Abstract
The sternomastoid (SM) muscle in rodents is known to have a peculiar distribution of fiber types with a steep gradient from surface to deep region. We here further characterize this peculiar regional distribution by quantitative histochemical morphometrys. In Hematoxylin-Eosin (H-E) stained transverse cryosections harvested in the medial portion of the muscle we counted around 10.000 myofibers with a mean diameter of 51.3±12.6 (μm). Cryisections of the SM stained by SDH reaction clearly show two distinct regions, toward the deep surface of the muscle a 40% area that contains packed SDH-positive myofibers, while the remaining area of the SM toward the external surface presents a more checker-board appearance. On the other hand, in the deep region of SM type 1 (slow contracting) muscle fibers, caracterized by positive acidic ATPase pH 4.35 reaction, are only the 24.5% of the fibers in the deep area of SM muscles, being restricted to the deepest region. The 75.5% of the myofibers in the deep region are of the fast contracting types (either 48.4% 2A, SDH –positive fibers or 27.1% 2B, SDH-negative fibers, respectively). As expected the 2B muscle fibers, acidic ATPase pH 4.3-negative and SDH-negative, present the largest size, while Type 1 fibers, acidic ATPase pH 4.3-positive and SDH-positive, present the smallest size in rat SM muscle. Based on present and previous observations, comparison of change in absolute number and/or percentage of the fiber types in any experimental model of muscle atrophy/hypertrophy/plasticity/pathology /recovery in the rat SM, and possibly of all mammals, will ask for morphometry of the whole muscle cross-sections, muscle sampling by bioptic approches will provide only comparable data on the size of the different types of muscle fibers.
Key Words: rat, Sternomastoid muscle, muscle fiber types, SDH, myofibrillar ATPases, regional distribution
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this typescript is consistent with those guidelines.
The sternomastoid muscle (SM) in rodents is known to have a peculiar distribution of fiber types with a steep gradient from surface to deep region.1 This behavior is also present in leg muscles (e.g., tibialis anterior muscle), but not at extent of the SM muscle.2-4 We are characterizing in normal animals this peculiar regional distribution by quantitative histochemical analyses of transverse sections in the third medial portion of the muscle. We will present results suggesting that comparison of change in absolute number and/or percentage of the fiber types in the rat SM will ask for morphometry of the whole muscle cross-section, while muscle bioptic samplings will provide only consistent results on size of muscle fibers.
Materials and Methods
Animals and harvesting of muscles
Adult females Wistar rats, 300 g of weight were used. These animals come from the vivarium of the Department of Neurosciences – University of Padova. The animals were kept in cages with light/dark cycle of 12h, room temperature (24±2), food and water ad libitum. All procedures in this study were according to the international ethical principles of animal experimentation and approved by the Ethics on Animal Experimentation Committee (EAEC) of University of Padova. Animals were killed by deep anaesthesia and muscles were excised, weighted, frozen in liquid nitrogen (-196°C) and kept at -80°C until use.
Histology and histochemistry of the sternomastoid muscle
Size, and absolute or percent contents of different muscle fiber types were determined in transverse cryosections of the SM muscle harvested in the medial third of the muscle. Serial 10μm cross-sections were cut on a cryostat microtome at -25°C, mounted on slides and stained in small glass jars. From stained sections digital images were collected at magnification of x 2.5 and x10 using a transmission light microscope mounting a HDMI camera. Quantitative evaluations were performed on the collected pictures using Scion Image for Windows version Beta 4.0.2 (2000 Scion Corporation). Muscle fiber sizes and regional distribution of the muscle fiber type were determined using the Adobe Photoshop software (Adobe Systems Incorporated, San Jose, CA).
Absolute number of muscle fibers in rat SM muscle
To determine the absolute number of muscle fibers in the rat SM muscle, cryosections were stained with standard Hematoxylin-Eosin (H-E) procedure.5
Fiber typing of the muscle fibers was performed by the following histochemical methods
Succinate Dehydrogenase (SDH) reaction
SDH reaction was performed to distinguish between oxidative, “less” oxidative and non-oxidative muscle fibers. Muscle sections were incubated in SDH incubation solution for 60 min a 37°C. Sections were rinsed in distilled H2O, 3x1 min and removed unbound NBT from sections with 3x1 min exchanges of acetone solutions 30, 60, 90 % in distilled H2O, in increasing then decreasing concentrations. Sections were rinsed in distilled H2O, 3x1 min and dehydrated in graded alcohol solutions (ethanol 70, 90, 100%) cleared in xylene and mounted in permanent medium: Canada balsam. SDH incubating solution: NBT (nitro blue tetrazolium): 10mg; SDH stock solution: 10ml. Adjust to pH 7.2 to 7.6 with 0.1 N HCl or NaOH. SDH stock solution: 0.2M sodium succinate solution 100ml, 0.2M Phosphate buffer, pH 7.4 100ml, make freshly at least every two weeks.
Histochemistry by Myofibrillar actomyosin ATPase
Two different procedures were used for staining myofibrillar actomyosin ATPase using essentially the same methods described by Brooke and Kaiser,6,7 and by Guth and Samaha.8-10 For determination of the alkali preincubated myofibrillar ATPase activity the following procedures were employed. cryosections were air dried for 10 min a room temerature and fixed for 3 min in 5% (w/v) formaldehyde in 200mM sodium cacodylate, 68mM CaCl2 2H2O and 340 mM sucrose (adjusted with HCl to pH 7.6 before adding the formaldehyde).After washing in wash solution 100mM Tris, 18mM CaCl2 (pH 7.8), an alkaline pre-incubation was performed for 15 min a room temperature in a solution containing 200mM 2-amino-3-methyl-1 propanol (adjusted with HCl to pH 10.4). After two successive 1 min incubation in the wash solution, sections were incubated for 45 min a 37°C in ATP solution (2,7mM ATP, 90mM CaCl2, 100mM sodium barbital adjusted with NaOH to pH 9.4). For the acid preincubation myofibrillar ATPase activity (10, 11), the following procedures were utilized. Sections were air dried for 10 min at room temperature and incubated in a solution consisting of 100mM sodium acetate (adjusted with HCl to pH 4.35) for 10 min a room temperature. After washing 2x1 min in 18 mM CaCl2 and 100mM Tris HCl pH 7.8, the sections were incubated a 37°C for 45 min in ATP solution together to serial sections with alkali preincubation. After washing 2x30 sec in distilled H2O, sections were incubated for 5 min in 2% (w/v) CoCl2, washed 2x30 sec in distilled H2O, sections were incubated 5 min in 2% (v/v) (NH4)2S, washed in distilled H2O 2x30 sec, and dehydrated in graded alcohol solution (ethanol 70-90-100%) cleared in xilene, and mounted with Canada balsam medium.
Results
Morphometry: number and size of rat SM muscle fibers in H-E stained cryosections.
In the rat SM muscle cryosections harvested in the medial portion of the muscle we counted around 10.000 myofibers. The mean diameter in these muscle sections stained by H-E was 51.3±12.7 μm (Table 1).
Table 1.
Mean myofiber diameter (μm) in the SM rat muscle stained by H-E. Values are mean±SD
| All muscle fibers | SM dx | SM sx |
| 51.6±26.7 | 50.9±12.1 | |
| Mean of two SM | 51.3±12.7 | |
| Total fibers in Deep and Superficial region | 9981 |
Histochemistry of sternomastoid rat muscle
Fiber typing by SDH reaction
In Fig.1A is presented a transverse section from the medial region of a rat SM muscle stained by SDH reaction. The cryosection clearly shows two distinct regions, a right part of the section that cover around 40% area that contains SDH positive myofibers (right part of Fig.1A) and a the left part of Fig.1A; the latter encopasses the other 60% area of the SM with a more checker-board appearance. The rigth part of the cryosection, peculiarly rich in SDH positive muscle fibers, corresponds to the deep part of the SM, while the left part of the figure correspond to the superficial region of the SM. Table 2 shows that SDH-positive myofibers are the 72.9% of the total myofibers of SM deep region. In the superficial region (left part of the cryosection) based on SDH staining (Fig.1A), the mitochondrial rich, 2A muscle fibers are 31.1%. Since very few or none of type 1 muscle fibers are present there (see ATPase histochemistry reaction). The 68.9% 2B negative fibers are that of the myofibers present in this region of the SM muscle. Figure 2 shows six rappresentative fields at highter magnification taken from periferal, intermediate, and deep portions of cryosections stained either by SDH (A, B, C) or acidic ATPase (D, E, F).
Table 2.
Mean myofiber diameter (μm) in deep and superficial region of rat SM stained by SDH or ATPase, pH 4.35 reactions. Values are mean±SD.
| SDH reaction | |||||
|---|---|---|---|---|---|
| Deep region | Superficial region | ||||
| Type fibers 1 + 2A | 33.8±7.5 | 35.8±9.2 | |||
| Type fibers 2B | 41.1±9.2 | 53.3±10.8 | |||
| Total number fibers | 5211 | 3975 | |||
| ATPase pH 4.35 | |||||
| Deep region | Superficial region | ||||
| Type fibers 1 | 29.2±6.5 | Type fibers 2A | 35.3±8.5 | ||
| Type fibers 2A + 2B | 36.8±9.2 | Type fibers 2B | 47.6±11.9 | ||
| Total number fibers | 5802 | 4179 | |||
Histochemistry by Myofibrillar actomyosin ATPase
In the deep region (right part of Fig.1B), the type 1 (slow contracting) muscle fibers, identified by positive acidic ATPase pH 4.35 (Fig.1B), are only the 24.5% of the fiber of this part of SM muscle (Table2). On the other hand, the slow type muscle fibers, i.e., those positive at the acidic ATPase pH 4.35 are restricted in the deepest region of SM muscle. Thus, 75.5% of the myofibers in the deep region are of the fast contracting types (either 48.4% 2A and 27.1% are 2B, respectively).
In the superficial region, left part of the cryosection, based on acidic ATPase pH 4.35, the type 1 positive fibers are absent, the type 2A and muscle fibers characterized by an intermediate intensity decrease to 20.3%. Therefore the vast majority of the muscle fibers present in the superficial region of the SM muscle are of the 2B type (68.9% as SDH- negative fibers or 79.7% when counted in the acidic ATPase pH 4.35).
Fiber size of the three different fiber types of the SM muscle
As expected, Table 1, shows that the acidic ATPase pH 4.35 negative and SDH-negative 2B type myofibers, present the largest size among the three types of muscle fiber of rat SM muscle. In the superficial region based on acid ATPase pH 4.35, the 2B fiber size result 47.6 μm and the 2A fiber 35.3 μm. In the deep region the fiber size of 1 fibers (slow contracting) result to be the smallest: 29.2 μm; while the 2A and 2B fiber size are 36.8 μm on average in size. Based on mitochondrial enzyme activities, SDH reaction, in the superficial region the size of 2B fiber recognizable as the larger ones are 53.3 μm and in the deep region 41.1μm, while the 1 and 2A fiber size in the superficial region and in the deep region is respectively 35.8μm and 33.8μm.
Discussion
The peculiarity of the SM rat muscle is the presence of two distinct regions, where the fiber type composition is characterized in both zone by 2B fibers that are easily recognizable from their major size, while the 1 and 2A are the smallest. The SM muscle presents a very heterogeneous distribution of muscle fiber types with a large predominance of the type 2B in the superficial region while moving to deep regions there is a progressive enrichment of 2A fibers. Only in the deepest region of the SM muscle there are type 1 muscle fibers (Fig 1B and Table 3).
Table 3.
Number and percentage of SM fiber types in deep or superficial regions by SDH or ATPase pH 4.3
| SDH reaction | |||
|---|---|---|---|
| Deep region | Superficial region | ||
| Type fibers 1+2A | |||
| Number | 3800 | 1235 | |
| Percentage | 72.9% | 31.1% | |
| Type fibers 2B | |||
| Number | 1411 | 2740 | |
| Percentage | 27.1% | 68.9% | |
| Total number fibers | 5211 | 3975 | |
| Total fibers Deep plus Superficial region | 9186 | ||
| ATPase pH 4.35 | |||
| Deep region | Superficial region | ||
| Type fibers 1 | Type fibers 2A | ||
| Number | 1422 | 849 | |
| Percentage | 24.5% | 20.3% | |
| Type fibers 2A+ 2B | Type fibers 2B | ||
| Number | 4380 | 3330 | |
| Percentage | 75.5% | 79.7% | |
| Total number fibers | 5802 | 4179 | |
| Total fibers Deep and Superficial region | 9981 | ||
It remains to be determined if the content of fiber types varies along the muscle length and if applying new imaging processing as the Machine-learning algorithms (Deep Neural Networks) that have been proved to be very powerful methods for automatic image segmentation, especially in the field of histological analyses,11 some of the probleams we are facing with non homogeneous muscles would be overcommed. Anyhow, as demonstrated here and by previously,1 in the rat SM muscle comparative analyses for experimental models of muscle plasticity, in normal and diseased muscles,12-26 will require morphometry of the whole cross-sections. In conclusion, in case of bioptic fragments of SM muscle, in small, but conceivably also large, mammals, only the size of the different fiber types could be evaluated to avoid systematic errors related to diferent regional sampling.
Fig 1.
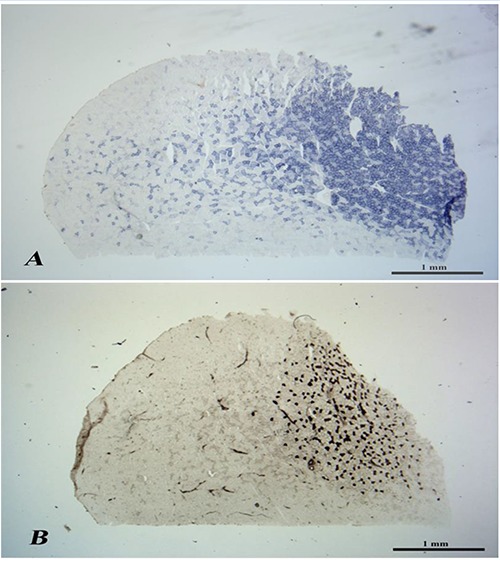
Transverse cross-section of normal rat SM muscle. Panel A, SDH reaction. In the deep region are present small diameter, intense blue fibers, while the superficial region presents higher diameter unstained fibers (type 2B) and some ligth to midle blue fibers (Type 2A). Panel B, ATPase pH 4.35 reaction. The black fibers (type1) are restricted to deepest region of the SM muscle. Scale Bar 1 mm.
Acknowledgments and Funding
BR thanks for support A&C M-C Foundation for Translational Myology, Padova, Italy.
List of acronyms
- ATPase
myofibrillar Actomyosin ATPase histochemistry
- EAEC
Ethics Committee on Animal Experimentation
- H-E
Hematoxylin-Eosin reaction
- SDH
succinate dehydrogenase reaction
- SM
sternomastoid muscle
Contributor Information
Valerio Gobbo, Email: gobbov@bio.unipd.it.
Damiana Incendi, Email: damiana.incendi@unipd.it.
Andrea Porzionato, Email: andrea.porzionato@unipd.it.
Veronica Macchi, Email: veronica.macchi@unipd.it.
Raffaele De Caro, Email: Raffaele.decar@unipd.it.
Dario Coletti, Email: dario.coletti@snv.jussieu.fr, dario.coletti@uniroma1.it.
Tiziana Martinello, Email: tiziana.martinello@unipd.it.
Marco Patruno, Email: marco.pat@unipd.it.
References
- 1.Polican Ciena A, Yokomito de Almeida SR, de Matos Alves PH, et al. Histological and ultrastructural changes of sternomastoid muscle in aged wistar rats. Micron 2011;42:871-6. [DOI] [PubMed] [Google Scholar]
- 2.Hiroux C, Vandoorne T, Koppo K, et al. Physical Activity Counteracts Tumor Cell Growth in Colon Carcinoma C26-Injected Muscles: An Interim Report. Eur J Transl Myol 2016;26:5958. doi: 10.4081/ejtm.2016.5958. eCollection 2016 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasic D, Dimov D, Gligorijevic J, et al. Muscle fibre types and fibre morphometry in the tibialis posterior and anterior of the rat: a comparative study. Medicine and Biology 2003;10:16-21. [Google Scholar]
- 4.Coletti D, Daou N, Hassani M, et al. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur J Transl Myol 2016;26:6008. doi: 10.4081/ejtm.2016.6008. eCollection 2016 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern H, Loefler S, Hofer C, et al. FES Training in Aging: interim results show statistically significant improvements in mobility and muscle fiber size. Eur J TranslMyology 2012;22:61-7. [Google Scholar]
- 6.Brooke MH, Kaiser KK. J. Some comments on the histochemical caracterization of muscle adenosin triphosphatase. Histochem Cytochem 1969;17:431-2. [DOI] [PubMed] [Google Scholar]
- 7.Brooke MH, Kaiser KK. Muscle fiber types: How many and what Kind? Arch Neurol 1970. 23; 369-79. [DOI] [PubMed] [Google Scholar]
- 8.Guth L, Samaha FJ. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscles Exp Neurol 1969;25:139-52. [DOI] [PubMed] [Google Scholar]
- 9.Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 1970;28: 365-7. [PubMed] [Google Scholar]
- 10.Hammalinen N, Pette D. The histochemical profiles of fast fibers IIB, IIA in skeletal muscle of mouse, rat and rabbit. Histochem Cytochem 1993;41:733-43. [DOI] [PubMed] [Google Scholar]
- 11.Ciresan DC, Giusti A, Gambardella LM, Schmidhuber J. Mitosis detection in breast cancer histology images with deep neural networks. Med Image Comput Assist Interv 2013;16:411-8. [DOI] [PubMed] [Google Scholar]
- 12.Seene T, Umnova M, Kaasik P. Morphological peculiarities of neuromuscular junctions among different fiber types: Effect of exercise. Eur J Transl Myol. 2017. Jun 27;27(3):6708. doi: 10.4081/ejtm.2017.6708. eCollection 2017 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samiee F, Zarrindast MR. Effect of electrical stimulation on motor nerve regeneration in sciatic nerve ligated-mice. Eur J Transl Myol. 2017. Sep 20;27(3):6488. doi: 10.4081/ejtm.2017.6488. eCollection 2017 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power GA, Dalton BH, Gilmore KJ, et al. Maintaining Motor Units into Old Age: Running the Final Common Pathway. Eur J Transl Myol 2017;27(1):6597. doi: 10.4081/ejtm.2017.6597. eCollection 2017 Feb 24. Pigna E Greco E Morozzi G.. Denervation does not Induce Muscle Atrophy Through Oxidative Stress. Eur J Transl Myol 2017;27(1): 6406. doi: 10.4081/ejtm.2017.6406. eCollection 2017 Feb 24. [Google Scholar]
- 15.Pette D, Vrbová G. The Contribution of Neuromuscular Stimulation in Elucidating Muscle Plasticity Revisited. Eur J Transl Myol. 2017. Feb 24;27(1):6368. doi: 10.4081/ejtm.2017.6368. eCollection 2017 Feb 24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carotenuto F, Coletti D, Di Nardo P, Teodori L. α-Linolenic Acid Reduces TNF-Induced Apoptosis in C2C12 Myoblasts by Regulating Expression of Apoptotic Proteins. Eur J Transl Myol 2016;26(4):6033. doi: 10.4081/ejtm.2016.6033. eCollection 2016 Sep 15.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riebold B, Nahrstaedt H, Schultheiss C, et al. Multisensor Classification System for Triggering FES in Order to Support Voluntary Swallowing. Eur J Transl Myol 2016;26(4):6224. doi: 10.4081/ejtm.2016.6224. eCollection 2016 Sep 15.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratton K, Faghri PD. Electrically and Hybrid-Induced Muscle Activations: Effects of Muscle Size and Fiber Type. Eur J Transl Myol 2016;26(3):6163. eCollection 2016 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willand MP. Electrical Stimulation Enhances Reinnervation After Nerve Injury. Eur J Transl Myol 2015;25(4):243-8. doi: 10.4081/ejtm.2015.5243. eCollection 2015 Aug 24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zampieri S, Mosole S, Löfler S, et al. Physical Exercise in Aging: Nine Weeks of Leg Press or Electrical Stimulation Training in 70 Years Old Sedentary Elderly People. Eur J Transl Myol 2015;25(4):237-42. doi: 10.4081/ejtm.2015.5374. eCollection 2015 Aug 24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber L, Scicchitano BM, Musaro A. Molecular and Cellular Mechanisms of Muscle Aging and Sarcopenia and Effects of Electrical Stimulation in Seniors. Eur J Transl Myol 2015;25(4):231-6. doi: 10.4081/ejtm.2015.5227. eCollection 2015 Aug 24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravara B, Gobbo V, Carraro U, et al. Functional Electrical Stimulation as a Safe and Effective Treatment for Equine Epaxial Muscle Spasms: Clinical Evaluations and Histochemical Morphometry of Mitochondria in Muscle Biopsies. Eur J Transl Myol 2015;25(2):4910. doi: 10.4081/ejtm.2015.4910. eCollection 2015 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa A, Rossi E, Scicchitano BM, et al. Neurohypophyseal Hormones: Novel Actors of Striated Muscle Development and Homeostasis. Eur J Transl Myol 2014;24(3):3790. doi: 10.4081/ejtm.2014.3790. eCollection 2014 Sep 23. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hockerman GH, Dethrow NM, Hameed S, et al. The Ubr2 Gene is Expressed in Skeletal Muscle Atrophying as a Result of Hind Limb Suspension, but not Merg1a Expression Alone. Eur J Transl Myol. 2014;24(3):3319. doi: 10.4081/ejtm.2014.3319. eCollection 2014 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomo T. The Response of Denervated Muscle to Long-Term Stimulation (1985, Revisited here in 2014). Eur J Transl Myol 2014;24:3294. doi: 10.4081/ejtm.2014.3294. eCollection 2014 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson BM. The Biology of Long-Term Denervated Skeletal Muscle. Eur J Transl Myol. 2014. Mar 27;24:3293. doi: 10.4081/ejtm.2014.3293. eCollection 2014 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]


