Abstract
Most heart failure (HF) related mortality is due to sudden cardiac death (SCD) and worsening HF, particularly in the case of reduced ejection fraction. Predicting and preventing SCD is an important goal but most works include no or few patients with advanced age, and the prevention of SCD in elderly patients with HF is still controversial. A recent reduction in the annual rate of SCD has been recently described but it is not clear if this is also true in advanced age patients. Age is associated with SCD, although physicians frequently have the perception that elderly patients with HF die mainly of pump failure, underestimating the importance of SCD. Other clinical variables that have been associated to SCD are symptoms, New York Heart Association functional class, ischemic cause, and comorbidities (chronic obstructive pulmonary disease, renal dysfunction and diabetes). Some test results that should also be considered are left ventricular ejection fraction and diameters, natriuretic peptides, non-sustained ventricular tachycardias and autonomic abnormalities. The combination of all these markers is probably the best option to predict SCD. Different risk scores have been described and, although there are no specific ones for elderly populations, most include age as a risk predictor and some were developed in populations with mean age > 65 years. Finally, it is important to stress that these scores should be able to predict any type of SCD as, although most are due to tachyarrhythmias, bradyarrhythmias also play a role, particularly in the case of the elderly.
Keywords: Heart failure, Prediction, Risk, Sudden death, The elderly
1. Introduction
Chronic heart failure (HF) represents a major health problem. Its prevalence is ≥ 10% among people older than 70 years,[1] implies a high rate of hospitalizations, a poor prognosis, and a great impact on quality of life, health-care costs, and families. The phenotype of patients with HF has changed, mainly due to the increase in age, and the consequent increase in the number of comorbidities and medications.[2] In any case, most deaths continue to be due to sudden cardiac death (SCD) and worsening HF, particularly in the case of patients with heart failure and reduced ejection fraction (HFREF). Data from the Framingham Heart Study showed that the causes of death were different in patients with HFREF and in those with preserved ejection fraction (HFPEF) where cancer, infection, and renal disease had a predominant role.[3] Predicting and preventing SCD is an important goal in patients with HF and, therefore, has been broadly studied.[4] However, most works include no or few elderly patients and the prevention of SCD in elderly patients with HF is still controversial. In this paper we review the evidence regarding this topic.
2. Definitions (ESC 2015)
Sudden death refers to a non-traumatic, unexpected fatal event occurring within one hour of the onset of symptoms in an apparently healthy subject. If death is not witnessed, the definition applies when the victim was in good health 24 h before the event.
SCD refers to sudden death in patients where a congenital, or acquired, potentially fatal cardiac condition was known to be present during life, or autopsy has identified a cardiac or vascular anomaly, or when an arrhythmic event is a likely cause of death.
3. Epidemiology
Cardiovascular diseases are the number one cause of death and about 25% of them are due to SCD. In the elderly, degenerative heart conditions that lead to HF are the main cause of SCD.[1] The risk of SCD is higher in men and its relation with age has been controversial, probably due to different etiologies that are age-related. However, a recent analysis of 40,195 patients with HFREF included in 12 clinical trials has clearly shown that older age is associated with SCD.[5] This association is of great importance for the clinical practice, as physicians may have the perception that advanced age patients with HF die mainly of pump failure, underestimating the importance of SCD in this group, and this may lead to suboptimal treatment.
4. Predictors of SCD in elderly patients with HF
SCD occurs in different population groups: (1) patients without a prior diagnosis of heart disease; (2) patients with a history of heart disease with no or mild cardiac dysfunction; (3) patients with a history of heart disease and severe cardiac dysfunction; and (4) those diagnosed with a defined genetically-based cause for a life-threatening cardiac arrhythmia.[6] SCD in elderly patients is mainly related to HF. The evidence to predict SCD in this age group is scarce and complex. The variables that may be used to predict SCD in elderly patients with heart failure are depicted in Table 1 and are described below.
Table 1. Variables that may be used to predict sudden cardiac death in elderly patients with heart failure.
| Clinical characteristics of heart failure |
| Etiology |
| Systolic and diastolic function |
| Functional capacity |
| Biochemical parameters |
| Autonomic abnormalities and electrical instability |
| Risk models |
4.1. Characteristics of heart failure
Left ventricular ejection fraction (LVEF), symptoms, and New York Heart Association (NYHA) class are related with SCD.[6] In patients with HFREF, a higher rate of SCD is observed in those with lower LVEF. Also, age, worse HF symptoms, and ischemic cause are associated with SCD.[7]–[17] A 50% reduction of the annual rate of SCD in these patients was been recently shown [from 6.5 % in the Randomized Aldactone Evaluation Study (RALES) trial to 3.3% in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial], probably due to the effects of medical treatment.[5] However, the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA),[14] that only included patients ≥ 60 years (mean age 73 years), was an outliner regarding this reduction in the annual rate of SCD, with 5.3%.
In fact, LVEF and NYHA class have been use as primary criteria in most clinical trials and have a central position in the guidelines regarding the use of implanted cardioverter defibrillators (ICD).[1],[18] Different trials investigated the utility of the ICD as primary prevention in ischemic HFREF [Multicenter Automatic Defibrillator Implantation Trial (MADIT), Multicenter Unsustained Tachycardia Trial Investigators (MUSTT), MADIT II, Defibrillation in Acute Myocardial Infarction Trial (DINAMIT) and Coronary Artery Bypass Graft (CABG) Patch Trial], and found a mortality reduction in the ICD arm.[19]–[23] Although these studies included some elderly patients, the MUSTT study was the only one with a mean age ≥ 65 years and reported a benefit in the electrophysiological-guided therapy group with ICD. In a post-hoc analysis of MADIT-II trial (patients ≥ 75 years), a tendency to an improved survival in the ICD group was found, although the benefit did not reach statistical significance.[24] The Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) has recently investigated the benefits of ICD in a non-ischemic population. Although the trial did not show a mortality benefit, ICD was associated with a reduction on SCD and subgroup analysis showed that patients younger than 59 years-old had a survival benefit, while patients ≥ 68 years-old did not, suggesting that the benefit of primary prevention in the elderly is questionable in non-ischemic patients.[25]
Fewer trials have investigated the efficacy of ICD to prevent SCD in secondary prevention. The Antiarrhythmics Versus Implantable Defibrillators (AVID) trial randomized patients to ICD or treatment with amiodarone/sotalol. This study had a mean age of 65 years and found a clear mortality benefit in the ICD group.[26] However, a combined analysis of AVID, Cardiac Arrest Study Hamburg (CASH), and Canadian Implantable Defibrillator Study (CIDS) trials found no benefit of ICDs in patients ≥ 75 years.[27]
Overall, these studies suggest that LVEF is a predictor of SCD in elderly patients. However, the fact that HFREF accounts only for < 20% of all SCD, the lack of evidence of causal relation LVEF-arrhythmia mechanisms, the variability of LVEF, and the lack of precision of its measure makes the use of LVEF to predict SCD controversial.[6],[28],[29] In the Oregon Sudden Unexpected Death Study,[28] the authors studied a cohort of patients who died of SCD in Oregon and retrospectively assessed LVEF. In this elderly population, with 38% > 75 years, only a third had a reduced LVEF. In the Candesartan in Heart failure―Assessment of moRtality and Morbidity (CHARM) program, that included HFPEF,[29] and had a mean age of 66 years, 35% of deaths were due to SCD.
As we will see in the end of this review, the inclusion of LVEF in prediction models is probably a better option to determine the risk of SCD. Comorbidities should be included in these models, including chronic obstructive pulmonary disease, renal dysfunction, and diabetes.[30],[31] Also HF-related factors as left ventricular diameters, natriuretic peptides, and non-sustained ventricular tachycardias may improve the prediction capability.[31]
As previously said, SCD is also an important cause of death in patients with HFPEF, which affects mainly elderly patients.[32],[33] Mortality rates have varied substantially across studies, probably due to the heterogeneity in the diagnosis of this condition, particularly when the studies did not include natriuretic peptides.[34] Population-based cohort studies related mortality in this group mainly with non-cardiac causes, while clinical trials reported higher rates of cardiovascular deaths, probably due to selection bias.[34] In the Irbesartan in Heart Failure With Preserved Ejection Fraction (I-PRESERVE) trial, with a mean age > 70 years, cardiovascular diseases were responsible for 60% of deaths, and 26% of all deaths were due to SCD.[35] In this trial, performed in patients with HFPEF, age, gender, diabetes mellitus, previous myocardial infarct, left bundle branch block, and the N-terminal pro B-type natriuretic peptide (NT-pro-BNP) were identified as risk factors of SCD over five years.[36]
Biochemical markers are used for the diagnosis of HF and are related to the prognosis and to SCD. BNP and Nt-proBNP have been broadly studied. In patients with LVEF < 35%, a BNP cut-off point 130 pg/mL had a 99% negative predictive value for SCD.[37] Although BNP is lower in a population with HFPEF, a study of 615 elderly patients (mean age 70 years) showed that when similar levels of BNP were compared across the whole spectrum of LVEF, and for different cut-off levels of LVEF, the associated risk of adverse outcomes was similar in HFPEF and HFREF.[38] As we have said, an association between Nt-proBNP and SCD in patients with HFPEF has also been found.[36] Moreover, significant associations between BNP/ Nt-proBNP levels and ventricular arrhythmias have been reported.[39],[40] A meta-analysis of 14 studies confirmed the relation between BNP/Nt-proBNP and SCD/ventricular arrhythmias.[39] Five of the studies used in this meta-analysis had a mean age ≥ 65 years.[31],[41]–[44] The association of natriuretic peptides with SCD is not merely due to a more advance HF situation.[40] However, the severity of the HF syndrome and the presence of comorbidities should be considered to predict SCD in elderly populations. BNP increases with ageing itself, probably due to age-related myocardial fibrosis and renal impairment, and with some comorbidities such as renal dysfunction, chronic obstructive pulmonary disease, low body mass index, and pulmonary hypertension.[45]–[48]
All the previous seen predictors of SCD are useful in elderly patients with HF but they also have important limitations that are depicted in Table 2.
Table 2. Main limitations of predictors of sudden cardiac death in elderly populations.
| Predictors | Elderly population |
| Heart failure signs and symptoms | Comorbidities may mimic (anemia, obesity, chronic obstructive pulmonary disease) |
| Left ventricularejection fraction | Usually preserved |
| Diastolic function | May be difficult to assess in elderly population |
| Functional class | Bad correlation |
| Natriuretic peptides | Increases with age and comorbidities(consider specific cut-offs) |
4.2. Autonomic abnormalities and electrical instability
Autonomic nervous system abnormalities may be caused by the response to disturbed homoeostasis caused by HF. Arrhythmic risk is enhanced when vagal activity decreases or sympathetic activity increases,[6],[49] and may increase the risk of ventricular fibrillation. The value of autonomic abnormalities to predict SCD is independent of electrical instability. There are different autonomic tests that study the variability of heart rate, arterial pressure behavior and QT interval variability that have been associated with a poor prognosis in HF patients (Table 3). Heart rate (HR) variability (R-R interval on the ECG/24 hours Holter) and baroreflex sensitivity (BRS) (provoked or spontaneous) are predictors of SCD.[50],[51] HR variability represents the neurohormonal interaction with the sinus node, and decreases with sympathetic activity and with age. Low HR variability has been related with poor outcomes in patients with chronic HF.[52]–[56] Depressed BRS is also an independent predictor of cardiovascular mortality in elderly patients with preserved ejection fraction.[57]
Table 3. Autonomic abnormalities and electrical instability as risk predictors in elderly populations with heart failure.
| Predictors | Associated with | Studies in elderly populations |
| HR variability | Poor outcomes and SCD | |
| HR deceleration capacity | SCD and mortality | |
| HR recovery after exercise | Mortality | Yes |
| BRS | Cardiovascular mortality and SCD | Yes |
| HRT | HF severity and SCD | Yes |
| QT variability | Cardiovascular mortality | Yes |
| TWA | SCD | Yes |
| TWR | SCD |
BRS: baroreflex sensitivity; HF: heart failure; HR: heart rate; HRT: heart rate turbulence; SCD: sudden cardiac death; TWA: T-wave alternans; TWR: T-wave morphology restitution.
Heart rate turbulence (HRT) describes short-term fluctuations in sinus cycle length that follow spontaneous ventricular premature complexes (VPCs). Usually, sinus rate initially briefly accelerates and subsequently decelerates compared with the pre-VPC rate, before returning to baseline.[58],[59] HRT is a vagally mediated phenomenon, reflecting baroreflex sensitivity. Increasing age is associated with a decrease in HRT.[60] In patients with HF, HRT is a predictor of HF severity and poor outcomes.[61] In the Sudden Death in Heart Failure [MUerte Subita en Insuficiencia Cardiaca (MUSIC)] registry, with a mean age of 63 years, HRT was strongly associated with SCD, also in patients > 65 years.[62] Adequate HR recovery after exercise depends on the vagal system. Impaired HR recovery after 1 min (≤ 12 beats) is a predictor of death, even in elderly population (≥ 65 years).[63] In patients with HF, it was associated independently with mortality.[64]
QT variability index is the ratio of normalized QT variability to normalized HR variation and is a non-invasive measure of repolarization lability due to autonomic abnormalities. Initially, it was related to SCD in a small-sample group of patients with HF,[65] but subsequent studies disagreed showing a positive association with cardiovascular death but not with SCD.[66],[67]
T wave-derived indices have been proposed as better markers of repolarization dispersion. T wave alternans (TWA), T peak-to-end restitution, and T wave morphology restitution are markers of ventricular instability and dispersion of repolarization that could predict SCD. TWA is a beat-to-beat alternation in the morphology of the ST segment and the T wave, which reflects the temporal and spatial heterogeneity of repolarization. TWA might be associated with arrhythmic risk[68] and SCD.[69]–[72] However, the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) trial failed to show a positive association between TWA and SCD.[73] However, in the Cardiovascular Health study TWA was independently associated with SCD in an elderly population.[74] T-wave morphology restitution (TWR) quantifies the morphological differences between T-wave, it measures T-wave morphological change per RR increment. In a recent analysis of the MUSIC registry study, TWR was the most significance variable associated with SCD in patients with HF and independent from other clinical and ECG variables.[75],[76] It was shown to be a better marker than other electrical and autonomic variables as TWA, HRT, QT variability index, T peak-to end or QRS duration. An integrated risk model with clinical (NYHA class and LVEF) and ECG derived parameters (TWA, TWR and T peak-to-end) to predict SCD has been proposed.[76],[77]
Other electrical parameters as QRS duration (specially left bundle branch block), QTc, microvolt electrical potentials in the terminal QRS complex and induced/spontaneous ventricular arrhythmias have been related with SCD in patients with HF.[78] However, it is the combination of them with other predictors in risk models what may predict accurately SCD. Moreover, abnormal electrocardiographic patterns in elderly population is very high. Increased QRS amplitude, QT prolongation and non-sustained monomorphic ventricular tachycardia may be present even in patients without structural heart disease,[79] so we must be careful when interpreting them as risk predictors for SCH in elderly patients with HF.
4.3. Risk models
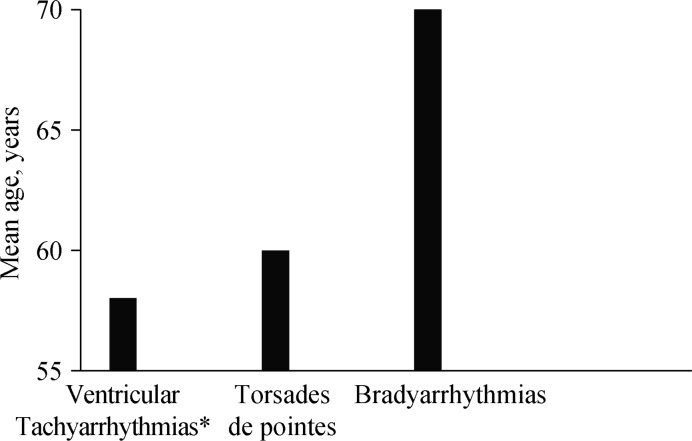
The combination of markers reflecting the impairment of different mechanisms based on clinical variables, biomarkers, and autonomic and electrical impairment is probably the best option to predict SCD prediction. Different risk scores have been described,[30],[31],[36],[76],[79] but there are no specific ones for elderly population, and some predictors may have important limitations in elderly populations. However, most of them include age as a risk predictor and some were developed in populations with mean age > 65 years. Interestingly several of them include comorbidities as predictors of SCD. Table 4 shows the variables most frequently included in these predictor scores. It is important to stress that these scores should be able to predict any type of SCD as, although most are due to tachyarrhythmias, bradyarrhythmias also play a role, particularly in the case of the elderly (Figure 1).[80],[81]
Table 4. Variables most frequently included in SCD predictor scores.
| Studies | Age, yrs | Male | NYHA | Echocardiography | Natriuretic peptides | Comorbidities | ECG | Etiology |
| Bilchick, et al.[30] | 75 | II | LVEF < 20% | COPD, kidney disease, diabetes | ||||
| Watanabe, et al.[31] | 66 | LVEF, LVEDD | BNP | Diabetes | NSVT | |||
| Adabag, et al.[36] | Male | Nt-proBNP | Diabetes | LBBB | Myocardial infarction | |||
| Ramírez, et al.[76] | Male | II | LVEF | TWA, TWR, T peak to end. | ||||
| Vázquez, et al.[79] | LA size > 26 mm/m2 | Nt-proBNP | LBBB, NSVT,Frequent VPBs | Atherosclerotic vascular events |
BRS: baroreflex sensitivity; BNP: B-type natriuretic peptide; COPD: chronic obstructive pulmonary disease; HR: heart rate; HRT: heart rate turbulence; LA: left atria; LBBB: left bundle branch block; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; NSVT: non-sustained ventricular tachycardia; NT-pro-BNP: N-terminal pro B-type natriuretic peptide; NYHA: New York Heart Association functional class; SCD: sudden cardiac death; VPBs: ventricular premature beats; TWA: T-wave alternans; TWR: T-wave morphology restitution.
Figure 1. Mean age of patients who died suddenly wearing a 24 hour ECG Holter recorder.
Data from Bayés de Luna, et al.[81] *Except torsade des pointes.
5. Conclusions
SCD is an important cause of death in elderly patients with HF. Predictors of SCD in this group are not well-defined and specific studies are needed. We cannot define the best risk marker to predict SCD, but rather a combination of clinical, biochemical, echocardiographic and electrical parameters. Specific characteristics and comorbidities of elderly population should be considered in prediction and prevention of SCD.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Jhund PS, Petrie MC, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 6.Wellens HJJ, Schwartz PJ, Lindemans FW, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–1651. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 8.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the betablocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 9.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 10.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metroprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 11.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 12.Granger CB, McMurray JJV, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJV, Östergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitor: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 14.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 15.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 20.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 21.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 22.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 23.Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery by-pass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 24.Huang DT, Sesselberg HW, McNitt S, et al. Improved survival associated with prophylactic implantable defibrillators in elderly patients with prior myocardial infarction and depressed ventricular function: a MADIT-II substudy. J Cardiovasc Electrophysiol. 2007;18:833–838. doi: 10.1111/j.1540-8167.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- 25.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;29:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 26.The Antiarrhythmics Versus Implantable Defibrillators (AVID) investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 27.Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J. 2007;28:1746–1749. doi: 10.1093/eurheartj/ehl438. [DOI] [PubMed] [Google Scholar]
- 28.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon sudden unexpected death study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 29.Solomon JD, Wang D, Finn P, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan In Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–2183. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 30.Bilchick KC, Stukenborg GJ, Kamatch S, et al. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes. 2016;9:23–30. doi: 10.1161/CIRCOUTCOMES.115.002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe J, Shinozaki T, Shiba N, et al. Accumulation of risk markers predicts the incidence of sudden death in patients with chronic heart failure. Eur J Heart Fail. 2006;8:237–242. doi: 10.1016/j.ejheart.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 2015:73–87. doi: 10.1016/j.yjmcc.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > = 65 years of age. CHS reserarch group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 34.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Hear Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation. 2010;30; 121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 36.Adabag S, Rector TS, Anand IS, et al. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Hear Fail. 2014;16:1175–1182. doi: 10.1002/ejhf.172. [DOI] [PubMed] [Google Scholar]
- 37.Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 38.Van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 39.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11:958–966. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 40.Levine YC, Rosenberg MA, Mittleman M, et al. B-type natriuretic peptide is a major predictor of ventricular tachyarrhythmias. Heart Rhythm. 2014;11:1109–1116. doi: 10.1016/j.hrthm.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Winkler K, Wanner C, Dreschsler C, et al. Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur Hear J. 2008;29:1092–1099. doi: 10.1093/eurheartj/ehn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blangy H, Sadoul N, Dousset B, et al. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Eurospace. 2007;9:724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 43.Budeus M, Reinsch N, Wieneke H, et al. The prediction of ICD therapy in multicenter automatic defibrillator implantation trial (MADIT) II trial like patients a retrospective analysis. Indian Pacing Electrophysiol J. 2008;8:80–93. [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H, Oswald H, Gardiwal A, et al. Comparison of N-terminal probrain natriuretic peptide versus electrophysiologic study for predicting future outcomes in patients with an implantable cardioverter defibrillator after myocardial infarction. Am J Cardiol. 2007;100:635–639. doi: 10.1016/j.amjcard.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 45.Iacoviello M, Antoncecchi V. Heart failure in the elderly: progress in clinical evaluation and therapeutic approach. J Geriatr Cardiol. 2013;10:165–177. doi: 10.3969/j.issn.1671-5411.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oudejans I, Mosterd A, Bloemen JA, et al. Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail. 2011;13:518–527. doi: 10.1093/eurjhf/hfr021. [DOI] [PubMed] [Google Scholar]
- 47.Vaes B, Gruson D, Van Pottelbergh G, et al. The impact of confounders on the test performance of natriuretic peptides for cardiac dysfunction in subjects aged 80 and older. Peptides. 2012;38:118–126. doi: 10.1016/j.peptides.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Wang TJ, Larson MG, Levy D, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study) Am J Cardiol. 2011;108:1341–1345. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 50.Barthel P, Bauer A, Müller A, et al. Spontaneous baroreflex sensitivity: prospective validation trial of a novel technique in survivors of acute myocardial infarction. Heart Rhythm. 2012;9:1288–1294. doi: 10.1016/j.hrthm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 51.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 52.Ponikowski P, Anker SD, Chua TP, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 53.La Rovere MT, Bigger JT, Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 54.La Rovere MT, Pinna GD, Hohnloser SH, et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 55.La Rovere MT, Pinna GD, Maestri R, et al. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193–199. doi: 10.1016/j.jacc.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 56.Mortara A, La Roverre MT, Pinna GD, et al. Arterial baroreflex modulation of heart rate in chronic heart failure. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 57.De Ferrari GM, Sanzo A, Bertoletti A, et al. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol. 2007;50:2285–2290. doi: 10.1016/j.jacc.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 58.Bauer A, Kantelhardt JW, Barthel P, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. 2006;367:1674–1681. doi: 10.1016/S0140-6736(06)68735-7. [DOI] [PubMed] [Google Scholar]
- 59.Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008;52:1353–1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 60.Schwab JO, Eichner G, Shlevkov N, et al. Impact of age and basic heart rate on heart rate turbulence in healthy persons. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S198–S201. doi: 10.1111/j.1540-8159.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 61.Cygankiewicz I, Zareba W, Vazquez R, et al. Relation of heart rate turbulence to severity of heart failure. Am J Cardiol. 2006;98:1635–1640. doi: 10.1016/j.amjcard.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 62.Cygankiewicz I, Zareba W, Vazquez R, et al. Heart rate turbulence predicts all-cause mortality and sudden death in congestive heart failure patients. Heart Rhythm. 2008;5:1095–1102. doi: 10.1016/j.hrthm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 64.Nanas S, Anastasiou-Nana M, Dimopoulos S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol. 2006;110:193–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 65.Barr CS, Naas A, Freeman M, et al. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 66.Dobson CP, La Rovere MT, Pinna GD, et al. QT variability index on 24-hour holter independently predicts mortality in patients with heart failure: analysis of Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca (GISSI-HF) trial. Heart Rhythm. 2011;8:1237–1242. doi: 10.1016/j.hrthm.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 67.Tereshchenko LG, Cygankiewicz I, McNitt S, et al. Predictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac death. Circ Arrhythm Electrophysiol. 2012;5:719–727. doi: 10.1161/CIRCEP.112.970541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klingenheben T, Zabel M, D'Agostino RB, et al. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure. Lancet. 2000;356:651–652. doi: 10.1016/s0140-6736(00)02609-x. [DOI] [PubMed] [Google Scholar]
- 69.Merchant FM, Ikeda T, Pedretti RF, et al. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm. 2012;9:1256–1264. doi: 10.1016/j.hrthm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monasterio V, Laguna P, Cygankiewicz I, et al. Average T-wave alternans activity in ambulatory ECG records predicts sudden cardiac death in patients with chronic heart failure. Heart Rhythm. 2012;9:383–389. doi: 10.1016/j.hrthm.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 71.Stein PK, Sanghavi D, Domitrovich PP, et al. Ambulatory ECG-based T-wave alternans predicts sudden cardiac death in high-risk post-MI patients with left ventricular dysfunction in the EPHESUS study. J Cadiovasc Electrophysiol. 2008;19:1037–1042. doi: 10.1111/j.1540-8167.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 72.Sakaki K, Ikeda T, Miwa Y, et al. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: a prospective study. Heart Rhythm. 2009;6:332–337. doi: 10.1016/j.hrthm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Gold MR, Ip JH, Costantini O, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;11:2022–2028. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stein PK, Sanghavi D, Sotoodehnia N, et al. Association of holter-based measures including T-wave alternans with risk of sudden cardiac death in the community-dwelling elderly: the Cardiovascular Health Study. J Electrocardiol. 2010;43:251–259. doi: 10.1016/j.jelectrocard.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramírez J, Orini M, Mincholé A, et al. T-wave morphology restitution predicts sudden cardiac death in patients with chronic heart failure. J Am Heart Assoc. 2017;6:e005310. doi: 10.1161/JAHA.116.005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramírez J, Orini M, Mincholé A, et al. Sudden cardiac death and pump failure death prediction in chronic heart failure by combining ECG and clinical markers in an integrated risk model. PLoS One. 2017;12:e0186152. doi: 10.1371/journal.pone.0186152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane RE, Cowie MR, Chow AWC. Prediction of sudden cardiac death in heart failure. Heart. 2005;91:674–680. doi: 10.1136/hrt.2003.025254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vicent L, Martínez-Sellés M. Electrocardiogeriatrics: ECG in advanced age. J Electrocardiol. 2017;50:698–700. doi: 10.1016/j.jelectrocard.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Vazquez R, Bayes-Genis A, Cygankiewicz I, et al. The MUSIC risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. 2009;30:1088–1096. doi: 10.1093/eurheartj/ehp032. [DOI] [PubMed] [Google Scholar]
- 80.Bayés de Luna A, Elosua R. Sudden death. Rev Esp Cardiol. 2012;65:1039–1052. doi: 10.1016/j.recesp.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 81.Bayés de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]