Abstract
Relationships between heart rate recovery after exercise (HRR, baseline heart rate variability measures (HRV), and time to perform a 10Km running trial (t10Km) were evaluated in "master" athletes of endurance to assess whether the measured indexes may be useful for monitoring the training status of the athletes. Ten “master” athletes of endurance, aged 40-60 years, were recruited. After baseline measures of HRV, the athletes performed a graded maximal test on treadmill and HRR was measured at 1 and 2 minutes from recovery. Subsequently they performed a 10Km running trial and t10Km was related to HRV and HRR indexes. The time to perform a 10Km running trial was significantly correlated with baseline HRV indexes. No correlation was found between t10Km and HRR. Baseline HRV measures, but not HRR, were significantly correlated with the time of performance on 10km running in “master” athletes. The enhanced parasympathetic function at rest appears to be a condition to a better performance on 10km running. HRV can be simple and useful measurements for monitoring the training stratus of athletes and their physical condition in proximity of a competition.
Key Words: heart rate recovery, heart rate variability, autonomic nervous system, endurance training, master athletes
Ethical Publication Statement
Institutional Review Board that approved the protocol for the study: Sport and Exercise Sciences Research Unit, University of Palermo, Italy.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The heart rate (HR) is controlled by the autonomic nervous system that, through the sympathetic and parasympathetic innervations, reaches the heart performing its function of excitation/inhibition, depending on the metabolic demands of the organism1,2. The heart rate variability (HRV) and the heart rate recovery after exercise (HRR) are both indirect evaluation marker of autonomic control of the heart3. The HRV describes the variations in the intervals between successive heartbeats and it can easily be determined from short-term ECG recording using methods of analysis in the time domain or frequency domain.4 Recently, instruments such as heart rate monitors are capable of recording intervals between two heartbeats (RR) for HRV analysis in sports sciences and medicine5. The analysis in the time domain provides the assessment of RR. The most commonly used measures derived from interval differences include the rMSSD (square root of the mean squared differences of successive RR intervals), which can be easily calculated during a short term recording4. The rMSSD expresses the levels of parasympathetic activity4 and has much greater reliability than other spectral indices6. It can be used to monitor the training status of endurance athletes7,8. The analysis in the frequency domain provides various spectral methods4. Three main spectral components are distinguished in a spectrum calculated from short-term recordings: very low frequency (VLF), low frequency (LF) and high frequency (HF) components; generally HF is expression of the parasympathetic, and LF of the sympathetic activity4. VLF represents a dubious measure and should be avoided when interpreting the power spectral density analysis4. The ratio LF/HF allows us to evaluate the cardiac sympatho-vagal balance: a value < 2 is generally associated with better cardiorespiratory fitness and exercise performance9,10. The HRR is defined as the rate at which the HR decreases, usually in a few minutes, after the end of an exercise, and is a result of reactivation of the parasympathetic system followed by reduction in the activity of the sympathetic system11. HRR at 1 and 2 minutes after maximal exercise test represent an important parameter to estimate the impaired autonomic function and is and a predictor of cardiovascular fitness12. It is also related to coronary artery disease13 and a very important marker of cardiovascular risk and mortality14,15. Moreover, several studies indicate that the HRR evaluation can be helpful in distinguishing a trained from a sedentary individual16. Recent reviews indicate that the HRR is sensitive to changes in the training status in athletes, and may be used as a valuable tool to monitor and optimize training programs17. The aim of this study was to assess, in "master" endurance athletes, the relationships between baseline HRV and HRR measures and the time to perform 10Km running, in order to evaluate adaptation to training, and to assess whether the measured parameters may be useful for monitoring the training status of the athletes.
Materials and Methods
Participants
The study was conducted on 10 male athletes aged between 40 and 60 years, who practice the specialty of endurance in club affiliated to the Italian Athletic Federation (FIDAL). At recruitment time, all enrolled athletes were at the top of the regional rankings in the “master” category, and all of them have a competitive experience of more than 20 years, training for 8/10 hours a week on average. Among the inclusion criteria it was required: to be "master" athletes aged between 40 and 60 years; to have a regular medical certificate for the current year; to practice specialties of endurance; to be at least moderately trained and in good physical status; to have several years of experience in endurance sports. Each athlete gave written informed consent to participate, following by an explanation of the nature and purpose of the study. The procedures were conducted in compliance with the Declaration of Helsinki and were approved by the IRB Committee at our Institution.
Measures
Every athlete has made the assessments on the same day in the Laboratory of Functional Assessment of our Institution, between 8 and 10 a.m., with the maintenance of the standard ambient conditions (temperature 22 ± 0.5 °C, humidity 55%). Before starting the evaluations, height and weight were measured using a stadiometer and an electronic scale (SECA, Germany). Body composition was assessed using an Akern Soft Tissue Analyzer with Bodygram software and the amount of body lean mass and the percentage of fat mass were calculated.
Procedures
The evaluations included baseline RHR measure and HRV analysis, followed by a maximal graded test for the evaluation of aerobic power (VO2max), ventilatory anaerobic threshold (VO2VAT), and heart rate recovery at 1 (HRR1) and 2 minutes (HRR2) after the end of the test. No athlete has been practicing a training session the day before the test, and we asked them to avoid taking food or drink coffee in the last three hours before the test. The day after the laboratory evaluations, every athlete performed an outdoor running trial over the distance of 10 Km, and the time of performance (t10km) was collected. The measurements of HRV parameters were performed with the athlete in supine position on an examination table for 10 minutes, during which RR interval recordings were obtained using a portable heart rate monitor (Polar V800, Polar, Finland)18. The last 5 minutes of RR recording were analyzed by means of Kubios HRV 2.2 software (Department of Applied Physics, University of Kuopio, Finland)19. The Kubios HRV software provides a wide variety of time-domain, frequency-domain and nonlinear HRV parameters. From the autoregressive power spectrum approach in the frequency domain, we got the powers of the very low frequency (VLF: < 0.04 Hz), low frequency (LF: from 0.04 to 0.15 Hz), and high frequency (HF: 0.15 to 0.40 Hz) components, in absolute (ms2) and in normalized units (nu) [LFnu: 100 x LF / (total power – VLF), and HFnu: 100 x HF / (total power - VLF)]2. From the values of LF and HF it was determined the LF/HF ratio. Through the statistical methods from the analysis in the time domain we got the mean squared differences of successive RR intervals (rMSSD). The validity of HRV parameters derived from V800 Polar heart rate monitor, e.g. small bias, strong ICC (≥ 0.99) and small ES (≤ 0.029), was confirmed by a recent study of Giles et al.18 The reference values of the indices of HRV in healthy untrained subjects are given in the review of Nunan et al.20 To assess the state of cardiorespiratory fitness, all subjects performed an incremental exercise test to exhaustion on motorized treadmill (Cosmed T-150). The protocol included:
Initial warm-up period lasting 4 minutes at 4 km / h;
1° step running at speed of 7 km / h and gradient of 2% for one minute;
Successive increments, for each minute of exercise, of 1 km / h of speed and 1% of inclination, until reaching the VO2max.
A disposable face mask with a dead space of 50-60 ml (Cosmed V2) was used to collect the exhaled air throughout the test. The oxygen consumption (VO2 in ml ∙ kg-1 ∙ min-1) was recorded with a breath by breath measurement system (Cosmed Quark CPET). VO2max was calculated as the highest consecutive 30-s average value achieved during the test. The ventilator anaerobic threshold (VO2VAT) was measured with V-slope method21. The flow meter and gas analyzers were calibrated before each test, according to the manufacturer's instructions.
The heart rate (HR) was recorded using a short-range radio system telemetry (Polar Electro Oy, Finland). The guidelines about the interruption of the tests provided by the American College of Sports Medicine were met22. VO2max was considered attained when one or more of the following criteria were achieved: attainment of a VO2max plateau < 2.2 ml ∙ kg-1 ∙ min-1; respiratory exchange ratio (RER) > 1.10; maximum heart rate (HRmax) close to the theoretical value of 220-age; athlete's inability to maintain the required work rate. The cool-down period after the end of the exercise consisted on walking on treadmill at 2.0 Km/h of speed and 0% grade for 6 minutes. Heart rate recovery was defined as the difference between heart rate at peak of exercise and heart rate at 1 (HRR1) and at 2 (HRR2) minutes after the end of the exercise, expressed in absolute terms (bpm).
Statistical analyses
All data are presented as mean ± standard deviation (s). A simple linear regression model was used to assess the relationships between t10Km and the results of the incremental exercise test. Pearson product moment correlation coefficient (r) was used to determine the association between considered parameters. The 95% confidence intervals (CI) were determined for each correlation coefficient and P value less than 0.05 was considered statistically significant. For the statistical analysis, GraphPad Prism 6.01 (GraphPad Software, Inc., USA) software was used.
Results
Enrolled athletes had mean age of 52.1 ± 6.4 years, weight of 69.3 ± 7.0 Kg, height of 171.3 ± 4.8 cm, and BMI of 23.6 ± 1.9. The others physiological parameters of athletes are shown in table 1. In our master athletes we found out sinus bradycardia at rest, with RHR of 49.6 ± 7.1 bpm. The athletes have been able to regularly end the test. Based on the intensity of the effort, they all have reached their VO2max. In all athletes we have detected high VO2max values, on average 52.7 ± 7.2 ml ∙kg-1 ∙min-1, corresponding to 138% of the theoretical VO2max, predicted with specific reference to age and sex23; 3 athletes have exceeded the value of 150%. Of particular note is the finding in a 47-year old athlete who reached a VO2max of 68.9 ml ∙kg-1 ∙ min-1, comparable to that of younger athletes of high national qualification. In the subjects we examined, the HRR1 averaged 25.3 ± 7.9 bpm and HRR2 averaged 53.6 ± 8.0 bpm; in one athlete we discovered a value of HRR1 lower than the standard (11 bpm), while his HRR2 was found in the standard (49 bpm). The HRV analysis showed rMSSD values of 34.8 ± 22.5 ms, LF and HF respectively 57.7 ± 22.2 and 31.6 ± 24.3 nu. The LF/HF ratio was 3.1 ± 2.1; 2/10 of the athletes had a value of LF/HF <1.0, whereas 7/10 had a LF/HF > 2.0. The statistical regression analysis showed significant correlations between t10Km and indexes of HRV (rMSSD, r = -0.710, p = 0.021; LF, r = 0.713, p = 0.020; HF, r = -0.700, p = 0.024) (Fig.1-3). Slight but not significant correlation was found between t10Km and LF/HF (r = 0.545, p = 0.103). As expected, we found out a significant correlation between t10Km and VO2VAT (r = -0.703, p = 0.023) and VO2max (r = -0.682, p = 0.029). No correlations were found out between t10Km and RHR, HRR1 and HRR2. All the considered parameters and their correlations with t10Km are shown in table 2.
Table 1.
Characteristics of athletes (n = 10; means ± s).
| Age (y) | 52.1 ± 6.4 |
|---|---|
| Body height (cm) | 171.3 ± 4.8 |
| Body weight (Kg) | 69.3 ± 7.0 |
| BMI (Kg ∙ m-2) | 23.6 ± 1.9 |
| Fat mass (%) | 13.7 ± 3.3 |
| Fat free mass (Kg) | 59.9 ± 6.4 |
| VO2rest (ml ∙ Kg-1 ∙ min-1) | 5.7 ± 2.3 |
| RHR (bpm) | 49.6 ± 7.1 |
BMI body mass index, VO2rest oxygen uptake at rest, RHR heart rate at rest
Table 2.
Indices of considered parameters (means ± s) and their correlation with 10Km running performance (r and P value).
| (n = 10) | r | P | |
|---|---|---|---|
| RHR | 49.6 ± 7.1 | 0.532 | 0.113 |
| LF (nu) | 57.7 ± 22.2 | 0.713 | 0.020 (*) |
| HF (nu) | 31.6 ± 24.3 | -0.700 | 0.024 (*) |
| LF/HF | 3.1 ± 2.1 | 0.545 | 0.103 |
| rMSSD (ms) | 34.8 ± 22.5 | -0.710 | 0.021 (*) |
| VO2VAT (ml ∙ Kg-1∙ min-1) | 43.5 ± 7.9 | -0.703 | 0.023 (*) |
| VO2max (ml ∙ Kg-1∙ min-1) | 52.2 ± 7.6 | -0.682 | 0.029 (*) |
| HRmax (bpm) | 156.6 ± 9.8 | 0.029 | 0.935 |
| HRR1 (bpm) | 25.3 ± 7.9 | -0.379 | 0.279 |
| HRR2 (bpm) | 53.6 ± 8.0 | -0.580 | 0.078 |
| t10Km (sec) | 2294.5 ± 173.3 |
LF low frequency power, HF high frequency power, VO2VAT ventilator anaerobic threshold, VO2max maximal oxygen uptake, HRmax maximal heart rate, HRR1 heart rate recovery at 1 minute, HRR2 heart rate recovery at 2 minutes, t10Km time to performing 10 Km running. (*) significant correlation with t10Km.
Discussion
Training, especially of endurance, is responsible for significant adaptations of the cardiovascular system, which mainly lead to a reduction in heart rate and peripheral resistance, and an increase in heart size associated with improved contractile capacity24. However, wide heterogeneity has been observed in the responsiveness of cardiorespiratory fitness to physical training25, as adaptations to physical training are the result of many factors26.
In athletes involved in our study the most evident adaptations are represented by bradycardia at rest (49.6 ± 7.1 bpm) and good aerobic power, expressed by measured VO2max (52.7 ± 7.2 ml ∙ kg-1 ∙ min-1), considered excellent in reference to the mean age of the athletes (ACSM’s normative table for maximum aerobic power by age and gender 2013). Of note are the good levels of ventilatory anaerobic threshold, that are on average around 83% of VO2max (VO2vat = 43.5 ± 7.9 ml ∙ kg-1 ∙ min-1). Moreover, the status of the autonomic nervous system plays an important role in the training response. The HRV measurements in athletes have yielded conflicting values when compared with data in the literature7. In healthy active or sedentary people, the LF/HF ratio derived from the spectral analysis of 5 min electrocardiographic recording in the supine position is between 1.5 and 220. In literature, endurance training and high levels of aerobic fitness expressed by high values of VO2max are associated with an increase of parasympathetic component, shown by an increase of rMSSD and HF values20,27-29, with a consequent reduction of the LF/HF ratio, up to ≤ 1. The values of LF/HF ratio > 2 found in a large number of athletes we examined, expression of a sympatho-vagal balance with prevalence of the sympathetic component, may depend on the stress conditions due to the training loads, such as "over-reaching" and/or "over-training"8. The over-reaching is considered a drop in performance of acute exposure to excessive training loads without adequate recovery, regressing after a period of slightly longer of the usual rest (few days)21. The over-training is considered a performance decline caused by excessive training loads and/or adequate recovery, which persists for a long time (usually weeks or months) after a period of rest29. Both syndromes can be associated with increased sympathetic adrenergic activation, with an increase of RHR and a simultaneous reduction of HRR, and/or a reduction of the activity of the parasympathetic system30-32. Based on these considerations, the finding of a LF/HF > 2 in several master athletes we examined could depend on precisely conditions of over-training, although the RHR and the parameters obtained from exercise testing (VO2max and HRR) indicate a high level of aerobic fitness and normal behavior of the autonomic cardiac system after effort. The significant correlation between the indices of HRV and the performance of the athletes expressed by time on the 10km running trial may support this hypothesis. This correlation shows an influence of the autonomic system, measured at rest before the test, on the performance: a predominance of the parasympathetic system on the autonomic balance at rest appears to predispose to a better performance on10km running. Although several longitudinal studies support the capacity of HRR to quantify differences in training status between trained and untrained healthy individuals17, in our study no correlation was found between HRR and the performance on 10km running. Recently, Aubry et al.33 reported that faster HRR does not systematically predict better physical performance and that its interpretation should always be made in relation to the specific training phase of an endurance training program, the perceived fatigue level of athletes and the performance response. This confirm the hypothesis that changes in HRR indices are strongly related to weekly training loads, while changes in HRV indices are mainly associated with cardiorespiratory fitness as assessed by maximal oxygen uptake34. However, the effect of confounding factors such as state of fatigue (over-reaching and/or over-training) on HRR in master endurance athletes needs to be further investigated.
Our study provides the following information regarding heart rate measures that seem to be important for “master” athletes of endurance:
The analysis of heart rate variability is able to evaluate complex adjustments charged to the cardiac autonomic system caused by training;
rMSSD and HF are negatively correlated with t10Km. LF is positively correlated with t10Km;
The increased activity of the parasympathetic function at rest seems to be a condition for a better performance to run a distance of 10km.
Measuring RHR and HRR after exercise is a common and pratical method of assessing cardiorespiratory fitness and may provide a good indication of training state of athletes.7 HRV data are perhaps a slighty more sensitive measure for tracking changes in fatigue and readiness status to a higher performance. Although further investigations involving grater subject samples are needed to confirm our conclusions, the analysis of heart rate variability can thus be a simple and useful tool for monitoring the training status of athletes and their physical condition in proximity of a competition, whether they are young and old high-level amateur sportspeople or athletes ready to participate to Paralympics or Cybathlon sports competitions.35-51
Fig 1.
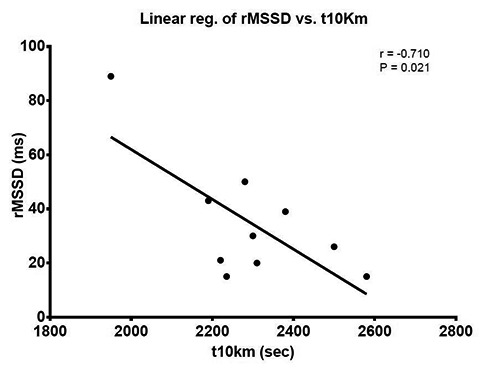
Relationship between time to run 10Km (t10Km) and parasympathetic activity expressed by the square root of the mean squared differences of successive RR intervals (rMSSD)
Fig 2.

Relationship between time to run (t10Km) and parasympathetic activity expressed by the high frequency power (HF)
Fig 3.

Relationship between time to run (t10Km) and sympathetic activity expressed by the low frequency power (LF)
Acknowledgments and Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
List of acronyms
- BMI
body mass index
- ECG
ElectroCardioGram
- HF
high frequency
- HR
heart rate
- HRR
heart rate recovery after exercise
- HRV
heart rate variability
- LF
low frequency
- RHR
heart rate at rest
- rMSSD
square root of the mean squared differences
- RR
recording intervals between two heartbeats
- VLF
very low frequency
- VO2max
maximal oxygen uptake
Contributor Information
Antonino Bianco, Email: antonino.bianco@unipa.it.
Antonio Paoli, Email: antonio.paoli@unipd.it.
Dario Cerasola, Email: cerada@icloud.com.
Saverio Alagna, Email: saverioalagna@virgilio.it.
Giuseppe Messina, Email: giuseppe.messina17@unipa.it.
Daniele Zangla, Email: daniele.zangla@unipa.it.
Marcello Traina, Email: marcello.traina@unipa.it.
References
- 1.La Rovere MT, Mortara A, Sandrone G, Lombardi F. Autonomic nervous system adaptations to short-term exercise training. Chest 1992;101(5 Suppl):299S-303S. [DOI] [PubMed] [Google Scholar]
- 2.La Rovere MT, Pinna GD, Maestri R, Sleight P. Clinical value of baroreflex sensitivity. Neth Heart J 2013;2:61-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achten J, Jeukendrup AE. Heart rate monitoring: applications and limitations. Sports Med 2003;33:517-38. [DOI] [PubMed] [Google Scholar]
- 4.Task Force. Heart rate variability . Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354-81. [PubMed] [Google Scholar]
- 5.Gamelin FX, Berthoin S, Bosquet L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med Sci Sports Exerc 2006;38:887-93. [DOI] [PubMed] [Google Scholar]
- 6.Al Haddad H, Laursen PB, Chollet D, et al. Reliability of resting and postexercise heart rate measures. Int J Sports Med 2011;32(8):598-605. [DOI] [PubMed] [Google Scholar]
- 7.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med 2003;33:889-919. [DOI] [PubMed] [Google Scholar]
- 8.Plews DJ, Laursen PB, Stanley J, et al. Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med 2013;43:773-81. [DOI] [PubMed] [Google Scholar]
- 9.Buchheit M, Gindre C. Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 2006;291:H451-8. [DOI] [PubMed] [Google Scholar]
- 10.Cataldo A, Zangla D, Cerasola D, et al. Influence of baseline heart rate variability on repeated sprint performance in young soccer players. J Sports Med Phys Fitness 2016;56:491-6. [PubMed] [Google Scholar]
- 11.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 2001;38:1980-7. [DOI] [PubMed] [Google Scholar]
- 12.Nanas S, Anastasiou-Nana M, Dimopoulos S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol 2006;110:393-400. [DOI] [PubMed] [Google Scholar]
- 13.Jae SY, Kurl S, Laukkanen JA, et al. Relation of heart rate recovery after exercise testing to coronary artery calcification. Ann Med 2017;49:404-410. [DOI] [PubMed] [Google Scholar]
- 14.Kokkinos P, Myers J, Doumas M, et al. Heart rate recovery, exercise capacity, and mortality risk in male veterans. Eur J Prev Cardiol 2012;19:177-84. [DOI] [PubMed] [Google Scholar]
- 15.Qiu S, Cai X, Sun Z, et al. Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. J Am Heart Assoc 2017;6(5). pii: e005505. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimpka U. Post-exercise heart rate recovery: an index of cardiovascular fitness. J Ex Physiol 2009;12:10-22. [Google Scholar]
- 17.Daanen HA, Lamberts RP, Kallen VL, et al. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int J Sports Physiol Perform 2012;7:251-60. [DOI] [PubMed] [Google Scholar]
- 18.Giles D, Draper N, Neil W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Eur J Appl Physiol 2016;116:563-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed 2014;113:210-20. [DOI] [PubMed] [Google Scholar]
- 20.Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol 2010;33:1407-17. [DOI] [PubMed] [Google Scholar]
- 21.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986;60:2020-2027. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sport Medicine. ACSM’s Guidelines for exercise testing and prescription. Lippincott Williams & Wilkins 9th ed. 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129(Suppl):S49-S55. [DOI] [PubMed] [Google Scholar]
- 24.Traina M, Cataldo A, Zangla D, et al. Il cuore dello sportivo, Chapter 20, in Principi di diagnostica per immagini in medicina dello sport. EdiSES, Italy: 2012. [Google Scholar]
- 25.Hautala AJ, Kiviniemi AM, Makikallio TH, et al. Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 2006;96:535-42. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Brown AM, Frontera WR. Principles of exercise physiology: responses to acute exercise and long-term adaptations to training. PM R 2012;4:797-804. [DOI] [PubMed] [Google Scholar]
- 27.Hedelin R, Bjerle P, Henriksson-Larsen K. Heart rate variability in athletes: relationship with central and peripheral performance. Med Sci Sports Exerc 2001;33:1394-8. [DOI] [PubMed] [Google Scholar]
- 28.Hautala AJ, Makikallio TH, Kiviniemi A, et al. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 2003;285:H1747-52. [DOI] [PubMed] [Google Scholar]
- 29.Meeusen R, Duclos M, Foster C, et al. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc 2013;45:186-205. [DOI] [PubMed] [Google Scholar]
- 30.Hynynen E, Uusitalo A, Konttinen N, Rusko H. Heart rate variability during night sleep and after awakening in overtrained athletes. Med Sci Sports Exerc 2006;38:313-7. [DOI] [PubMed] [Google Scholar]
- 31.Hynynen E, Uusitalo A, Konttinen N, Rusko H. Cardiac autonomic responses to standing up and cognitive task in overtrained athletes. Int J Sports Med 2008;29:552-8. [DOI] [PubMed] [Google Scholar]
- 32.Bosquet L, Merkari S, Arvisais D, Aubert AE. Is heart rate a convenient tool to monitor over-reaching? A systematic review of the literature. Br J Sports Med 2008;42:709-14. [DOI] [PubMed] [Google Scholar]
- 33.Aubry A, Hausswirth C, Louis J, Aaron J, Coutts AJ, Buchheit M, Le Meur Y. The development of functional overreaching is associated with a faster heart rate recovery in endurance athletes. PLoS One 201521;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchheit M, Papelier Y, Laursen PB, Ahmaidi S. Noninvasive assessment of cardiac parasympathetic function: postexercise haert rate recovery or heart rate variability? Am J Physiol Heart Circ Physiol 2007;293:H8-H10. [DOI] [PubMed] [Google Scholar]
- 35.Wilson D, Ramchandani G. An investigation of home advantage in the Summer Paralympic Games. Sport Sci Health 2017;13:625-33. doi: 10.1007/s11332-017-0393-2. Epub 2017 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz S, Blauwet CA. Implications of altered autonomic control on sports performance in athletes with spinal cord injury. Auton Neurosci. 2017 Apr 4. pii: S1566-0702(17)30081-4. doi: 10.1016/j.autneu.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Krassioukov A, West C. The role of autonomic function on sport performance in athletes with spinal cord injury. PM R 2014;6(8 Suppl):S58-65. doi: 10.1016/j.pmrj.2014.05.023. Review. [DOI] [PubMed] [Google Scholar]
- 38.Squair JW, Phillips AA, Currie KDG, et al. Autonomic testing for prediction of competition performance in Paralympic athletes. Scand J Med Sci Sports. 2017. Apr 27. doi: 10.1111/sms.12900. [DOI] [PubMed] [Google Scholar]
- 39.Azevedo Coste C, Mayr W, Bijak M, et al. FES in Europe and Beyond: Current Translational Research. Eur J Transl Myol 2016;26:6369. eCollection 2016 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riener R. The Cybathlon promotes the development of assistive technology for people with physical disabilities. J Neuroeng Rehabil 2016;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimarães JA, da Fonseca LO, Dos Santos-Couto-Paz CC, et al. Towards Parameters and Protocols to Recommend FES-Cycling in Cases of Paraplegia: A Preliminary Report. Eur J Transl Myol 2016;26:6085. eCollection 2016 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padilha Lanari Bo AP, Fonseca L, Guimaraes J., Fachin-Martins E, Gutierrez Paredes ME, Brindeiro GA, Cardoso de Sousa AC, Cristina Dorado M, Ramos F. Cycling with Spinal Cord Injury: A Novel System for Cycling Using Electrical Stimulation for Individuals with Paraplegia, and Preparation for Cybathlon 2016. IEEE Robotics & Automation Magazine 2017; PP(99):1-1. [Google Scholar]
- 43.Azevedo Coste C, Bergeron V, Berkelman R, et al. Comparison of strategies and performance of functional electrical stimulation cycling in spinal cord injury pilots for competition in the first ever Cybathlon. Eur J Transl Myol 2017;27:251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkelmans R, Woods B. Strategies and perfor-mances of Functional Electrical Stimulation Cycling using the BerkelBike with Spinal Cord Injury in a competition context (CYBATHLON). Eur J Transl Myol 2017;27:255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laubacher M, Aksöz EA, Bersch I, Hunt KJ. The road to Cybathlon 2016 - Functional electrical stimulation cycling Team IRPT/SPZ. Eur J Transl Myol 2017;27:259-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sijobert B, Fattal C, Daubigney A, Azevedo-Coste B. Participation to the first Cybathlon: an overview of the FREEWHEELS team FES-cycling solution. Eur J Transl Myol 2017;27:265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guimarães JA, Oliveira da Fonseca L, de Sousa AC, et al. FES Bike Race preparation to Cybathlon 2016 by EMA team: a short case report. Eur J Transl Myol 2017;27:272-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metani A, Popovi?-Maneski L, Mateo S, et al. Functional electrical stimulation cycling strategies tested during preparation for the First Cybathlon Competition - a practical report from team ENS de Lyon. Eur J Transl Myol 2017;27:279-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDaniel J, Lombardo LM, Foglyano KM, et al. Cycle Training Using Implanted Neural Prostheses: Team Cleveland. Eur J Transl Myol 2017;27:289-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnin J, Yamsa-ard T, Triponyuwasin P, Wongsawat Y. Development of practical functional electrical stimulation cycling systems based on an electromyography study of the Cybathlon 2016. Eur J Transl Myol 2017;27:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung WC, Tong RKY, Wang X, et al. The Effectiveness of Functional Electrical Stimulation (FES) in On-Off Mode for enhancing the cycling performance of Team Phoenix at 2016 Cybathlon. Eur J Transl Myo 2017;27:302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


