Abstract
Objective
To investigate whether electronically measured medication adherence is associated with vision-related quality of life (VRQoL) in patients with open-angle glaucoma.
Methods and analysis
This is a 3-year prospective cohort study of 79 subjects with open-angle glaucoma at a Veterans Affairs medical centre. Participants returned a medication event monitoring system (MEMS) for their glaucoma eye-drops and had at least two visits with glaucoma during the study period. Those taking less than 80% of prescribed glaucoma medication doses were considered to be non-adherent. Subjects were interviewed using the National Eye Institute’s Visual Function Questionnaire-25 (VFQ-25) at baseline and after 3 years.
Results
Thirty per cent (n=24/79) of participants took less than 80% of prescribed doses of their glaucoma medications at baseline. Patients who did not adhere to their medications at baseline had lower mean composite VFQ-25 scores at baseline (70.66±20.50 vs 75.91±19.12, standardised mean difference=0.27) and after 3 years (71.68±21.93 vs 76.25±21.67, standardised mean difference=0.21). Visual acuity (P=0.03), but not visual field severity (P=0.13) or medication adherence (P=0.30), was significantly associated with composite VFQ-25 score in an adjusted model.
Conclusions
Subjects who were non-adherent to their glaucoma medications at baseline as assessed by a MEMS device reported lower VRQoL than adherent subjects at baseline and after 3 years. However, visual acuity was significantly associated with VRQoL. Future studies should assess whether improved adherence to eye-drops impacts VRQoL in patients with glaucoma.
Keywords: open angle glaucoma, vision-related quality of life, electronically measured medication adherence
Key messages.
What is already known about this subject?
Patient self-reported non-adherence to glaucoma eye-drops has been associated with worse vision-related quality of life (VRQoL) in cross-sectional studies.
What are the new findings?
We found that electronically measured poor adherence to glaucoma drops was associated with lower scores on the National Eye Institute’s Visual Function Questionnaire-25 both at baseline and at 3 years, but that visual acuity and visual field severity were more significant drivers of VRQoL.
How might these results change the focus of research or clinical practice?
Future studies should assess whether improving adherence to eye-drops impacts VRQoL in patients with glaucoma.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide,1 making it a significant public health issue. Reduction in intraocular pressure through topical eye-drops has been shown to prevent vision loss from glaucoma.2 Nevertheless, numerous studies have shown that patients with glaucoma frequently do not take their eye-drops as prescribed.3 4
Reasons for poor medication adherence are multifactorial. Low health literacy5 and poor understanding that glaucoma can lead to blindness may impact adherence to medical therapy.6 Patients whose glaucoma is undertreated are more likely to suffer visually significant field loss.2 More severe visual field defects due to glaucoma are associated with lower vision-related quality of life (VRQoL) scores and a decrease in quality of life over time.7 8 VRQoL is an important patient-centred metric that complements traditional clinical assessments of glaucomatous damage, such as automated perimetry and optical coherence tomography.9 10 In two cross-sectional studies, patients who self-reported poor adherence to glaucoma medications or difficulty self-administering eye-drops were more likely to have lower VRQoL scores.11 12 However, these studies relied on subjective measures of medication adherence rather than more objective measures like electronic monitoring, and only evaluated a single time-point.11 12 We hypothesised that patients who are non-adherent to their glaucoma regimen as measured by a medication event monitoring system (MEMS) would have lower VRQoL scores at baseline, and that this relationship would persist at 3 years.
Methods
We conducted a prospective cohort study of participants with open-angle glaucoma (OAG) at the Durham Veterans Affairs Medical Center Eye Clinic.
Patient charts were reviewed for the following inclusion criteria: (1) age ≥18 years, (2) diagnosis of OAG (primary OAG, pigment dispersion, pseudoexfoliation, combined mechanism, low-tension glaucoma), (3) treatment with glaucoma eye-drops and (4) Humphrey visual fields (HVF) performed within the prior 9 months. Patients were excluded if they had any condition that would impact the frequency of prescribed drops, such as active uveitis, ocular infection and/or intraocular surgery in the preceding 3 months or anticipated in the next 3 months. To ensure completion of vision-dependent tasks in the study, patients with best corrected Snellen visual acuity worse than 20/70 in the better-seeing eye were excluded. Potential participants were sent a letter explaining the nature of the study and offering the patient the ability to opt out of further study-related contact by calling the study coordinator. On the day of their appointment, the study coordinator took the potential subject to a private room in the clinic for informed consent and data collection while the patient was waiting for their regularly scheduled appointment. Baseline Mini-Mental Status Exam was performed and patients with scores <18 were excluded from ultimate enrolment in the study since significant cognitive dysfunction could confound the assessment of medication adherence.13
Subjects were given a MEMS (MEMS 6 SmartCap; Aardex, Union City, California, USA). This device has been used in more than 300 studies of oral medications and more recently in ophthalmic studies.3 14 If the patient had been prescribed more than one glaucoma eye-drop, then the study coordinator placed into the MEMS device whichever eye-drop bottle had the most frequent number of doses prescribed per day. Participants needed to return their MEMS to the study team after 3 months of use and have at least two visits with someone from the division of glaucoma within the 3-year study period in order to be included in analyses. Adherence data were downloaded from the MEMS device and analysed using PowerView software. All data were collected at the eye level, but medication adherence data were summarised at the subject level if both eyes were enrolled. Subjects were categorised as having good adherence if they took >80% of prescribed doses of glaucoma drops, a common metric in the literature.15 16 We have previously shown that self-reported adherence with glaucoma drops is associated with taking at least 80% of prescribed doses using electronic medication monitors.17
Description of variables
Demographic and clinical data were collected at the time of study enrolment. Baseline HVFs were scored by a single reviewer (KWM) blinded to the patient’s medical history, based on the American Academy of Ophthalmology Glaucoma Preferred Practice Guidelines (table 1).18
Table 1.
American Academy of Ophthalmology Glaucoma Preferred Practice Guidelines
| Visual field severity | |
| Mild | Normal visual field |
| Moderate | Visual field abnormalities in one hemifield that are not within 5 degrees of fixation |
| Severe | Visual field abnormalities in both hemifields and/or loss within 5 degrees of fixation in at least one hemifield |
| Indeterminate | Inability of patient to perform visual field testing, unreliable/uninterpretable visual field test results |
HVFs with >33% false-positives, false-negatives and fixation losses were considered unreliable. Any HVF that met the reliability criteria but whose pattern was non-glaucomatous (eg, cloverleaf pattern or lens rim artefact) was categorised as ‘indeterminate’. The person-level severity was defined as the severity of the worse eye.
The best corrected Snellen visual acuity of each eye was also collected, and a dichotomous variable was created at the patient level for acuity ≥20/50 or <20/50 in either eye.
The Rapid Estimate of Adult Literacy in Medicine (REALM), a 2–3 minute word-recognition test, was administered at baseline to identify patients with limited reading skills and to estimate patient reading levels.19 The sum score ranges from 0 to 66 and counts the number of words pronounced correctly by the patient. The score was dichotomised into high literacy level (≥61 and roughly equivalent to a high school reading level) and low literacy level (≤60).
To assess VRQoL, subjects were interviewed in person at baseline using the National Eye Institute’s Visual Function Questionnaire-25 (VFQ-25).20 This instrument is designed to measure various aspects of self-reported vision-targeted health statuses that are most important for people with chronic eye diseases, and has been validated in patients with glaucoma.21 The overall composite score is the mean of 11 of the subscales, excluding general health. At the end of the 3-year study period, subjects were recontacted by telephone to repeat the VFQ-25. Subjects’ charts were also reviewed to determine whether they had any intensification in their treatment, including increased medication use, laser therapy or surgery, or any progression on their HVF.
Statistical analysis was completed with SAS V.9.4. Descriptive statistics and plots were used to describe demographics and VFQ-25 scores by adherence status. In particular, the standardised mean differences (SMD) were used to quantify how patients who were adherent or non-adherent differed on the VFQ-25 at baseline and after 3 years. The SMD is the difference of sample means for two variables divided by the square root of the summed sample variances divided by 2. Unlike P values, this quantity indicates standardised differences regardless of sample size and is often used to quantify differences between non-randomised groups. Interpreting the results of SMD in a clinical setting may be more useful than P values alone for this study as they indicate both the magnitude and direction of the change in the two groups. In the literature, an SMD greater than or equal to 0.1 is considered a meaningful difference between two groups.22 Finally, unadjusted and adjusted generalised linear model regression was performed to evaluate the association between the composite VFQ-25 and adherence. Only covariates that were potential confounders because they reached a P value <0.10 and/or had an effect size >0.10 in univariate analysis were included in the adjusted model. In order to allow comparison of the strength of the association between each of the covariates and the composite VFQ-25 score, effect sizes were also calculated by dividing the point estimate by the SD.
Results
A total of 83 subjects were recruited and 95.18% (n=79/83) returned the MEMS device after 3 months. The four subjects who did not return their MEMS device had the following characteristics: gender (4 male), race (3 non-Hispanic white, 1 black or African–American) and age (52, 62, 71 and 75 years). Participants who returned the MEMS device took an average of 79.74% (SD 25.35) of prescribed glaucoma medication doses. Thirty per cent (n=24/79) of the participants took less than 80% of prescribed doses of their glaucoma medications. A majority of subjects saw 20/20–20/30 in at least one eye (94.94%, n=75/79). Clinical and demographic variables are presented stratified by adherence group in table 2.
Table 2.
Baseline demographic and clinical variables by medication adherence*
| Variable | ≥80% adherence | <80% adherence | Total | P value† |
| Male, n (%) | 53 (96.36) | 21 (87.50) | 74 (93.67) | 0.16 |
| White, n (%) | 24 (43.64) | 6 (25.00) | 30 (37.97) | 0.14 |
| Visual field‡ baseline severity: uninterpretable, n (%) | 5 (9.80) | 4 (18.18) | 9 (12.33) | 0.69 |
| Visual field baseline severity: severe, n (%) | 16 (31.37) | 5 (22.73) | 21 (28.77) | |
| Visual field baseline severity: moderate, n (%) | 14 (27.45) | 5 (22.73) | 19 (26.03) | |
| Visual field baseline severity: mild, n (%) | 16 (31.37) | 8 (36.36) | 24 (32.88) | |
| Baseline visual acuity ≥20/50 in either eye, n (%) | 14 (25.45) | 6 (25.00) | 20 (25.32) | 1.0 |
| Subject age, mean (SD) | 67.78 (8.79) | 63.96 (10.10) | 66.62 (9.31) | 0.11 |
| Number of medications, mean (SD) | 2.37 (1.15) | 1.92 (1.14) | 2.23 (1.16) | 0.10 |
| Number of days monitored, mean (SD) | 176.5 (44.37) | 212.7 (93.13) | 187.5 (64.81) | 0.33 |
| REALM score§, mean (SD) | 60.98 (6.56) | 57.73 (13.68) | 60.03 (9.25) | 0.50 |
There was one subject missing medication data and four subjects missing REALM data.
*≥80% of prescribed doses taken according to electronic monitor; <80% of prescribed doses taken according to medication monitor.
†P value from χ2 for categorical variables and t-test for continuous variables.
‡Visual fields with >33% fixation losses, false-positives or false-negatives and/or with artefact such as a cloverleaf pattern or rim defect were labelled uninterpretable.
§REALM, Rapid Estimate of Adult Literacy in Medicine, ranges 0–66, >60 is equivalent to ninth-grade reading level or above.
A scatterplot of the VFQ-25 composite score against the per cent medication adherence showed a cluster above the 80% cut-off for adherence (online supplementary figure 1). Boxplots demonstrated that the VFQ-25 composite score was greater for those taking ≥80% versus <80% of prescribed doses (online supplementary figure 2). Non-adherent subjects had lower baseline mean composite VFQ-25 scores (70.66±20.50 vs 75.91±19.12, SMD=0.27). Non-adherent subjects also had lower VFQ-25 subscale scores, with SMD of greater than 0.1 in all subscales except for driving and colour vision (table 3).
Table 3.
Baseline Visual Function Questionnaire scores, mean (SD)
| Subscale | ≥80% adherence | <80% adherence | Total | Standardised difference |
| Composite | 75.91 (19.12) | 70.66 (20.50) | 74.32 (19.57) | 0.27 |
| Peripheral vision | 71.36 (26.10) | 65.63 (25.34) | 69.62 (25.84) | 0.22 |
| General vision | 68.73 (17.96) | 64.17 (19.54) | 67.34 (18.45) | 0.24 |
| Colour vision | 83.65 (23.68) | 81.52 (28.42) | 83.00 (25.05) | 0.08 |
| Ocular pain | 78.86 (22.81) | 72.40 (22.11) | 76.90 (22.65) | 0.29 |
| Near activities | 73.41 (22.57) | 64.06 (25.29) | 70.57 (23.67) | 0.39 |
| Distance activities | 78.11 (24.78) | 72.74 (21.03) | 76.48 (23.70) | 0.23 |
| Social functioning | 86.59 (19.22) | 80.21 (26.04) | 84.65 (21.55) | 0.28 |
| Mental health | 74.09 (27.17) | 66.93 (30.48) | 71.91 (28.22) | 0.25 |
| Role difficulties | 72.95 (27.93) | 69.79 (31.48) | 71.99 (28.88) | 0.11 |
| Dependency | 83.94 (23.34) | 80.90 (27.42) | 83.02 (24.51) | 0.12 |
| Driving | 64.71 (34.43) | 62.50 (31.93) | 64.08 (33.54) | 0.07 |
| General health | 46.82 (21.55) | 36.46 (26.56) | 43.67 (23.50) | 0.43 |
bmjophth-2017-000114supp001.jpg (36.6KB, jpg)
bmjophth-2017-000114supp002.jpg (32.3KB, jpg)
Subjects were asked about their use of eye-drops. Medication adherence ≥80% using the MEMS device was significantly associated with answering ‘very confident’ to the question ‘How confident are you that you can carry out the following task—always remembering to use your glaucoma medications’ (P=0.01), and was also associated with answering ‘No’ to the question ‘In the past four weeks, did you ever forget to take your medicine?’ (P=0.08).
Subjects with good adherence had approximately a five-unit increase in composite VFQ-25 score compared to those with poor adherence, in both unadjusted and adjusted models, although this relationship was underpowered to reach statistical significance (table 4). The effect size for good adherence was approximately half as large as the effect size for better visual acuity (0.27 vs 0.68), and two-thirds as large as the effect size for mild versus moderate or severe visual field severity (0.27 vs 0.45) in unadjusted analysis, and this effect size remained similar after controlling for visual acuity and visual field severity in multivariate analysis. Only visual acuity was significantly associated with composite VFQ-25 in the unadjusted and adjusted models, independent of visual field or medication adherence (table 4). Age, gender, race and REALM score had neither a large effect size (<0.10) nor a potentially significant P value (all P>0.40) in univariate analysis, and thus were not included in the adjusted model.
Table 4.
Unadjusted and adjusted generalised linear models of Visual Function Questionnaire-25 composite score regressed over per cent adherence and covariates at baseline (n=79)
| Characteristics | Unadjusted estimate (95% CI) |
Unadjusted effect size* | Unadjusted P value | Adjusted estimate (95% CI) | Adjusted effect size* | Adjusted P value |
| Percent adherence: ≥80% vs <80% | 5.26 (0.48 to 10.04) | 0.27 | 0.27 | 4.98 (0.20 to 9.76) | 0.25 | 0.30 |
| Visual field: mild vs severe/moderate | 8.89 (3.97 to 13.81) | 0.45 | 0.08 | 7.54 (2.68 to 12.40) | 0.39 | 0.13 |
| Visual field : uninterpretable† vs severe/moderate | 12.26 (5.23 to 19.29) | 0.63 | 0.09 | 15.22 (8.26 to 22.18) | 0.78 | 0.03 |
| Visual acuity <20/50 vs ≥20/50 in either eye | 13.24 (8.37 to 18.11) | 0.68 | <0.01 | 11.77 (6.59 to 16.95) | 0.60 | 0.03 |
| Age (years) | 0.01 (−0.23 to 0.25) | 0.0 | 0.98 | |||
| Gender: male vs female | 1.75 (−7.34 to 10.85) | 0.09 | 0.85 | |||
| Race: white vs non-white | −1.04 (−5.61 to 3.52) | −0.05 | 0.82 | |||
| REALM score | 0.18 (0.48 to 10.04) | 0.01 | 0.45 |
*Effect size was calculated by dividing the point estimate by the SD.
†Visual fields with >33% fixation losses, false-positives or false-negatives and/or with artefact such as a cloverleaf pattern or rim defect were labelled uninterpretable.
REALM, Rapid Estimate of Adult Literacy in Medicine.
The mean composite score remained relatively stable for both the adherent and non-adherent subjects, with adherent subjects generally maintaining higher VFQ-25 scores at both time-points. When evaluating subjects who completed the study, subjects with good baseline adherence showed an increase, while those with poor adherence showed a decrease at 3 years for the ocular pain, peripheral vision, general vision and near activities subscales (online supplementary table 1). Trajectory plots of the VFQ-25 score and subscales were plotted for the 47 adherent and 15 non-adherent subjects who completed the VFQ-25 survey both at baseline and at 3 years (figure 1). There was no significant difference in intensification of medication, laser or surgical therapy (P=0.75), or visual field progression (P=0.50) between the two adherence groups at 3 years using 80% (online supplementary table 2) as well as several lower cut-offs for adherence (online supplementary table 3).
Figure 1.
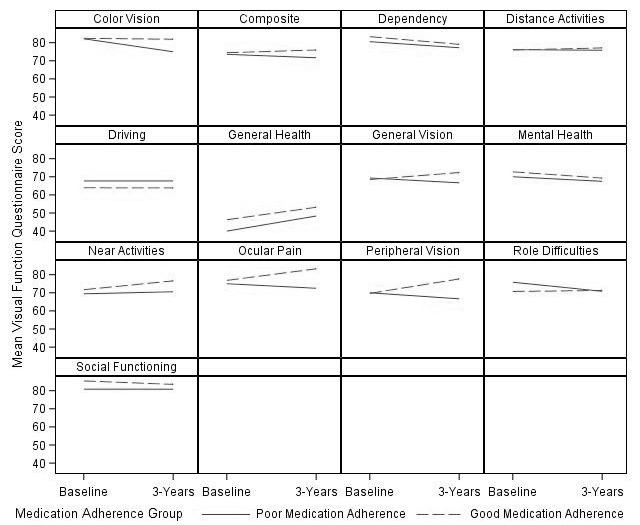
Plots of mean visual function subscale and composite scores at baseline and after 3 years among subjects who completed the study.
bmjophth-2017-000114supp003.docx (25.7KB, docx)
Discussion
Our study suggests that medication non-adherence measured with a MEMS device may be a marker of poor long-term VRQoL in patients with glaucoma. Patients who took less than 80% of prescribed doses of their glaucoma drops had lower mean VQF-25 scores when compared with those who took at least 80% of their prescribed doses both at baseline and at 3 years. In adjusted analysis, baseline visual field severity and visual acuity were more strongly associated with VRQoL than medication adherence. However, the effect size for medication adherence remained fairly stable despite adjustment for visual field severity and visual acuity, which suggests that it may uniquely contribute to VRQoL.
Quality of life is an important patient-centred metric that is of significant interest in the field of glaucoma. We observed that electronically measured adherence was associated with VRQoL, with an effect size approximately half as large as that of visual acuity and two-thirds the size of that of visual field severity in both unadjusted and adjusted analyses. The relationship was not statistically significant due to the modest sample size, but still warrants attention given the strength of the association. Our finding is similar to that of two prior studies that were limited by their use of self-reported medication adherence. In one cross-sectional study by Loon et al11, patients with glaucoma in Singapore were interviewed about their adherence to glaucoma medications and their quality of life was evaluated. Non-adherent patients were more likely to have lower quality of life (P=0.014). Balkrishnan et al analysed mailed self-administered surveys on medication-taking behaviours and health-related quality of life among Medicare beneficiaries with primary OAG. They found that self-reported difficulty using eye-drops was associated with lower health-related quality of life (P<0.05).12 Self-reported adherence tends to overestimate true medication-taking behaviour.23 Physician reports of non-adherence likewise do not correlate well with electronic non-adherence.23 We have also previously shown that medication possession ratio (MPR) based on pharmacy records is not an accurate representation of medication usage.17 The mean MPR in our Veterans Affairs population is greater than 1.5, likely due to frequent refills ordered by physicians during follow-up visits. Other groups have similarly found that the MPR for other chronic diseases, like hypertension, is high among veterans.24 Electronic measurement tools, such as the MEMS device that we used, provide a more objective means of estimating how adherent patients are to their medication schedule,3 14 23 although even MEMS can overestimate adherence if patients fail to properly instil their eye-drops. We also sought to confirm whether those with poor baseline medication adherence would continue to have poor VRQoL after a 3-year interval. To our knowledge, our study is the first to show that electronically measured non-adherence with eye-drops may be associated with poor long-term VRQoL in patients with glaucoma.
Whether patients with worse VRQoL are less likely to take their medications or whether non-adherent behaviour leads to poor VRQoL is not clear. In our study, subjects with poor adherence to their eye-drops had lower VFQ-25 scores at baseline compared with those with good medication adherence. Those subjects with poor baseline adherence also persisted in having lower VFQ-25 scores after 3 years. This finding may suggest that poor adherence may be a risk factor for long-term poor VRQoL. In previous studies, patients who self-reported difficulty using their eye-drops were more likely to have lower VRQoL scores.12 It is possible that poor adherence to drops over time could lead to worse control of intraocular pressure on average, which could put one at risk for glaucomatous progression. Medication non-adherence is also associated with patterns of non-adherence to scheduled follow-up appointments for glaucoma, which could further increase one’s risk of progression and adversely impact VRQoL.6
In our study, baseline visual field severity (mild vs moderate/severe) was associated with the VFQ-25 composite score (P=0.08) with a fairly large effect size. However, it was not associated with adherence (P=0.69), and did not substantially confound the relationship between adherence and the VFQ-25 score. Nevertheless, subjects with poor medication adherence self-reported lower VRQoL on the peripheral visual field subscale both at baseline and at 3 years (SMD>0.10). In fact, while the mean peripheral vision score slightly improved at 3 years for those with good adherence, it declined for those with poor medication adherence even though neither treatment intensification nor progression of glaucomatous visual field loss significantly differed between the adherent and non-adherent patients at the end of the study (P>0.05). Thus, patients’ subjective perception of peripheral visual dysfunction on the VFQ-25 did not correspond to their objective performance on visual field testing. These findings surprised us as several other groups have suggested that declines in VRQoL parallel objective measures of glaucoma progression. In one study, greater visual field defects were associated with worse scores on the VFQ-25 and VF-14 (P<0.05).25 Location of visual field loss has also been shown to differentially impact quality of life in glaucoma, with superior field loss impacting near activities and inferior field loss impacting general and peripheral vision quality of life scores.9 Progressive retinal nerve fiber layer (RNFL) loss has been shown to be associated with longitudinal decreases in quality of life, even after adjusting for visual field deficits.10 We may have been underpowered to detect a statistically significant association between visual field severity and VFQ-25 composite score. Psychosocial influences may also partly explain the discrepancy between the objective severity of visual field loss and subjective peripheral vision score in our study. For example, subjects who did not take their eye-drops may have under-reported their visual function on the questionnaire. A 3-year follow-up period may also be insufficient to detect significant glaucomatous progression.
In our study, the ocular pain subscale was also consistently lower at baseline and after 3 years for subjects with poor adherence to eye-drops. The VFQ-25’s ocular pain subscale has previously been shown to be significantly related to abnormal tear film break-up time.26 Ocular surface disease can be exacerbated in patients with glaucoma by chronic exposure to preservatives or allergens in their eye-drops, which may deter subjects from taking their eye-drops as prescribed.
There are several limitations to this study. This was a small sample of patients drawn from a single Veterans Affairs medical centre, which limits our power and generalisability. Although medication adherence had a smaller relative effect size, adjustment for visual acuity and visual field did not substantially alter the point estimate, which suggests that it may contribute uniquely to VRQoL. Because this was a modest sample, we also did not control for other factors that may impact VRQoL, such as media opacity or other ocular pathology, and instead corrected for visual acuity. However, best corrected visual acuity did not significantly differ between the two groups at baseline and a minority of patients saw 20/50 or worse in one eye. By including patients with other ocular diseases, our results more closely approximate the true ophthalmic experiences of patients with glaucoma who often have numerous reasons for limited vision. Because patients needed to be able to complete vision-related tasks, such as use of the MEMS device, we excluded patients whose vision was worse than 20/70 in both eyes. Thus, the findings are not relevant to patients with bilaterally very low vision. It is possible that subjects in this study modified their behaviour because they were being monitored, that is, the Hawthorne effect. However, such an effect would tend towards an underestimation of the degree of non-adherence, and thus bias the findings towards the null. Defining adherence using a particular cut-off may not reflect the range of non-adherence that exists among patients with glaucoma. The decision to use an 80% cut-off was based on the current literature, as well as driven by the data. The mean adherence rate was 79.8% (SD 25.3%), and dichotomising at 80% generated a 30% prevalence of non-adherence, which is similar to what others have found.27 An 80% adherence using MEMS was also significantly associated with patient self-report on adherence. Longer, larger studies are needed with longitudinal medication adherence data to determine how changes in medication adherence impact disease progression over time.
Conclusions
The effect size for the association between electronically measured non-adherence to glaucoma medications and lower VRQoL scores is approximately half the effect size of visual acuity and two-thirds the effect size of visual field severity, but was not statistically significant due to limited sample size. Non-adherence to eye-drops may be associated with the ocular pain and peripheral vision VFQ-25 subscales. Visual field severity and visual acuity are strongly associated with VRQoL in patients with glaucoma. Future evaluations of interventions to improve adherence to glaucoma eye-drops should assess the impact on VRQoL measures.
Footnotes
Contributors: ACT: study design, data collection, manuscript preparation and revision, manuscript submission. SW: study design, statistical analysis, manuscript revision. MKO: study design, statistical analysis, manuscript revision. SD: study design, data collection. HBB: study design, manuscript revision. KWM: study design, data collection, manuscript revision.
Funding: This research was funded by the VA Health Services Research & Development Career Development Award (VA HSR&D 10/019/2) (KWM) and the American Glaucoma Society Clinician Scientist Award (KWM). HBB is supported by a Senior Career Scientist Award (VA HSR&D 08/027).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study was approved by the Institutional Review Board of the Durham Veterans Affairs Medical Center and was compliant with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. . Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–51. doi:/S0042-96862004001100009 [PMC free article] [PubMed] [Google Scholar]
- 2.Heijl A, Leske MC, Bengtsson B, et al. . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of ophthalmology 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, Wolfs RC, O’Colmain BJ, et al. . Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol 2004;122:532–8. doi:10.1001/archopht.122.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SC, Spaeth GL. Compliance in patients prescribed eye drops for glaucoma. Ophthalmic Surg. May 1995;26:233–6. [PubMed] [Google Scholar]
- 5.Muir KW, Christensen L, Bosworth HB. Health literacy and glaucoma. Curr Opin Ophthalmol 2013;24:119–24. doi:10.1097/ICU.0b013e32835c8b0e [DOI] [PubMed] [Google Scholar]
- 6.Lee BW, Sathyan P, John RK, et al. . Predictors of and barriers associated with poor follow-up in patients with glaucoma in South India. Arch Ophthalmol 2008;126:1448–54. doi:10.1001/archopht.126.10.1448 [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez P, et al. . Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthal 1997;115:777–84. doi:10.1001/archopht.1997.01100150779014 [DOI] [PubMed] [Google Scholar]
- 8.Medeiros FA, Gracitelli CP, Boer ER, et al. . Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122:293–301. doi:10.1016/j.ophtha.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HC, Guo CY, Chen MJ, et al. . Patient-reported vision-related quality of life differences between superior and inferior hemifield visual field defects in primary open-angle glaucoma. JAMA Ophthalmol 2015;133:269–75. doi:10.1001/jamaophthalmol.2014.4908 [DOI] [PubMed] [Google Scholar]
- 10.Gracitelli CP, Abe RY, Tatham AJ, et al. . Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol 2015;133:384–90. doi:10.1001/jamaophthalmol.2014.5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loon SC, Jin J, Jin Goh M. The relationship between quality of life and adherence to medication in glaucoma patients in Singapore. J Glaucoma 2015;24:e36–e42. doi:10.1097/IJG.0000000000000007 [DOI] [PubMed] [Google Scholar]
- 12.Balkrishnan R, Bond JB, Byerly WG, et al. . Medication-related predictors of health-related quality of life in glaucoma patients enrolled in a medicare health maintenance organization. Am J Geriatr Pharmacother 2003;1:75–81. doi:10.1016/S1543-5946(03)90003-1 [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR, et al. . A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 14.Lichter P, et al. . Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943–53. doi:10.1016/S0161-6420(01)00873-9 [DOI] [PubMed] [Google Scholar]
- 15.Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma 2012;21:234–40. doi:10.1097/IJG.0b013e31821dac86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleath B, Blalock S, Covert D, et al. . The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology 2011;118:2398–402. doi:10.1016/j.ophtha.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar JB, Bosworth HB, Sleath B, et al. . Quantifying glaucoma medication adherence: the relationship between self-report, electronic monitoring, and pharmacy refill. J Ocul Pharmacol Ther 2016;32:346–54. doi:10.1089/jop.2015.0102 [DOI] [PubMed] [Google Scholar]
- 18.Ophthalmology AAo. American academy of ophthalmology preferred practice pattern primary open angle glaucoma. 2015.
- 19.Davis TC, Long SW, Jackson RH, et al. . Rapid estimate of adult literacy in medicine: a shortened screening instrument. Family medicine 1993;25:391–5. [PubMed] [Google Scholar]
- 20.Nassiri N, Mehravaran S, Nouri-Mahdavi K, et al. . National Eye Institute Visual Function Questionnaire: usefulness in glaucoma. Optom Vis Sci 2013;90:745–53. doi:10.1097/OPX.0000000000000003 [DOI] [PubMed] [Google Scholar]
- 21.Mangione CM, et al. . Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthal 2001;119:1050–8. doi:10.1001/archopht.119.7.1050 [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. doi:10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okeke CO, Quigley HA, Jampel HD, et al. . Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology 2009;116:191–9. doi:10.1016/j.ophtha.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Thorpe CT, Bryson CL, Maciejewski ML, et al. . Medication acquisition and self-reported adherence in veterans with hypertension. Med Care 2009;47:474–81. doi:10.1097/MLR.0b013e31818e7d4d [DOI] [PubMed] [Google Scholar]
- 25.McKean-Cowdin R, Wang Y, Wu J, et al. . Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008;115:941–8. doi:10.1016/j.ophtha.2007.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi GC, Pasinetti GM, Scudeller L, et al. . Ocular surface disease and glaucoma: how to evaluate impact on quality of life. J Ocul Pharmacol Ther 2013;29:390–4. doi:10.1089/jop.2011.0159 [DOI] [PubMed] [Google Scholar]
- 27.Cate H, Bhattacharya D, Clark A, et al. . Patterns of adherence behaviour for patients with glaucoma. Eye 2013;27:545–53. doi:10.1038/eye.2012.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2017-000114supp001.jpg (36.6KB, jpg)
bmjophth-2017-000114supp002.jpg (32.3KB, jpg)
bmjophth-2017-000114supp003.docx (25.7KB, docx)


