Abstract
Our previous studies have shown that severely atrophic Quadriceps muscles of spinal cord injury (SCI) patients suffering with complete conus and cauda equina lesions, and thus with permanent denervation-induced atrophy and degeneration of muscle fibers, were almost completely rescued to normal size after two years of home-based Functional Electrical Stimulation (h-bFES). Since we used large surface electrodes to stimulate the thigh muscles, we wanted to know if the skin was affected by long-term treatment. Here we report preliminary data of morphometry of skin biopsies harvested from legs of 3 SCI patients before and after two years of h-bFES to determine the total area of epidermis in transverse skin sections. By this approach we support our recently published results obtained randomly measuring skin thickness in the same biopsies after H-E stain. The skin biopsies data of three subjects, taken together, present indeed a statistically significant 30% increase in the area of the epidermis after two years of h-bFES. In conclusion, we confirm a long term positive modulation of electrostimulated epidermis, that correlates with the impressive improvements of the FES-induced muscle strength and bulk, and of the size of the muscle fibers after 2-years of h-bFES.
Key Words: spinal cord injury, denervated-degenerating muscle, long-term effects, h-bFES, skin biopsy, morphometry of epidermis
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
We previously showed that atrophic leg muscles from spinal cord injury patients suffering with complete conus and cauda equina lesions, and thus with permanent-denervation atrophy/degeneration of muscles (DDM), were almost completely rescued to normal size after two years of home-based Functional Electrical Stimulation (h-bFES) with the impressive improvements of the FES-induced muscle strength, of the muscle bulk and of the size of the muscle fibers.1-13 Since we used large surface electrodes to stimulate the thigh muscles,14 we wanted to know if the electrical stimulated skin was either negatively or positively affected.15 To evaluate neuronal and trophism biomarkers in the skin biopsies of those patients we are performing immunohistochemistry of skin cross sections from a subsets of patients enrolled in the EU Project RISE.2 Here we report preliminary measurements of the area of epidermis in cross sections of the skin harvested from both legs of 3 SCI patients before and after two years of h-bFES. Morphometric analyses of the area of epidermis determined in skin cross sections before and after two years of h-bFES support our recently published evidence of an increase of skin thickness randomly quantified in the biopsies.15
Materials and Methods
Histologic morphometry of skin biopsies
The harvested skin biopsies were fixed in 10% buffered formalin and embedded in paraffin. Histological sections of 5 μm thickness were then collected and stained with immunohistochemical procedures to analyze in the skin biopsies some biomarkers (see below). Three sections per sample were considered for the analysis and from each transverse section 3-5 digital images were collected at a primary magnification of x10 using a light microscope (B-293 PLi, Optika Srl, Ponteranica, BG, Italy) mounting a HDMI camera (Optika 4083.13E HDMI Easy camera). Image of 1024x576 pixel size were then captured and stored as TIFF files on the workstation associated with the instrument. Care was taken to accurately align the epidermal layer parallel to the largest side of the frame before acquiring each image. Images were analyzed by using the ImageJ software (available at http://rsb.info.nih.gov/ij/).16 The boundary of the epidermal layer, from the outermost surface of the epidermis (but excluding the stratum corneum) to the dermo–epidermal junction, was interactively traced to estimate its area. One example is presented in figure 1.
Fig 1.
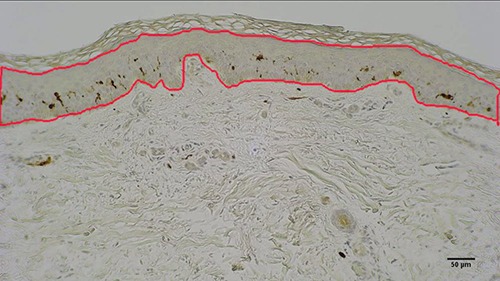
Skin biopsy from a SCI subject after 2 years of h-bFES. The immuno-stained skin cross section present in orange the outlined epidermis area quantified by ImageJ. Bar = 50 μm.
Immunohistochemistry
Immunological characterization was performed by Dako Autostainer/Autostainer Plus (Dako, Milan, Italy) with the following antibodies diluted in EnVision™ FLEX: S100 diluted 1:4000 (Z0311 anti-S100 Dako), CD1a 1:100 (M3571 anti-human CD1a Dako) and TUBB3 1:5000 (poly18020 BioLegend). Every section was incubated with peroxidase-blocking serum (EnVision™ FLEX Peroxidase-Blocking Reagent; Dako) for 5 min to remove unspecific bindings and for 30 min with the primary antibody. After, it was used a labeled polymer (EnVision™ FLEX /HRP, Dako) for 20 min and 3,3’-diaminobenzidine (EnVision™ FLEX Substrate buffer, + DAB + Chromogen; Dako) in order to show the positivity of the reactions, that is display the primary antibody binding. All the sections were finally counterstained with hematoxylin (EnVision™ FLEX Hematoxylin, Dako) for 5 min to reveal the presence of nuclei, dehydrated with a increasing scale of alcohol solutions (70%, 95%, 99%), cleared with xylene and mounted. These steps were performed at RT.17
Statistical analyses
Statistical analyses were performed by using GraphPad Prism 5.0 software (GraphPad software, La Jolla, CA, USA). Area values from each biopsy were first averaged to obtain a representative value for each leg and the values before h-bFES and those observed (on the same legs) after two years of h-bFES were then compared by paired Student’s t-test. Unpaired Student’s t-test comparing leg-by-leg the set of measurements before h-bFES with the one obtained after h-bFES was also applied. Results are reported as absolute area in μm2, and as %, i.e., the percent difference comparing before and after 2-years of h-b FES. The limit for statistical significance was always considered p<0.05 (Table 1).
Table 1.
Area of epidermis (μm2) in transverse sections of thigh skin biopsies. Skin biopsies were harvested from denervated SCI-patients before and after 2 years of home-based functional electrical stimulation (h-bFES, 5 days per week) using large surface electrodes and a purpose-developed electrical stimulator. The column of “Years from SCI” shows the number of years from SCI to the beginning of h-bFES.
| Subjects | Years from SCI | Before FES | Mean±SD | After FES | Mean±SD | Δ % | P |
|---|---|---|---|---|---|---|---|
| 1 | 3.3 | Left | 39558±6074 | Left | 54953±14034 | +39 | 0.001 |
| Right | 37143±8044 | Right | 49154±10821 | +32 | 0.01 | ||
| 2 | 3.2 | Left | 39683±2682 | Left | 66070±8530 | +66 | <0.001 |
| Right | 42780±2512 | Right | 54139±4544 | +26 | <0.001 | ||
| 3 | 0.8 | Left | 44265±10870 | Left | 54532±14552 | +23 | 0.159 |
| Right | 42940±14239 | Right | 42010±9780 | -2 | 0.875 | ||
| ALL-Before | 41062±7404 | ALL-After | 53477±10378 | +30 | 0.02 |
Results are reported as absolute area in μm2, and as Δ%, i.e., the percent difference comparing before and after 2-years of h-b FES. The limit for statistical significance was always considered p<0.05. The data in bold are significant or highly significant.
Results and Discussion
The twelve skin biopsies here analyzed were harvested from both the right and left thighs of 3 SCI patients before and after approximately two years of h-bFES. All the twelve biopsies presented with a regular stratified squamous epithelium rich in cells. The epidermis, consisting of several layers of cells, was distinguishable from the underlying dermis and exhibited a regular and consistent basal membrane. The H-E staining revealed the various layers of keratinocytes with small, nucleated basal cells (see Figure 1. in Albertin et al. 2018)15. The surface layer is the stratum corneum which sloughs away naturally and is evenly colored, thus it was not included in the measured cross area of the epidermis. Although some samples shown the skin organization as having ridges and papillae, in most samples the epidermis appears thinner and flattened, in particular in the skin biopsies harvested before h-bFES, that is two years after SCI. We performed the skin morphometry, measuring the total area of epidermis in transverse sections of skin biopsies (Figure1), by acquiring ImageJ pictures. Each skin biopsy taken before h-bFES was evaluated versus its corresponding post h-bFES biopsy. Despite the heterogeneity of gender and time from SCI of these three cases, the results in Table 1 show that the two SCI persons have a significantly increased epidermal area after two years of electrical stimulation. More important, the grouped statistical analysis of the epidermal area in the three subjects show a significant 30 % increase after h-bFES (Table 1). The skin is a sensory organ that protect the internal body from chemical, physical and biological insults and several disorders may affect epidermis and dermis due to physical, chemical, metabolic and genetic factors.18,19 Different studies have shown that electrical stimulation has positive effects, specifically on wound closure.20-22. Based upon this evidence we were encouraged to study the effects of functional electrical stimulation on the skin of patients with spinal cord injury (SCI), specifically in persons suffering with permanent denervation of the leg muscles as the result of conus and cauda equina complete lesion. Earlier, we demonstrated by sound functional, structural, and cellular approaches that 2-years of home-based functional electrical stimulation (h-bFES) produced significant improvements in muscle size and assisted function.1-13 We have indeed shown that denervated, degenerating muscles (DDM), of patients suffering with permanent disconnection of muscle fibers from the nervous system were rescued by two years of h-bFES, using very large skin-contact electrodes and a new electrical stimulator designed in Vienna, Austria.2-8. The use of those equipments and the related protocols of stimulation of DDM has been validated by the successful EU Program: RISE [Use of electrical stimulation to restore standing in paraplegics with long-term DDMs (QLG5-CT-2001-02191)].2 Thanks to the generous efforts of the Schuhfried Company based in Vienna, Austria the needed stimulators and large electrodes that recover DDM muscles are now commercially available .14 We have previously demonstrated that the positive effects of h-bFES are extended to the epidermal layer of the skin. The aim of the present short report was to describe a different approach to hopefully confirm the improvement of epidermis after two years of h-bFES.15 The previously study evaluated the histological structure of skin and the extent of changes that occurred in the thickness of the epidermis of three human SCI subjects before and after two years of continuous treatments of thigh muscles with h-bFES by surface stimulation revealing that there was a significant 28% overall increase in the thickness of the epidermis in response to h-bFES. Here with the evaluation of the epidermis area in cross sections of the skin and an unpaired statistical analysis (Table 1) the results are that: i) in two subjects (1 and 2) the skin biopsies from both the right and left legs show a significant increase in cross area of epidermis; ii) in the third subject there is a non statistically significant increase in epidermis area in the right leg, while there are no changes in the left leg; iii) the analysis of the three grouped subjects shows a significant 30 % increase after h-bFES. Whether those differential behaviors are related to the time from SCI, as reported in table 1, will be tested analyzing more subjects (research in progress). If confirmed by further analyses in the whole series of skin biopsies of the RISE Project, the results will provide conclusive evidence that electrical stimulation could also be of foremost importance in prevention/management of decubitus ulcers in SCI, metabolic diseases like diabetes, age-related sarcopenia and in wound healing.20-29 Furthermore, the evidence of a FES-induced modulations of the skin suggests to extend similar studies to adaptation of the skin to other physical and pharmacological therapies, based on rehabilitation managements applied to the skin, specifically in decubitus ulcer recovery and pain relief.
Acknowledgments and Funding
The support of the European Regional Development Fund-Cross Border Cooperation Program SLOVAKIA–AUSTRIA (Interreg- Iva) project ‘Mobilität im Alter’ MOBIL N_00033; Austrian Federal Ministry of Science and Research; Ludwig Boltzmann Society (Vienna) is gratefully acknowledged. Supported also by institutional funds of the Department of Neuroscience, Section of Human Anatomy, University of Padova, Italy; Interdepartmental Research Center of Myology, Department of Biomedical Sciences of the University of Padova, the IRCCS Fondazione Ospedale San Camillo, Venice, Italy. SZ thanks for support A&C M-C Foundation for Translational Myology, Padova, Italy.
List of acronyms
- DDM
denervated, degenerating muscles
- FES
Functional Electrical Stimulation
- h-b FES
home-based FES
- RISE Project
EU Program: RISE [Use of electrical stimulation to restore standing in paraplegics with long-term DDMs (QLG5-CT-2001-02191)]
- SCI
Spinal Cord Injury
Contributor Information
Helmut Kern, Email: helmut@kern-reha.at.
Christian Hofer, Email: stefan.loefler@kern-reha.at.
Diego Guidolin, Email: diego.guidolin@unipd.it.
Andrea Porzionato, Email: andrea.marcante@ospedalesancamillo.net.
Anna Rambaldo, Email: anna.rambaldo@unipd.it.
Raffaele De Caro, Email: raffaele.decaro@unipd.it.
Francesco Piccione, Email: Francesco.piccione@ospedalesancamillo.net.
Andrea Marcante, Email: andrea.porzionato@unipd.it.
Sandra Zampieri, Email: sanzamp@unipd.it.
References
- 1.Kern H, Hofer C, Löfler S, et al. Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res 2017;39:660-6. doi: 10.1080/01616412.2017.1314906. Epub 2017 Apr 13. [DOI] [PubMed] [Google Scholar]
- 2.Kern H, Carraro U, Adami N, et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair. 2010;24:709-721. DOI:10.1177/1545968310366129. Epub 2010 May 11. [DOI] [PubMed] [Google Scholar]
- 3.Kern H, Carraro U, Adami N, et al. One year of home-based functional electrical stimulation (FES) in complete lower motor neuron paraplegia: recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol Res 2010;32:5-12. DOI:1 0.1189/184313209X385644 [DOI] [PubMed] [Google Scholar]
- 4.Fiorucci R, Piscioneri A. FES for large denervated muscles: comments of patients and practical demonstrations. Eur J Transl Myol 2013;23:162–4. [Google Scholar]
- 5.Kern H, Carraro U. Home-based functional electrical stimulation (h-b FES) for long- term denervated human muscle: history, basics, results and perspectives of the Vienna rehabilitation strategy. Eur J Transl Myol 2014;24:27-40. DOI: 10.4081/ejtm.2014.3296 eCollection 2014 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern H, Boncompagni S, Rossini K, et al. Long-term denervation in humans causes degeneration of both contractile and excitation contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): A role for myofiber regeneration? J Neuropathol Exp Neurol. 2004;63:919-31. [DOI] [PubMed] [Google Scholar]
- 7.Boncompagni S, Kern H, Rossini K, et al. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci USA 2007;104:19339-44. DOI: 10.1073/pnas.0709061104 PubMed PMID: 18042706; PubMed Central PMCID: PMC 2148291.2008;18:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carraro U, Boncompagni S, Gobbo V, et al. Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J Transl Myol2015;25:77-92. DOI: 10.4081/ejtm.2015.4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carraro U, Kern H. Severely atrophic human muscle fibers with nuclear misplacement survive many years of permanent denervation. Eur J Transl Myol 2016;26:76–80. DOI:10.4081/ejtm.2016.5894. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargiulo P, Reynisson PJ, Helgason B, et al. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res 2011;33:750-8. doi: 10.1179/1743132811Y .0000000007. [DOI] [PubMed] [Google Scholar]
- 11.Edmunds KJ, Gíslason MK, Arnadottir ID, et al. Quantitative computed tomography and image analysis for advanced muscle assessment. Eur J Transl Myol 2016 Jun 22;26:6015. DOI:10.4081/ejtm.2016.6015. eCollection 2016 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carraro U, Edmunds KJ, Gargiulo P. 3D False Color Computed Tomography for Diagnosis and Follow-Up of Permanent Denervated Human Muscles Submitted to Home-Based Functional Electrical Stimulation. Eur J Transl Myol 2015;25:5133. doi: 10.4081/ejtm.2015.5133. eCollection 2015 Mar 11. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortolan P, Zanato R, Coran A, et al. Role of Radiologic Imaging in Genetic and Acquired Neuromuscular Disorders. Eur J Transl Myol 2015;25:5014. doi: 10.4081/ejtm.2015.5014. eCollection 2015 Mar 11. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Available: https://www.schuhfried.com/umbraco/Surface/AuthenticationSurface/Login?returnUrl=%2Fportal [Google Scholar]
- 15.Albertin G, Hofer C, Zampieri S, et al. In complete SCI patients, long-term functional electrical stimulation of permanent denervated muscles increases epidermis thickness. Neurol Res 2018. Feb 15:1-6. doi: 10.1080/01616412.2018.1436877. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Available at http://rsb.info.nih.gov/ij/). [Google Scholar]
- 17.Porzionato A, Macchi V, Guidolin D, et al. Histopathology of carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology. 2005;46:296-306. [DOI] [PubMed] [Google Scholar]
- 18.Bloom W, Fawcett DW. A textbook of histology, 12th ed., Chapman and Hall, 1994. [Google Scholar]
- 19.Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009; 54:273-285. DOI: 10.1111/j.1365-2559.2008.03096.x. Epub 2008 Jul 15. [DOI] [PubMed] [Google Scholar]
- 20.Hunckler J, de Mel A. A current affair: electrotherapy in wound healing. J Multidiscip Healthc 2017:10:179-94. DOI: 10.2147/JMDH.S 127207. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, Bai H, Wang E, et al. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 2004;117(Pt 3):397-405. Epub 2003 Dec 16 DOI: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayat M, Asgari-Moghadam Z, Maroufi M, et al. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev 2006;43:219-26. [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738-746. Review. [DOI] [PubMed] [Google Scholar]
- 24.Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321-6. Epub 2004 Feb 17. Review. [DOI] [PubMed] [Google Scholar]
- 25.Boyer B, Kern P, Fourtanier A, et al. Age-dependent variations of the biosyntheses of fibronectin and fibrous collagens in mouse skin. Exp Gerontol 1991;26:375-83. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006;442:457-60. [DOI] [PubMed] [Google Scholar]
- 27.Norman RA, Henderson JN. Aging: an overview. Dermatologic Therapy 2003;16:181–185 DOI: 10.1046/j.1529-8019.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- 28.Sauermann K, Clemann S, Jaspers S, et al. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res Technol 2002;8:52-56. DOI: 10.1046/J.0909-752x.2001.10297.x [DOI] [PubMed] [Google Scholar]
- 29.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405. PubMed PMID: 12366692 DOI: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]


