vemulapalli S, et al. Should transcatheter aortic valve replacement be performed in nonagenarians? Insights from the STS/ACC TVT registry. J Am Coll Cardiol. 2016;67:1387–1395. doi: 10.1016/j.jacc.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weintraub WS, Clements SD, Ware J, et al. Coronary artery surgery in octogenarians. Am J Cardiol. 1991;68:1530–1534. doi: 10.1016/0002-9149(91)90291-r. [DOI] [PubMed] [Google Scholar]
- 38.McNeely C, Zajarias A, Robbs R, Markwell S, Vassileva CM. Transcatheter aortic valve replacement outcomes in nonagenarians stratified by transfemoral and transapical approach. Ann Thorac Surg. 2017;103:1808–1814. doi: 10.1016/j.athoracsur.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28:539–553. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto M, Mouillet G, Meguro K, et al. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry. Ann Thorac Surg. 2014;97:29–36. doi: 10.1016/j.athoracsur.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 41.Doshi R, Shah P, Meraj P. Gender disparities among patients with peripheral arterial disease treated via endovascular approach: A propensity score matched analysis. J Interv Cardiol. 2017;30:604–611. doi: 10.1111/joic.12431. [DOI] [PubMed] [Google Scholar]
- 42.Udell JA, Koh M, Qiu F, et al. Outcomes of women and men with acute coronary syndrome treated with and without percutaneous coronary revascularization. J Am Heart Assoc. 2017:e004319. doi: 10.1161/JAHA.116.004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suri RM, Gulack BC, Brennan JM, et al. Outcomes of patients with severe chronic lung disease who are undergoing transcatheter aortic valve replacement. Ann Thorac Surg. 2015;100 doi: 10.1016/j.athoracsur.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 44.Tjang YS, van Hees Y, Korfer R, et al. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg. 2007;32:469–474. doi: 10.1016/j.ejcts.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Peura JL, Colvin-Adams M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–2667. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 46.Singh V, Damluji AA, Mendirichaga R, et al. Elective or emergency use of mechanical circulatory support devices during transcatheter aortic valve replacement. J Interv Cardiol. 2016;29:513–522. doi: 10.1111/joic.12323. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Shoshan J, Konigstein M, Zahler D, et al. Comparison of the Edwards SAPIEN S3 versus medtronic evolut-r devices for transcatheter aortic valve implantation. Am J Cardiol. 2017;119:302–307. doi: 10.1016/j.amjcard.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Spertus JA, Jones PG. Development and validation of a short version of the kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. International classification of diseases, ninth revision, clinical modification codes.
| Variable name | ICD 9 CM Code Used |
| Smoking | v15.82, 305.1 |
| Family history of coronary artery disease | v17.3 |
| Prior myocardial infarction | 412 |
| Ischemic cardiomyopathy | 414.8 |
| Mitral stenosis | 396.0 |
| Mitral insufficiency | 396.2, 396.8, 394.2, 424.0 |
| Chronic kidney disease | 585.x |
| Dialysis (procedural code) | Haemodialysis: 39.95Peritoneal dialysis: 54.98 |
| IABP (procedural code) | 37.61 |
| pLVAD (procedural code) | 37.68 |
| ECMO (procedural code) | 39.65 |
| Trans-femoral approach for TAVR (procedural code) | 35.05 |
| Transapical approach for TAVR (procedural code) | 35.06 |
| Composite of stroke (ischemic and hemorrhagic combined) | 430, 431, 432, 997.02, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, or 436 |
| Acute renal failure | 584 |
| Cardiac arrest | 427.5 |
| Blood transfusion | 998.11, 998.12, 285.1, 99.0 |
| Iatrogenic cardiac complication | 997.1 |
| Vascular injury requiring surgery (procedure code) | 39.31, 39.41, 39.49, 39.52, 39.53, 39.56, 39.57, 39.58, 39.59, 39.79 |
| Perioperative stroke | 997.02 |
| Perioperative infection | 998.5, 998.59, 998.62 |
| Postoperative shock | 998.0 |
| Permanent pacemaker implantation (procedural code) | 37.70 to 37.79, 37.80 to 37.89, 00.50, 00.52, 00.53 |
| Atrial fibrillation | 427.31 |
IABP: intra-aortic balloon pump; ICD 9 CM: international classification of diseases, ninth revision, clinical modification codes; ECMO: extra-corporeal membrane oxygenation; pLVAD: percutaneous left ventricle assist device; TAVR: transcatheter aortic valve replacement.
Table 2S. Comorbidities in hospitalizations with transcatheter aortic valve replacement (unmatched).
| Variable name | Octogenarians (n = 4002) | Nonagenarians (n = 1201) | P value |
| Weighted numbers | 20010 | 6005 | N/A |
| Diabetes mellitus (uncomplicated) | 26.2% | 15.8% | < 0.001 |
| Diabetes mellitus (with chronic complications) | 4.7% | 2% | < 0.001 |
| Hypertension | 80.6% | 78.9% | 0.24 |
| Liver disease | 1% | 0.6% | 0.15 |
| Fluid and electrolytes disorder | 26.5% | 25.6% | 0.53 |
| Other neurological disorder | 6.8% | 6.1% | 0.36 |
| Obesity | 9.5% | 2.7% | <0.001 |
| Peripheral vascular disease | 30.2% | 25.7% | 0.002 |
| Smoking | 1.7% | 1% | 0.08 |
| Alcohol | 0.5% | 0.4% | 0.71 |
| Prior MI | 12.2% | 11.5% | 0.53 |
| Family history of CAD | 1.7% | 1% | 0.08 |
| Coagulopathy | 23.9% | 27.7% | 0.009 |
| Chronic pulmonary disease | 31% | 20.6% | <0.001 |
| Congestive heart failure | 12.5% | 11% | 0.21 |
| AIDS | 0% | 0% | N/A |
| Ischemic cardiomyopathy | 7% | 4.5% | 0.001 |
| Mitral stenosis | 1.1% | 1.3% | 0.58 |
| Mitral insufficiency | 18.2% | 18.7% | 0.68 |
| Chronic kidney disease | 37.9% | 38.5% | 0.66 |
| Composite of hemo and peritoneal dialysis | 3.4% | 2.1% | 0.014 |
HMOs: health maintenance organizations; PPOs: Preferred Provider Organizations, MI: Myocardial Infarction, CAD: Coronary Artery Disease, N/A: Not Applicable