Abstract
1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (ectoine) functions as a compatible osmolyte in the moderate halophile Halomonas elongata OUT30018. Ectoine is biosynthesized by three successive enzyme reactions from aspartic β-semialdehyde. The genes encoding the enzymes involved in the biosynthesis, ectA, ectB, and ectC, encoding l-2,4-diaminobutyric acid acetyltransferase, l-2,4-diaminobutyric acid transaminase, and l-ectoine synthase, respectively, have been previously cloned. To investigate the function of ectoine as a compatible solute in plant cells, the three genes were individually placed under the control of the cauliflower mosaic virus 35S promoter and introduced together into cultured tobacco (Nicotiana tabacum L.) cv Bright Yellow 2 (BY2) cells. The transgenic BY2 cells accumulated a small quantity of ectoine (14–79 nmol g−1 fresh weight) and showed increased tolerance to hyperosmotic shock (900 mOsm). Furthermore, the transgenic BY2 cells exhibited a normal growth pattern even under hyperosmotic conditions (up to 530 mOsm), in which the growth of the untransformed BY2 (wild type) cells was obviously delayed. These results suggest that genetically engineered synthesis of ectoine results in the increased hyperosmotic tolerance of cultured tobacco BY2 cells despite the low level of accumulation of the solute.
Environmental stresses such as drought, high salinity, and low temperature are major factors that limit plant growth and productivity by disturbing the intracellular water balance (Epstein et al., 1980; Boyer, 1982; Yancey et al., 1982). Most plants synthesize and accumulate osmolytes as a response to these abiotic stresses. The osmolytes, or the so-called compatible solutes (Brown, 1976), are neutral under physiological pH, have a low molecular mass, a high solubility in water, and are nontoxic to the organisms even when accumulated at a high concentration. Polyols (e.g. glycerol, sorbitol, and mannitol), non-reducing sugars (e.g. Suc and trehalose), and amino acids (e.g. Glu, Pro, and betaine) are some of the known organic compatible solutes. Transgenic plants harboring genes for the biosynthesis of mannitol (Tarczynski et al., 1993), ononitol (Sheveleva et al., 1997), trehalose (Holmström et al., 1996; Romero et al., 1997), Pro (Kishor et al., 1995), betaine (Lilius et al., 1996; Hayashi et al., 1997; Sakamoto et al., 1998), or fructan (Pilon-Smits et al., 1995) showed significant improvement in water stress tolerance.
1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (ectoine) was identified as a compatible solute in Ectothiorhodospira halochloris, an extremely halophilic phototrophic eubacterium (Galinski et al., 1985), and is also found in various moderately halophilic eubacteria (Ronit et al., 1990; Wohlfarth et al., 1990; Bernard et al., 1993; Del Moral et al., 1994; Farwick et al., 1995; Malin and Lapidot, 1996). We have isolated Halomonas elongata OUT30018 (formerly designated strain KS3), which synthesizes and accumulates ectoine as a compatible solute, from a salty soil in northeastern Thailand (Okuda et al., 1989; Ono et al., 1998). The usefulness of the compatible solute ectoine as an enzyme protectant against heat, freezing, and drying were demonstrated (Lippert and Galinski, 1992). The biosynthetic pathway of ectoine, which comprises three steps of enzyme reactions, as shown in Figure 1A, has been elucidated in gram-negative halophilic eubacteria (Peters et al., 1990; Tao et al., 1992; Galinski and Trüper, 1994; Ono et al., 1999). Interestingly, non-halophilic eubacteria accumulate by taking up extracellular ectoine as a compatible solute under hyperosmotic conditions (Jebbar et al., 1992; Peter et al., 1998). It has been reported in Rhizobium meliloti that ectoine can induce the synthesis of endogenous compatible solutes such as Glu, N-acetylglutaminyl-Gln amide, and trehalose (Talibart et al., 1994).
Figure 1.
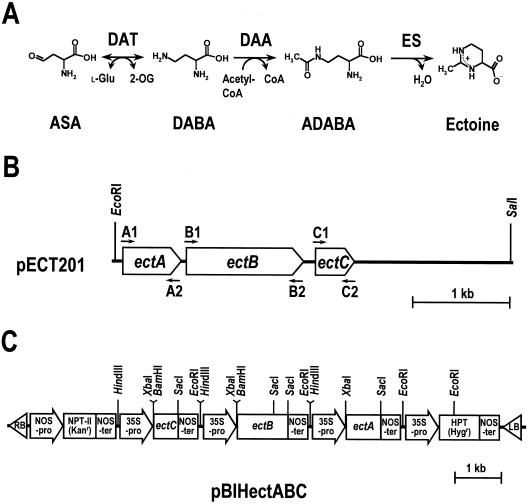
H. elongata genes encoding the enzymes involved in ectoine synthesis introduced into BY2 cells. A, Ectoine biosynthetic pathway in H. elongata OUT30018. The first step is catalyzed by DAT, which converts ASA, an intermediate in amino acid metabolism, to l-2,4-diaminobutyric acid (DABA). The second step, which is the acetylation of DABA to Nγ-acetyl l-2,4-diaminobutyric acid (ADABA), is promoted by DAA. In the last step, ES catalyzes the cyclic condensation of l-2,4-diaminobutyric acid to yield tetrahydropyrimidine ectoine. B, Structure of the 4.1-kb DNA fragment containing the ect operon. ectA, ectB, and ectC genes encode DAA, DAT, and ES, respectively. The arrows show the approximate positions of the PCR primers used to amplify each ect gene. C, Structure of the plasmid pBIHectABC for expression of the ect genes in the transgenic BY2 cells. NPT-II, Neomycin phosphotransferase gene; Kanr, kanamycin-resistance gene; HPT, hygromycin phosphotransferase gene; Hygr, hygromycin-resistance gene; 35S-pro, 35S promoter of cauliflower mosaic virus; NOS-pro, nopaline synthase promoter; NOS-ter, nopaline synthase terminator; LB, left border; RB, right border.
We cloned a 4.1-kb DNA fragment involving the ectC gene encoding l-ectoine synthase (ES), which catalyzes the final reaction step of ectoine biosynthesis in H. elongata OUT30018 (Fig. 1, A and B). The 4.1-kb DNA fragment was introduced into Escherichia coli and the resulting clones exhibited accumulation of ectoine and increased salt tolerance (Min-Yu et al., 1993). The ectA and ectB genes, encoding l-2,4-diaminobutyric acid acetyltransferase (DAA) and l-2,4-diaminobutyric acid transaminase (DAT), respectively, were also found in the 4.1-kb DNA fragment (H. Ono and Y. Murooka, unpublished data). The ect genes were also cloned from Marinococcus halophilus (Louis and Galinski, 1997; accession no. U66614), H. elongata DSM 2581T (Göller et al., 1998; accession no. AF031489), and H. elongata DSM 3043 (Cánovas et al., 1998; accession no. AJ011103). Although the ect genes are considered to be powerful tools for the molecular breeding of salt-tolerant plants, there is still no evidence either for ectoine biosynthesis or for the functional role of ectoine in plants.
In the present work, we have taken a transgenic approach to investigate the function of ectoine as a compatible solute in plant cells. Constitutive expression of the genes encoding the enzymes involved in ectoine synthesis, ectA, ectB, and ectC, with the cauliflower mosaic virus 35S (CaMV 35S) promoter in cultured tobacco (Nicotiana tabacum L.) cv Bright Yellow 2 (BY2) cells allowed us to examine the role of ectoine in water stress tolerance. We found that ectoine conferred increased hyperosmotic tolerance in transgenic BY2 cells, and that the extent of hyperosmotic tolerance was correlated with the level of ectoine accumulation.
MATERIALS AND METHODS
Construction of the Binary Plasmids for ect Gene Expression
The ect genes, designated as ectA, ectB, and ectC, for the enzymes involved in ectoine biosynthesis were obtained from Halomonas elongata OUT30018 genomic DNA clone pECT201 (Min-Yu et al., 1993) by PCR using primers designed to contain restriction enzyme sites (Fig. 1B). The ectA gene was amplified by PCR using the forward primer A1 with a XbaI site (5′-GCGAACCTCTAGAATGAACGCAACC-3′) and the reverse primer A2 with a SacI site (5′-CGGCGTCCGAGCTCAGATCTG-3′). The ectB gene was amplified by PCR using the forward primer B1 with a BamHI site (5′-ACAGGAGGATCCAATGCAGACCC-3′) and the reverse primer B2 with a SacI site (5′-CCTCAGGAGCTCAGCTAAAGGCC-3′). The ectC gene was amplified from pECT201 by PCR using the forward primer C1 with a BamHI site (5′-CACTGGAGGATCCACATGATCGTTC-3′) and a reverse primer C2 with a SacI site (5′-CAGAATAGAGCTCCGGGTTACAGCG-3′). The following profile was used for these reactions: 94°C/1 min, followed by 30 cycles of 98°C/20 s, 68°C/1 min 30 s, and a final extension at 72°C/10 min. Each amplified DNA fragment of the ect genes was cloned into the SmaI site of pBluescriptII (SK−, Stratagene, La Jolla, CA) and was confirmed by DNA sequencing. The GUS gene between the CaMV 35S promoter and the nos terminator in the binary vector pBI121 (Jefferson et al., 1987) was replaced by a 590-bp XbaI-SacI ectA fragment, a 1276-bp BamHI-SacI ectB fragment, and a 432-bp BamHI-SacI ectC fragment to generate pBIectA, pBIectB, and pBIectC, respectively. Each HindIII-EcoRI fragment of the CaMV 35S promoter-ect gene-nos terminator cassette from these plasmids was separately inserted into the HindIII-EcoRI gap of pUC19. The HindIII-SacI fragment of the CaMV 35S promoter-ectA insert in pUC19 was subcloned into the HindIII-SacI gap of pBI101HmB (Akama et al., 1992) to generate pBIHectA. Each PvuII fragment of the ectB and ectC cassette in pUC19 was inserted into the EcoRV site of SK−. The HindIII fragment of the ectB cassette in SK− was inserted into the HindIII site of pBIHectA to generate pBIHectAB, then the HindIII fragment of the ectC cassette in SK− was inserted into the HindIII site of pBIHectAB to generate pBIHectABC (Fig. 1C).
Plant Materials and Culture Conditions
Tobacco (Nicotiana tabacum L.) cv Bright Yellow 2 (BY2) suspension-cultured cells were maintained in a modified liquid Linsmaier and Skoog (LS) medium (pH 5.8) (Nagata et al., 1981). The cells were cultivated in this medium at 27°C in the dark on an orbital shaker (BR-3000, Taitec, Saitama, Japan) at 130 rpm. Cells were subcultured by regularly transferring 2 mL of a 7-d-old culture into 95 mL of fresh medium in a 300-mL Erlenmeyer flask. The composition of the solid medium for callus culture was identical to that of the liquid medium, except that 0.3% (w/v) gellan gum was added before sterilization. Callus was cultivated on solid medium in a 90-mm Petri dish at 25°C in a dark incubator (MIR-553, Sanyo Medicasystems, Osaka).
Transformation of Plant Cells
Transformation of BY2 cells was carried out by a variation of a method described previously (An, 1985). Eight-milliliter aliquots of a 4-d-old, exponentially growing suspension of BY2 cells were transferred to a 90-mm Petri dish and incubated at 25°C with 100 μL of an overnight culture of Agrobacterium tumefaciens EHA105 (Hood et al., 1993) harboring the binary plasmid. After 2 d of co-cultivation, the cells were washed with a modified LS liquid medium and plated on a modified LS solid medium containing 250 μg mL−1 carbenicillin and 100 μg mL−1 kanamycin. After 4 weeks of the first selection, the kanamycin-resistant calli were collected and transferred onto solid medium containing 100 μg mL−1 kanamycin and 20 μg mL−1 hygromycin. After 2 weeks of the second selection, the kanamycin- and hygromycin-resistant calli were subjected to genomic PCR analysis to confirm the existence of the transgenes. The selected transformants were then transferred to a modified LS liquid medium containing 100 μg mL−1 kanamycin and 20 μg mL−1 hygromycin.
Genomic PCR Analysis
The genomic DNA of BY2 cells was isolated with a DNA isolation kit (ISOPLANT, Nippon Gene, Tokyo), and analyzed by PCR using the specific primers. The following profile was used for this reaction: 94°C/1 min, followed by 30 cycles of 98°C/20 s, 68°C/1 min 30 s, and a final extension at 72°C/10 min.
RNA-Blot Analysis
Exponentially growing, 5-d-old suspensions of BY2 cells were harvested and ground in liquid nitrogen and extracted with 1 mL of 50 mm Tris-HCl (pH 8.0) containing 300 mm NaCl, 5 mm EDTA, 2% (w/v) SDS, 2% (w/v) Na-triisopropylnaphthalene sulfonate, 2 mm aurintricarboxylic acid, and 12.8 mm 2-mercaptoethanol. After the addition of 140 μL of 3 m KCl, the mixture was incubatedon ice for 15 min and then centrifuged at 9,000g for 5 min. RNA was precipitated from the supernatant by the addition of 440 μL of 10 m LiCl and incubation on ice for 1 h. The precipitate was pelleted by centrifugation at 12,000g for 20 min and then resuspended in 400 μL of water. The suspension was extracted with phenol and chloroform and RNA was precipitated from the aqueous phase with ethanol. The pellet was washed with 70% (v/v) ethanol, dried, and resuspended in 30 μL of water. The total RNA (20 μg) was separated on a 1% (w/v) formaldehyde agarose gel and blotted onto a blotting membrane (Zeta-Probe GT, Bio-Rad, Hercules, CA). The blots were then hybridized with the 32P-labeled DNA probes. Hybridizations were carried out at 43°C in accordance with the formamide protocol described in the blotting membranes instruction manual (Bio-Rad).
Analysis of Ectoine
For the identification of intracellular ectoine, 5-d-old suspensions of BY2 cells were harvested, and then 1 g fresh weight of the cells was transferred to a 15-mL centrifuge tube, suspended in 5 mL of extraction buffer (ethanol:chloroform:water, 12:5:2, v/v), and sonicated. The cell extract was separated from the cell pellet by centrifugation at 3,000g for 5 min. The pellet was re-extracted twice by the same method and all of the cell extracts were pooled in a 50-mL centrifuge tube. After the addition of 10 mL of chloroform and 5 mL of water and centrifugation at 3,000g for 5 min, the aqueous layer was collected in a 15-mL centrifuge tube and evaporated at 80°C. The residue was then dissolved in 5 mL of water and filtered through a 1.2-μm-pore syringe filter (Whatman, Clifton, NJ). The filtered extract was then passed through an ion-exchange column containing AG50W-X8 (H+ form, Bio-Rad), washed with two bed volumes of water, and eluted with 3 n NH4OH. The eluate was evaporated at 80°C, and then the residue was dissolved in 1 mL of water. The dissolved solution was filtered through centrifugal filter units (0.2-μm pore; Ultrafree-MC, Nihon Millipore, Yamagata, Japan) and subjected to liquid chromatography/electrospray ionization mass spectrometry (LC/ESIMS) (LCMS-QP8000, Shimadzu, Kyoto). The samples were loaded onto a column (2 × 250 mm; YMC-Pack ODS-AQ, YMC, Kyoto) at 40°C and eluted over 30 min at a flow rate of 0.2 mL min−1 with 0.1% (v/v) aqueous formic acid. The effluent was fed directly to the electrospray interface of the mass spectrometer. Ions were detected throughout the entire LC step over a m/z (mass-to-charge ratio) range of 100 to 300. Authentic ectoine was purified from the cells of H. elongata OUT30018 by a method described previously (Ono et al., 1998).
Analysis of Hyperosmotic Shock Tolerance
Exponentially growing, 5-d-old suspensions of BY2 cells were harvested in a 50-mL centrifuge tube and centrifuged for 5 min at 800g. After removal of the culture medium, the cell density was adjusted to 50% (v/v) with fresh medium. A 5-mL aliquot of the cell suspension was then transferred to a 15-mL centrifuge tube and centrifuged for 5 min at 800g. The pellet was washed with 10 mL of 180 mm mannitol solution (200 mOsm) that was iso-osmotic relative to the culture medium, and incubated in a hyperosmotic solution of 620 mm mannitol (700 mOsm) or 500 mm NaCl (900 mOsm) for 20 min, and then washed with and resuspended in fresh medium. The tolerance of the cells to hyperosmotic shock was determined by the growth and viability of the cells treated with the hyperosmotic solution relative to those of the control cells treated with the iso-osmotic solution. The osmolarities of the solutions used in this paper were measured using a freezing-point osmometer (model OM801, Vogel GMBH, GieBen, Germany).
Determination of the Fresh Weight of Cells
The fresh weight of BY2 cells was measured by measuring the weight of cell pellets precipitated by centrifugation at 800g for 5 min.
Estimation of Cell Viability with Fluorescein Diacetate (FDA) Staining
Cell viability was determined by staining of cells with FDA according to a method described previously (Ono et al., 1995). Five-milliliter aliquots of the suspension of BY2 cells were withdrawn after various treatments. Cells were washed twice with fresh medium and resuspended in 5 mL of medium to which 100 μL of a 0.5% solution (w/v) of FDA in acetone was added. The cells were then incubated for 20 min at room temperature, washed twice with fresh medium, and observed under a fluorescence microscope (Axiophoto, filter sets: excitation BP450–490, beamsplitter FT510, emission LP520; Zeiss, Jena, Germany).
Analysis of Tolerance to Hyperosmotic Stress
BY2 cells grown for 5 d in a modified LS medium were harvested in the exponentially growing phase in a 50-mL centrifuge tube and centrifuged for 5 min at 800g. After removal of the culture medium, the cell density was adjusted to 50% (v/v) with fresh medium. A 2-mL aliquot of the cell suspension was then transferred to a 300-mL Erlenmeyer flask containing 95 mL of modified LS medium with 100, 200, or 300 mm mannitol (310, 420, or 530 mOsm, respectively). The tolerance to hyperosmotic stress of the cells was determined by comparing the growth of the cells in the hyperosmotic medium with mannitol with that of the control cells in the medium without mannitol.
RESULTS
Expression of the H. elongata ect Genes in Transgenic BY2 Cells
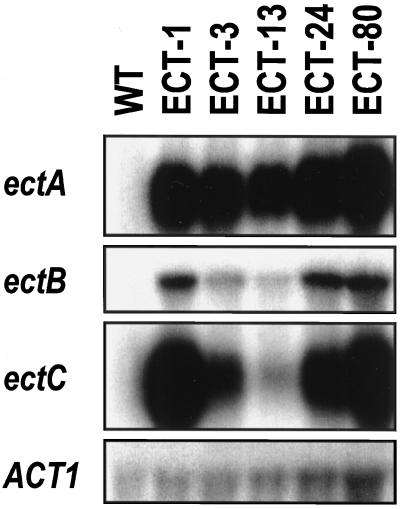
The three H. elongata genes encoding the enzymes involved in ectoine synthesis (ectA, ectB, and ectC) were individually placed under the control of the CaMV 35S promoter (pBIHectABC, Fig. 1C) and introduced together into cultured tobacco BY2 cells by A. tumefaciens-mediated transformation. One-hundred kanamycin-resistant calli were isolated and transferred onto a modified LS medium containing both kanamycin and hygromycin, and then 10 drug-resistant calli were randomly selected. The presence of transgenes in the selected transgenic clones (ECT clones) was confirmed by genomic PCR using primers amplifying a 420-bp DNA fragment corresponding to the ectC gene. Since northern-blot analysis showed that the levels of mRNA accumulation of the ect genes in each of the ECT clones varied, we selected five ECT clones (ECT-1, -3, -13, -24, and -80) for further analyses. The ect genes were strongly expressed in the ECT-1, -24, and -80 clones (high expression) and weakly expressed in the ECT-13 clone (low expression) (Fig. 2). Moderate levels of accumulation of the mRNA from ect genes were detected in the ECT-3 clone, and no ect gene mRNA was detected in untransformed BY2 wild-type (WT) cells as the negative control (Fig. 2).
Figure 2.
Accumulation of ect mRNA in the transgenic BY2 cells as detected by northern hybridization. RNA was isolated from 5-d-old untransformed BY2 (WT) cells and the transgenic clones ECT-1, -3, -13, -24, and -80. Each lane was loaded with 20 μg of total RNA and, after electrophoresis, hybridized with a 32P-labeled DNA fragment of the ectA, ectB, or ectC gene. A rice ACT1 cDNA (Sano and Youssefian, 1991) was used as the control probe.
Accumulation of Ectoine in the Transgenic BY2 Cells
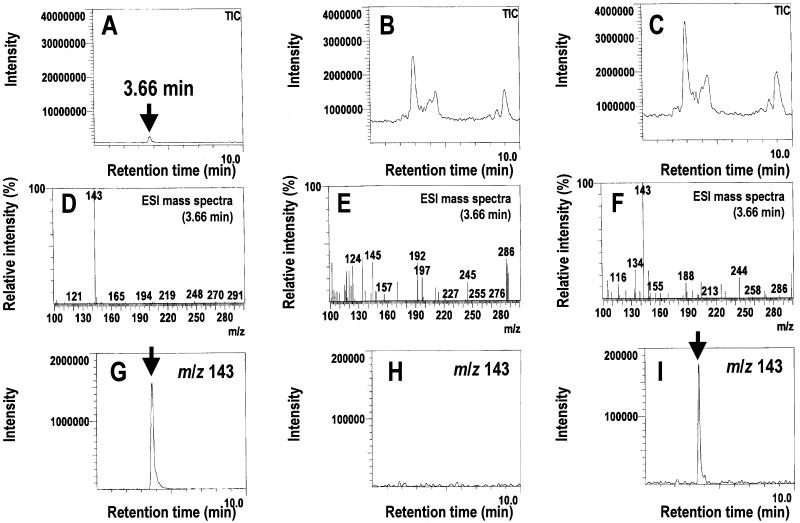
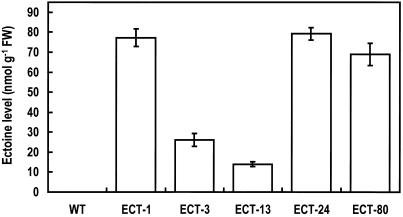
To identify and quantitate ectoine accumulation in the transgenic BY2 cells, the cell extracts were analyzed using LC/ESIMS. In the total ion chromatogram (TIC), purified ectoine had a retention time of 3.66 min (Fig. 3A), and the ESI mass spectra at 3.66 min showed that protonated ectoine had a m/z of 143 (Fig. 3D). In the TICs, ectoine was not clearly detected in the cell extracts of either the WT cells or the ECT clones (Fig. 3, B and C). However, the ESI mass spectra at 3.66 min confirmed the existence of ectoine (m/z 143) in the cell extracts of the ECT clone (Fig. 3F), but not of the WT cells (Fig. 3E). Finally, the ectoine contents in the cell extracts were quantitated by a computer-reconstructed mass chromatogram of m/z 143 (Fig. 3, G–I). The levels of ectoine accumulated in the clones exhibiting high levels of expression ranged from 69 to 79 nmol g−1 fresh weight of cells, compared with 14 nmol g−1 fresh weight in the clone exhibiting a low level of expression; the accumulation of ectoine was undetectable (<0.1 nmol g−1 fresh weight) in the WT cells (Fig. 4).
Figure 3.
Detection of ectoine accumulated in transgenic BY2 cells using LC/ESIMS. A, TIC of standard ectoine. B, TIC of untransformed BY2 (WT) cells. C, TIC of the ECT-80 clone. D, ESI mass spectra at 3.66 min of standard ectoine. E, ESI mass spectra at 3.66 min of the WT cells. F, ESI mass spectra at 3.66 min of the ECT-80 clone. G, Computer-reconstructed mass chromatogram of m/z 143 of standard ectoine. H, Computer-reconstructed mass chromatogram of m/z 143 of the WT cells. I, Computer-reconstructed mass chromatogram of m/z 143 of the ECT-80 clone. The arrows indicate the ectoine peak.
Figure 4.
Ectoine levels in the untransformed BY2 (WT) cells and transgenic BY2 cells. Ectoine levels in 5-d-old cultured cells of the WT cells and five ECT clones. Error bars represent ±sd (n = 3).
Increased Tolerance to the Hyperosmotic Shock Conferred by Ectoine Production in BY2 Cells
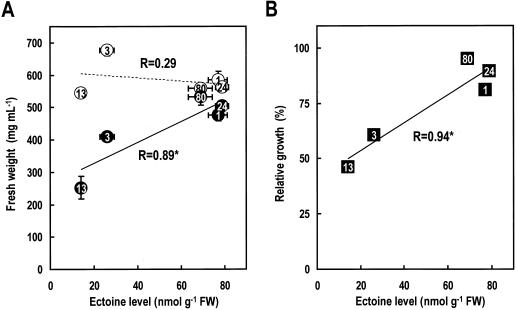
In a preliminary experiment to examine the phenotypic changes of the transgenic cells, the growth of ECT clones subjected to transient hyperosmotic shock for 20 min was analyzed. The iso-osmotic treatment with 180 mm mannitol solution (200 mOsm) could not inhibit the growth of all ECT clones. There was no correlation between ectoine level and the cell growth (Fig. 5A). However, hyperosmotic shock treatment with 620 mm mannitol solution (700 mOsm) inhibited the growth of the WT cells (data not shown), and the clones exhibiting moderate (ECT-3) and low levels of expression (ECT-13), but not in those exhibiting a high level of expression (ECT-1, -24, and -80). There were significant correlations between ectoine level and the cell growth after hyperosmotic shock (Fig. 5A) and between ectoine level and tolerance to the hyperosmotic shock (Fig. 5B).
Figure 5.
Relationship between ectoine level and cell growth in transgenic BY2 cells. A, Fresh weight of BY2 cells was measured at 5 d after iso-osmotic treatment with 180 mm mannitol solution (control, ○), and hyperosmotic shock treatment with 620 mm mannitol solution (●). Data for ectoine level are from Figure 4. Error bars represent ±sd (n = 3). B, Relative growth of each clone was determined as the ratio of the fresh weight of cells that had been treated with hyperosmotic shock to the fresh weight of control cells. Asterisks indicate P < 0.05. The numbers in circles and squares correspond to the ECT clone numbers.
Viability of the Transgenic BY2 Cells after Exposure to Hyperosmotic Shock
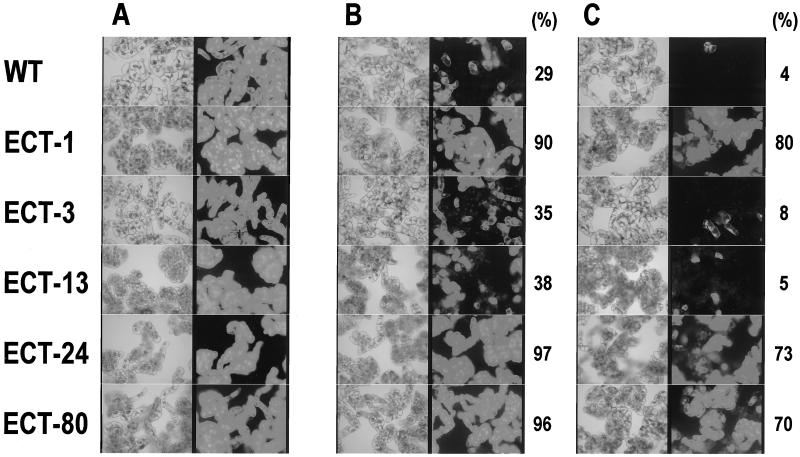
FDA was used to detect the viability of BY2 cells because it stains cells with normal membrane permeability. As shown in Figure 6, FDA stained all cells of both the WT and ECT clones after iso-osmotic treatment with 180 mm mannitol solution (200 mOsm) as a non-stress control. Treatment with 620 mm mannitol solution (700 mOsm), however, reduced the number of FDA-stained cells of the WT and the ECT-3, and -13 clones, while more than 90% of ectoine-accumulated cells and the ECT-1, -24, and -80 clones were stained. Furthermore, to mimic the high-salt conditions of seawater, BY2 cells were treated with 500 mm NaCl solution (900 mOsm). Most cells of the WT and the ECT-3 and -13 clones were not stained with FDA after the salt treatment, while more than 70% of cells of the ECT-1, -24, and -80 clones were stained.
Figure 6.
Viability of untransformed BY2 (WT) cells and transgenic BY2 cells after hyperosmotic shock treatment. Five-day-old BY2 cells were treated with 180 mm mannitol (200 mOsm; A), 620 mm mannitol (700 mOsm; B), or 500 mm NaCl (900 mOsm; C) at a cell density of 50% (v/v) for 20 min, and then stained with FDA as described in “Materials and Methods.” Viable cells with normal membrane permeability were detected in fluorescence micrographs (right image) by comparison with corresponding phase-contrast micrographs of the cells (left image). Each value indicates the percentage of cell survival quantified from the photographs.
Growth Performance of Transgenic BY2 Cells under Hyperosmotic Stress
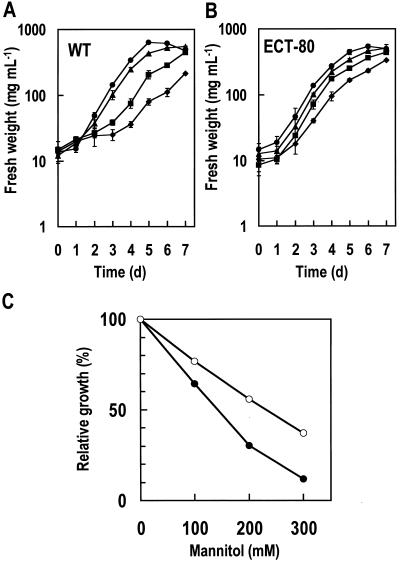
To investigate the effect of ectoine on the growth of BY2 cells under continuous hyperosmotic stress, we compared the growth of the WT (sensitive to hyperosmotic shock) and the ECT-80 clone (tolerant to hyperosmotic shock) cells under mannitol stress (Fig. 7, A and B, respectively). In modified LS medium containing 200 or 300 mm mannitol, the ECT-80 clone, with a shorter lag phase, showed a significant growth advantage over the WT cells. The concentration of mannitol that decreased the cell yield by 50% relative to that in a medium without mannitol (I50) was estimated. The I50 concentrations for the WT and the ECT-80 clone were 140 and 230 mm, respectively (Fig. 7C). Thus, the ECT-80 clone was 1.6-fold more tolerant to the hyperosmotic stress generated by mannitol than the WT cells.
Figure 7.
Growth of untransformed BY2 (WT) cells and transgenic BY2 cells under hyperosmotic stress. A and B, Growth curve of the WT cells (A) and the ECT-80 clone (B). ●, Modified LS medium (200 mOsm); ▴, modified LS medium containing 100 mm mannnitol (310 mOsm); ▪, modified LS medium containing 200 mm mannnitol (420 mOsm); ♦, modified LS medium containing 300 mm mannnitol (530 mOsm). C, Hyperosmotic stress tolerance of the WT cells and the ECT-80 clone. ●, WT; ○, ECT-80. Error bars represent ±sd (n = 3).
DISCUSSION
We have demonstrated in plant cells, for the first time to our knowledge, that the accumulation of ectoine confers increases tolerance to hyperosmotic stress. In this study, three genes encoding the enzymes involved in the ectoine biosynthetic pathway were introduced into plant cells with a binary vector harboring two antibiotic-resistant genes. Since the CaMV 35S promoter was used to drive the expression of each of the ect genes and the hygromycin-resistant gene, the binary plasmid pBIHectABC (Fig. 1C) had the same four promoters in the T-region. Although it is generally known that the expression of multiple CaMV 35S promoters is easily silenced in a transgenic plant cell, our results show that at least four CaMV 35S promoters can be transcribed at the same time. Furthermore, detection of ectoine synthesis in only transgenic cells with pBIHectABC showed that each mRNA derived from the three ect genes was correctly translated to each enzyme of the ectoine synthetic pathway.
Extremely low levels of accumulation of ectB mRNA compared with the other two ect genes were detected in all five transgenic clones examined in this study (Fig. 2). Since the first enzyme of the ectoine synthetic pathway, DAT, is encoded by the ectB gene and leads Asp-β-semialdehyde (ASA) into this pathway, a reduction of ASA as the precursor of Lys, Thr, and Met possibly results from overexpression of ectB gene. Therefore, transgenic clones in which the ectB gene is strongly expressed do not grow well. However, the reason for the variation in the amounts of mRNA accumulation of the four chimeric genes driven by the same promoter in the same T-region (Fig. 2) is unclear.
Plant cells that undergo plasmolysis on exposure to hyperosmotic stress such as 0.6 m saccharose exhibit irreversible damage of the membranes (Adamec, 1984). BY2 cells were also irreversibly damaged by transient exposure to hyperosmotic shock with 620 mm mannitol or 500 mm NaCl for 20 min, and cell death followed (Figs. 5 and 6). This indicates that the plasmolysis of BY2 cells occurs during the initial stages of hyperosmotic shock, even in the case of exposure to osmotic stresses such as salinity or drought, when the cells become lethally damaged. Since the intracellular ectoine level (14–79 nmol g−1 fresh weight, Fig. 4) in the ECT clones was insufficient for the solute to function as an osmolyte, and no difference in the concentration of NaCl causing plasmolysis was detected between the WT cells and the ECT clones (data not shown), it was considered that ectoine did not directly contribute to the intracellular osmotic adjustment. However, tolerance of BY2 cells harboring ect genes to hyperosmotic shock was increased in proportion to the level of ectoine accumulation (Figs. 5 and 6). As shown in Figure 7, hyperosmotic stress had a more negative effect on the growth of the WT cells than that of ECT clones. Furthermore, the lag time of the WT cells under stress was longer than that of the WT cells not exposed to stress (Fig. 7A), while the lag time of ECT clones is the same in both conditions (Fig. 7B). These results suggest that the accumulated ectoine functioned to improve the tolerance of the cells to hyperosmotic shock.
It has been reported that drought tolerance in transgenic tobacco is improved by genetically engineering the synthesis of trehalose or fructan (Pilon-Smits et al., 1995; Holmström et al., 1996; Romero et al., 1997). However, the concentrations of these sugars in transgenic plants were too low to confer a conventional osmolyte effect, which engineered mannitol and Pro have been shown to confer (Tarczynski et al., 1993; Kishor et al., 1995). Crowe et al. (1984) described that the interaction between trehalose and dipalmitoyl phosphatidylcholine was an important factor in the ability of trehalose to stabilize the dry membranes in anhydrobiotic organisms. It was also reported that the water-stress protective effect of fructans was induced by membrane-fructan interaction (Demel et al., 1998). These reports suggested that a low-level accumulation of trehalose or fructan contributed to protect the functions of the cell membrane in transgenic plants rather than conferring an osmolyte effect. Although the interaction between ectoine and the cell membrane was not studied, the FDA staining experiment showed that ectoine synthesis allowed the maintenance of the normal permeability of the cell membrane even upon exposure to hyperosmotic shock (Fig. 6). This suggested that, like trehalose and fructan, ectoine functions to protect the cell membrane from the effects of hyperosmotic shock in transgenic BY2 cells.
Plant cells exposed to hyperosmotic stress such as salinity and drought are considered to be able to adapt to the environment by the overproduction of Pro, one of the natural osmolytes (Yancey et al., 1982). Our results suggested that only cells surviving the irreversibly lethal damage induced by the initial hyperosmotic shock could adapt to the hyperosmotic stress. Although the usual strategies for increasing hyperosmotic tolerance in plants are engineering constitutively by increasing the content of osmolytes, the stress-inducible system of osmolyte production, such as using the rd29A promoter (Kasuga et al., 1999), will be more useful in the next generation. Improved tolerance to the initial hyperosmotic shock is an important factor in this system; therefore, genetically engineered ectoine synthesis in plants is an effective strategy to serve this purpose. The multigene introduction system, in which the accumulation of osmolytes such as Pro and mannitol for intracellular osmotic adjustment and ectoine production to protect the cell membrane from initial damage are simultaneously achieved, will be a powerful strategy of metabolic engineering to grow useful salt- and drought-tolerant plants.
ACKNOWLEDGMENTS
We are grateful to Shin-ichiro Kajiyama and Dr. Eiichiro Fukusaki of Osaka University for excellent instruction on LC/ESIMS analysis of ectoine. We thank Dr. Akira Isogai of the Nara Institute of Science and Technology (NAIST) for helpful technical advice for the analytical experiments. We also thank Drs. Masami Sekine and Ko Kato of NAIST for helpful discussions.
Footnotes
This work was supported by the “Research for the Future” Program of the Japan Society for the Promotion of Science (JSPS–RFTF96R16001) and by a JSPS Research Fellowship for Young Scientists to H.N.
LITERATURE CITED
- Adamec L. The effect of plasmolysis and deplasmolysis on the permeability of plant membranes. Biol Plant. 1984;26:128–131. [Google Scholar]
- Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y. Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep. 1992;12:7–11. doi: 10.1007/BF00232413. [DOI] [PubMed] [Google Scholar]
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard T, Jebbar M, Rassouli Y, Himdi-Kabbab S, Hamelin J, Blanco C. Ectoine accumulation and osmotic regulation in Brevibacterium linens. J Gen Microbiol. 1993;139:129–136. [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Brown AD. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas D, Vargas C, Calderón MI, Ventosa A, Nieto JJ. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst Appl Microbiol. 1998;21:487–497. doi: 10.1016/S0723-2020(98)80060-X. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Del Moral A, Severin J, Ramos-Cormenzana A, Trüper HG, Galinski EA. Compatible solutes in new moderately halophilic isolates. FEMS Microbiol Lett. 1994;122:165–172. [Google Scholar]
- Demel RA, Dorrepaal E, Ebskamp MJM, Smeekens JCM, de Kruijff B. Fructans interact strongly with model membranes. Biochim Biophys Acta. 1998;1375:36–42. doi: 10.1016/s0005-2736(98)00138-2. [DOI] [PubMed] [Google Scholar]
- Epstein E, Norlyn JD, Rush DW, Kingsbury RW, Kelley DB, Cunningham GA, Wrona AF. Saline culture of crops: a genetic approach. Science. 1980;210:399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- Farwick M, Siewe RM, Krämer R. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J Bacteriol. 1995;177:4690–4695. doi: 10.1128/jb.177.16.4690-4695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski EA, Pfeiffer HP, Trüper HG. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid: a novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem. 1985;149:135–139. doi: 10.1111/j.1432-1033.1985.tb08903.x. [DOI] [PubMed] [Google Scholar]
- Galinski EA, Trüper HG. Microbiol behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- Göller K, Ofer A, Galinski EA. Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol Lett. 1998;161:293–300. doi: 10.1111/j.1574-6968.1998.tb12960.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997;12:133–142. doi: 10.1046/j.1365-313x.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- Holmström K-O, Mäntylä E, Welin B, Mandal A, Palva ET, Tunnela OE, Londesborough J. Drought tolerance in tobacco. Nature. 1996;379:683–684. [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2:208–218. [Google Scholar]
- Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol. 1992;174:5027–5035. doi: 10.1128/jb.174.15.5027-5035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kishor PBK, Hong Z, Miao G-H, Hu C-AA, Verma DPS. Overexpression of Δ1-pyrrolin-5-carboxylate synthase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius G, Holmberg N, Bülow L. Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase. Biotechnology. 1996;14:177–180. [Google Scholar]
- Lippert K, Galinski EA. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37:61–65. [Google Scholar]
- Louis P, Galinski EA. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology. 1997;143:1141–1149. doi: 10.1099/00221287-143-4-1141. [DOI] [PubMed] [Google Scholar]
- Malin G, Lapidot A. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J Bacteriol. 1996;178:385–395. doi: 10.1128/jb.178.2.385-395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Yu L, Ono H, Takano M. Gene cloning of ectoine synthase from Halomonas sp. Annu Rep Int Cent Coop Res Biotechnol Jpn. 1993;16:193–200. [Google Scholar]
- Nagata T, Takabe I, Matsui C. Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes) Mol Gen Genet. 1981;184:161–165. [Google Scholar]
- Okuda M, Sakuta S, Tongpin S, Imai K, Shinmyo A, Takano M. Compatible solute of a halotolerant bacterium, Halomanas sp. Microbial Utilization of Renewable Resources. 1989;6:129–135. [Google Scholar]
- Ono H, Okuda M, Tongpim S, Imai K, Shinmyo A, Sakuda S, Kaneko Y, Murooka Y, Takano M. Accumulation of compatible solutes, ectoine and hydroxyectoine, in a moderate halophile, Halomonas elongata KS3 isolated from dry salty land in Thailand. J Ferment Bioeng. 1998;85:362–368. [Google Scholar]
- Ono H, Sawada K, Khunajakr N, Tao T, Yamamoto M, Hiramoto M, Shinmyo A, Takano M, Murooka Y. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J Bacteriol. 1999;181:91–99. doi: 10.1128/jb.181.1.91-99.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yamamoto Y, Hachiya A, Matsumoto H. Synergistic inhibition of growth by aluminium and iron of tobacco (Nicotiana tabacum L.) cells in suspension culture. Plant Cell Physiol. 1995;36:115–125. [Google Scholar]
- Peter H, Weil B, Burkovski A, Krämer R, Morbach S. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J Bacteriol. 1998;180:6005–6012. doi: 10.1128/jb.180.22.6005-6012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P, Glanski EA, Trüper HG. The biosynthesis of ectoine. FEMS Microbiol Lett. 1990;71:157–162. [Google Scholar]
- Pilon-Smits EAH, Ebskamp MJM, Paul MJ, Jeuken MJW, Weisbeek PJ, Smeekens SCM. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995;107:125–130. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Bellés JM, Vayá JL, Serrano VR, Culiáñez-Macià FA. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta. 1997;201:293–297. doi: 10.1007/s004250050069. [DOI] [PubMed] [Google Scholar]
- Ronit R, Peri I, Gilboa H, Avi-Dor Y. 13C NMR study of the interrelation between synthesis and uptake of compatible solutes in two moderately halophilic eubacteria: bacterium Ba1 and Vibrio costicola. Arch Biochem Biophys. 1990;278:106–112. doi: 10.1016/0003-9861(90)90237-s. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Murata A, Murata N. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol. 1998;38:1011–1019. doi: 10.1023/a:1006095015717. [DOI] [PubMed] [Google Scholar]
- Sano H, Youssefian S. A novel ras-related rgp1 gene encoding a GTP-binding protein has reduced expression in 5-azacytidine-induced dwarf rice. Mol Gen Genet. 1991;228:227–232. doi: 10.1007/BF00282470. [DOI] [PubMed] [Google Scholar]
- Sheveleva E, Chmara W, Bohnert HJ, Jensen RG. Increased salt and drought tolerance by d-ononitol production in transgenic Nicotiana tabacum L. Plant Physiol. 1997;115:1211–1219. doi: 10.1104/pp.115.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wróblewski H, Blanco C, Bernard T. Osmoadaptation in Rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Yasuda N, Ono H, Shinmyo A, Takano M. Purification and characterization of 2,4-diaminobutyric acid transaminase from Halomonas sp. Annu Rep Int Cent Coop Res Biotechnol Jpn. 1992;15:187–199. [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science. 1993;259:508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- Wohlfarth A, Severin J, Galinski EA. The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. J Gen Microbiol. 1990;136:705–712. [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte system. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]