Abstract
Background
Preclinical research has demonstrated a causal relationship between medial prefrontal cortex activity and cocaine self-administration. As a step towards translating those data to a neural circuit-based intervention for patients, this study sought to determine if continuous theta burst stimulation (cTBS) to the left frontal pole (FP), would attenuate frontal-striatal activity in two substance-dependent populations.
Methods
Forty-nine substance dependent individuals (25 cocaine, 24 alcohol) completed a single-blind, sham-controlled, crossover study wherein they received 6 trains of real or sham cTBS (110% resting motor threshold, FP1) each visit. Baseline evoked BOLD signal was measured immediately before and after real and sham cTBS (interleaved TMS/BOLD imaging: single pulses to left FP; scalp-to-cortex distance covariate, FWE correction p<0.05)
Results
Among cocaine users, real cTBS significantly decreased evoked BOLD signal in the caudate, accumbens, anterior cingulate, orbitofrontal (OFC) and parietal cortex relative to sham cTBS. Among alcohol users, real cTBS significantly decreased evoked BOLD signal in left OFC, insula, and lateral sensorimotor cortex. There was no significant difference between the groups.
Conclusions
These data suggest that 6 trains of left FP cTBS delivered in a single day decreases TMS-evoked BOLD signal in the OFC and several cortical nodes which regulate salience and are typically activated by drug cues. The reliability of this pattern across cocaine- and alcohol-dependent individuals suggests that cTBS may be an effective tool to dampen neural circuits typically engaged by salient drug cues. Multiday studies are required to determine it this has a sustainable effect on the brain or drug use behavior.
Keywords: neuromodulation, functional MRI, orbitofrontal, mesolimbic
1. Introduction
Through technical and experimental advances in preclinical neuroscience research over the last 10 years, we have an increasingly sophisticated understanding of the neural circuitry of substance dependence. Through optogenetics (Cao et al., 2011; Ferguson and Neumaier, 2012; Steinberg and Janak, 2013) and designer receptors exclusively activated by designer drugs (DREADDs;(Ferguson and Neumaier, 2012)), it is possible to directly increase or decrease cocaine self-administration via stimulation or inhibition of the nucleus accumbens, a core brain region facilitating reinforced behavior and reward saliency. This causal relationship has also been demonstrated in alcohol self-administration (Bass et al., 2013; Cassataro et al., 2014). Beyond direct stimulation of the ventral striatum however, it is also possible to change cocaine self-administration through infralimbic cortical stimulation (Peters et al., 2008). The rodent infralimbic cortex (IL) has strong projections to the multiple regions that modulate arousal, including the medial prefrontal, insular, entorhinal, and amydala cortex (Vertes, 2004). The IL is functionally and anatomically similar to the orbitomedial prefrontal cortex in primates (aka orbitofrontal cortex (OFC)) (Barbas, 1995; Barbas, 2000; Groenewegen and Uylings, 2000)
Given these promising preclinical data, there is strong momentum to develop a neural circuit-based treatment for clinical substance abuse. Transcranial magnetic stimulation (TMS) allows researchers to selectively activate or inhibit populations of neurons in humans. Through electromagnetic induction, repetitive pulses of TMS to the scalp will induce long-term potentiation-like (LTP-like) or long-term depression-like (LTD-like) effects in the cortical area beneath the coil in a frequency-dependent manner. Furthermore, 10 Hz rTMS to the frontal cortex induces a change in dopamine binding (Cho and Strafella, 2009; Strafella et al., 2001) in monosynaptic striatal targets. By applying either a single high frequency (> 10 Hz) or intermittent bursting frequency (intermittent theta burst stimulation; iTBS) to the cortex, it is possible to induce an LTP-like effect on both behavior and neural activity (as measured through neuroimaging (Cho and Strafella, 2009; Siebner et al., 2009), as well as electrophysiological recordings (Mueller et al., 2014)). By applying either a single low frequency (1–5 Hz) or continuous bursting frequency (cTBS), it is possible to induce an LTD-like effect. The effects these forms of stimulation have on neural circuits may be investigated using TMS/BOLD imaging. Single pulses of TMS causes a transient increase in BOLD signal beneath the TMS coil and in regions monosynaptically connected to the stimulated area (Baudewig et al., 2001; Bestmann et al., 2003; Bestmann et al., 2005; Bohning et al., 1999; Bohning et al., 2000a; Bohning et al, 1998; Bohning et al., 2000b). In addition to a cortical BOLD response, this technique has been previously shown to target anatomically distinct dorsal and ventral striatal targets in the absence of task engagement (Hanlon et al., 2013).
When considering treatment development for addiction, one potential strategy is to attenuate activity in the frontal-striatal reward-motivation circuitry that is engaged by drug-related cues. Elevated resting state functional connectivity among brain regions typically involved in arousal and craving – including the nucleus accumbens, amygdala, cingulate cortex, parahippocampal gyrus, and ventral prefrontal cortex – have all been associated with poor abstinence rates (Camchong et al., 2014; McHugh et al., 2014). As with the IL in rodents, the OFC in primates has direct synaptic projections to a wide distribution of brain regions involved in regulating arousal including the insula, parahippocampal gyrus, and ACC. Consequently, if we could decrease connectivity in this circuit through LTD-like rTMS, we may be able to reduce substance induced pathological connectivity and ultimately dampen craving and improve clinical outcomes. However, before the field moves forward and initiates large scale clinical trials, it is scientifically prudent to determine if, in fact, rTMS to the mPFC can induce a causal change in baseline mPFC-striatal connectivity in a controlled manner is non-treatment seeking adult AUD and SUD individuals..
To address this question, we designed a single-blind, sham-controlled crossover study of 50 substance-dependent individuals who all received 6 trains of LTD-like cTBS (or sham) to the left frontal pole in a single day while viewing drug cues. Immediately before and after cTBS or sham, the brain response to mPFC stimulation was measured via interleaved TMS/BOLD imaging. This study design was based on pilot data from our laboratory that demonstrated that this paradigm induced selective decrease in OFC and striatal BOLD signal in a small cohort of chronic cocaine users. In the present study, we aimed to determine if this would generalize to a new, larger group of cocaine-dependent individuals and if it would affect alcohol-dependent individuals in a similar manner.
2. Methods
2.1. Overall protocol design
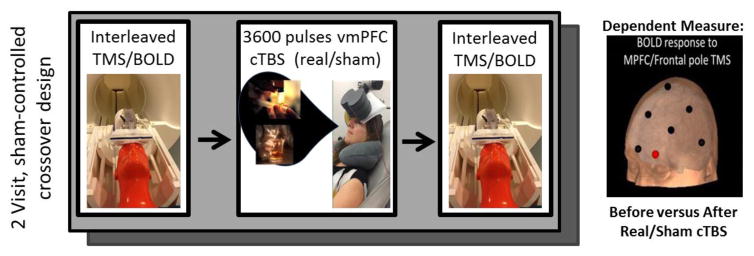
This single-blind, sham-controlled pilot study involved 1 Screening visit and 2 Scanning/Stimulation visits (occurring within 7–14 days of each other). At each scanning/stimulation visit, interleaved TMS/BOLD imaging data was acquired before and after exposure to 6 trains of real or sham continuous theta burst stimulation (cTBS). The order of real and sham visits was counterbalanced across study participants. Continuous TBS was applied over the left FP (landmark based on EEG 10–20 system: FP1). This landmark was coregistered with the T1 MRI for each individual in order to calculate the scalp-cortex distance. Immediately prior to the cTBS procedure, participants were prompted to recall their most recent experience using cocaine or alcohol through a standardized series of cue-induction questions. During the cTBS procedure they were instructed to imagine themselves in that scenario again (See Supplemental Data for standardized scripts1). We tested the hypothesis that cTBS over the FP would induce LTD-like stimulus evoked brain activity in the FP and its projection regions, including cortical limbic areas and the ventral striatum, using interleaved TMS/BOLD imaging.
2.2. Participants and screening
Non-treatment seeking chronic cocaine users (n=25) and alcohol-dependent individuals (n=24) were recruited from the Charleston, SC metropolitan area using word-of-mouth advertising and digital and print media. Individuals were prescreened for TMS and MRI safety over the phone prior to being invited to the Center for Biomedical Imaging at the Medical University of South Carolina (MUSC). Participants signed informed consent documents approved by the MUSC Institutional Review Board. Following informed consent, participants completed several screening assessments including a brief medical history, an MRI/TMS safety screen, the DSM-IV based Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), the Becks Depression Inventory (Beck et al., 1996), and the Alcohol Use Disorders Identification Test (AUDIT) (Babor, 2001). Participants were required to be TMS naïve, which was verified by participant report and prior study logs in our center. A multidrug urine panel (Quikvue 5-panel urine drug screen, Quidel, San Diego, CA) was given to all participants at the screening and scanning/stimulation visits. Participants were informed that they would be required to abstain from cocaine use for 48 hours prior to cTBS due to the sensitivity of the test. Participants were allowed to drink alcohol in their typical patterns, but were required to have an undetectable breath alcohol level (p<0.001) upon their arrival to the laboratory. This was done to minimize the risk of acute withdrawal. Participants were required to have a negative urine drug screen (including cocaine, methamphetamine, benzodiazepines, opiates) at the Scanning/Stimulation visits. Eleven of the cocaine-dependent participants, and none of the alcohol-dependent participants, had a positive UDS for cannabinoids at screening. Demographics and drug/alcohol use variables are described in Supplementary Table 12 (cocaine users), Supplementary Table 22 (alcohol users). For cocaine SUD gender was equally represented and they used cocaine on average for 19 +/− 10 years on an average 3.6 +/− 3.9 days a week but had less alcohol use but more smoking severity than AUD individuals. AUD individuals were younger (27 +/− 5.7 yrs) more males than females, drank on average 7.4 +/− drinks per drinking day and had AUDIT scores in the mild-moderate range. The MINI exam tested for potential influences of major depression, PTSD, panic disorder, manic episodes, social phobia, OCD, anorexia, general anxiety, and personality disorder. No participants met criteria for current diagnoses in any of these domains.
2.3. Scanning and stimulation visits
During both the real and sham stimulation visits, interleaved TMS/BOLD imaging data was acquired immediately before and after the cTBS protocol. Interleaved TMS/BOLD was acquired through a Magstim SuperRapid stimulator. The cTBS protocol was given via a Magventure X100-Magoption. Resting Motor Threshold (rMT) was determined in the MRI scanning room while the participant sat upright on the retracted bed (Figure 1). Resting Motor Threshold (rMT) was determined separately for the cTBS. A thin foam sheet was placed under the coil for both hygiene purposes and patient comfort (Staples ©, Item: 425888, 0.02″/0.5 mm thick). The presence of this sheet likely caused a small decrease in the dose of rTMS delivered to all individuals. This was present for all of the real and sham rTMS sessions in all participants. Self-reported craving was recorded at several timepoints throughout each visit (visual analogue scale: 0–10; before cTBS, immediately after cTBS, at conclusion of Visit).
Figure 1.
Experimental Design.
2.3.1. Interleaved TMS/Functional MRI protocol
2.3.1.1 Coil Positioning
Participants were positioned supine on the scanner bed and the TMS coil (Magstim double 70mm coil, part # 9925-00) was mounted in the MR head coil with a custom TMS coil holder with 6 degrees of freedom (Bohning et al., 1999). The standardized international 10–20 EEG system was used as the basis for positioning the TMS coil as it accounts for variability in participant skull size and is consistently used in clinical TMS applications. The coil was centered over Fp1 (10% dorsal from the nasion to inion, 10% lateral), a location approximating the left frontal pole.
2.3.1.2 Image acquisition
This study was performed on a Siemens 3T TIM trio scanner (Siemens, Erlangen, Germany). Following high resolution anatomical image acquisition (Siemens 3T Tim Trio, TR = 1900 ms, TE = 4 ms, voxel dimensions 1.0 × 1.0 × 1.0 mm, 160 slices, 32 Channel head coil), participants received 2 interleaved TMS-BOLD imaging runs with the coil on Fp1 (biphasic TMS pulses (250μs), 110% rMT, 10.18s interpulse interval, applied during a 100ms gap, flip = 90degrees, TR = 2.5 s, TE = 0.023 s, FOV = 192 mm, voxel size= 3×3×3, 12-channel RAPID Biomedical coil [Rimpar, Germany]). Participants received a total of 80 pulses in the MRI scanner (20 before and after real and sham cTBS). To deal with T1 equilibrium volumes the Siemens TimTrio discards a series of ‘dummy scans’ before it begins to record data. For this study, 2 scans (5 seconds) were discarded. For this protocol, 4 volumes were collected prior to the first TMS pulse, with sufficient time for the rise and fall of the hemodynamic response between pulses (10 seconds). Additionally, as customary with this technique, the BOLD response to the first TMS pulse was not included in the analysis in order to account for startle effects.
2.3.2. Continuous Theta Burst protocol
Following TMS/BOLD acquisition, participants walked to an adjacent room for the cTBS procedure. Six trains of either real or sham LTD-like cTBS were applied over the left frontal pole (Fp1) (3 pulse bursts presented at 5Hz, 15 pulses/sec, 600 pulses/train; 110% rMT, 60-sec interval after 3rd train, MagPro Coil Cool-B65 A/P; 3600 total pulses per visit). During the real and sham cTBS procedure the amplifier output was escalated during the first train (over 30 sec) from 80% to 110% rMT to enhance tolerability. The coil was left in position during the 60-sec intertrain interval. The integrated sham stimulation system in the Magventure X10 system, enabled the sham stimulation level through the electrodes to be proportional to the stimulation intensity of the real condition. The electrodes were placed on the left frontalis muscle under the coil though they were only active during sham stimulation. Preliminary testing in 9 healthy individuals determined that with an amplifier calibration to 9 on the Magventure electrode system individuals were unable to perceive the difference between real and sham stimulation. Real and sham stimulation were well tolerated. Subjective reports indicated that the painfulness of the protocol subsided after the first 15–30 sec, consistent with prior studies showing an endogenous opiate effect of prefrontal rTMS (Taylor et al., 2012; Taylor et al., 2013). At the conclusion of each visit, the participants filled out a form indicating their confidence (scale 1–10) on whether they received sham or real cTBS. A one sample t-test indicated that there was no significance difference between participants’ accuracy in guessing TMS stimulation type and chance [t(45) = 1.19]. This confirms the integrity of the sham system and suggests that individuals were not aware of the protocol administered.
A timer was started after the final cTBS pulse and the participants were led back to the scanner to begin the second TMS/BOLD procedure (which included a new low-resolution T1 image for localization). There were no significant differences in the time interval between conclusion of cTBS and initiation of TMS/BOLD for the real versus sham visits (sham: 7 ± 2 mins (range: 5–13 mins), real cTBS: 7 ± 2 mins (range: 5–10 mins), (p = .65)).
2.4. Imaging analysis
Preprocessing and functional imaging analysis was performed using standard parametric mapping techniques (SPM12, London, UK) in Matlab R2012a (MathWorks, Natick, MA). Images were first converted from DICOM format to 4D NIfTI files, and motion corrected (Realign: 6 parameter, rigid body realignment to first image in each time series using a least squares approach). Normalization parameters, bias correction and anatomical tissue maps were determined simultaneously, using the Segment toolbox. Individual anatomical images were stripped of their skulls by masking the bias corrected image with the combined tissue masks of grey matter, white matter and CSF. The functional images derived from realignment were coregistered, through the mean image, to the skull stripped anatomical image (Coregister: Estimate, using normalized mutual information). Coregistered images were then normalized (Normalize: Write) to MNI template space with the nonlinear warps derived from the Segment tool. Functional images were masked (to remove the skull) and smoothed (8mm FWHM Gaussian kernel) prior to any between-group analyses. The motion was limited to 3mm and residual movement parameters (X, Y, Z, pitch, yaw, roll) were included as regressors on first-level within subject analyses. The TMS/BOLD data were modeled as an event-related design with TMS pulses as instantaneous events and subsequently convolved with the canonical hemodynamic response function. A full factorial design matrix was created for each group and contained the parameter estimates for interleaved TMS/BOLD datasets (before real cTBS, after real cTBS, before sham cTBS, after sham cTBS). Statistical maps were created for both the within-condition (real or sham) and between-condition contrasts, and clusters that exceeded false discovery rate correction were reported (FDR adjusted p<0.05, also referred to as q-values) (Chumbley et al. 2010).
2.5 Scalp-Cortex distance quantification
Given that the effects of TMS on cortical depolarization are proportional to the distance between the skull and the cortex (Kozel et al., 2000; Stokes et al., 2005), we calculated the distance from the scalp to the cortex on the transverse plane on MPRAGE images of each individual (Mango ver. 3.7; Research Imaging Institute, UTHSA, Lancaster and Martinez, 2005)(Figure S1). The average distance from the participant-specific placement of FP1 to the nearest cortex (Cocaine: 18.5 +/− 5.8; Alcohol: 16.37 mm +/− 3.18) was not significantly different between the groups. As in previous studies (Hanlon et al., 2016), all analyses for Interleaved TMS/BOLD were done with these distances as covariates.
3. Results
3.1. TMS-evoked BOLD signal before and after real and sham Theta Burst stimulation
3.1.1 Cocaine users
At baseline, before cTBS the frontal pole stimulation (single pulse) led to elevated BOLD signal in multiple large clusters, including the temporal cortex and insula bilaterally, and the rostromedial cingulate cortex (Supplementary Table 33).
Following real cTBS treatment, cocaine users had significantly lower activity after cTBS in 3 distinct clusters (Table 2): 1) left anterior/subcallosal cingulate, 2) right orbitofrontal cortex, and 3) left precentral gyrus. There were no regions in which real cTBS led to an increase in BOLD signal. Relative to sham stimulation, real cTBS led to significantly lower BOLD signal (in response to single TMS pulses) in 1 large cluster (Positive interaction of time x condition; FDR corrected p-value< 0.001, 4198 voxels) which had local maxima in several discrete areas: 1) the left caudate, ventral striatum and anterior cingulate cortex, 2) the left precuneus, and 3) the left inferior parietal cortex. (Table 1, Figure 2). There were no regions in which sham stimulation led to a significant decrease in BOLD signal. Sham cTBS did, however, lead to an increase in BOLD signal in 1 large cluster located in the posterior cingulate cortex, spreading to the occipital cortex. There no difference in the primary auditory cortex response to TMS pulses across conditions (a positive control region for interleaved TMS/BOLD studies).
Table 2.
Cocaine users: The effect of left FP cTBS treatment, by condition.
| Contrast | Cluster | Maxima | MNI coordinates | Description | |||
|---|---|---|---|---|---|---|---|
| p-value& | size | Z | x | y | z | Label^ | |
| Real cTBS, Before greater than After | |||||||
| 0.003 | 1054 | 3.26 | −57 | 11 | −7 | Temporal Pole | |
| 3.2 | −6 | 20 | −10 | Subcallosal Cingulate | |||
| 3.19 | 0 | 35 | −1 | Cingulate | |||
| 0.01 | 876 | 3 | 66 | −37 | 11 | Supramarginal Gyrus | |
| 3 | 21 | 29 | −19 | Frontal Orbital Cortex | |||
| 2.97 | 24 | 20 | −19 | Frontal Orbital Cortex | |||
| 0.01 | 1392 | 2.93 | −6 | −25 | 41 | Thalamus | |
| 2.89 | −45 | −4 | 50 | Precentral Gyrus | |||
| 2.87 | −6 | −31 | 62 | Precentral Gyrus | |||
|
| |||||||
| Real cTBS, Before less than After | |||||||
| no significant clusters | |||||||
|
| |||||||
| Sham cTBS, Before greater than After | |||||||
| no significant clusters | |||||||
|
| |||||||
| Sham cTBS, Before less than After | |||||||
| <0.001 | 5581 | 4.50 | 0 | −43 | 14 | Posterior Cingulate | |
| 4.10 | −6 | −49 | 20 | Posterior Cingulate | |||
| 3.63 | 36 | −64 | 47 | Lateral Occipital | |||
These data reflect the results of secondary T-tests within the factorial design {Treatment x Time}.
Clusters displayed meet Family Wise Error multiple comparison correction p<0.05.
Labels reflect consensus results from 6mm sphere around local maxima, Harvard-Oxford Atlas and Automated Anatomical Labeling atlas
Table 1.
Cocaine users: The effect of left FP cTBS treatment (LTD-like) on the BOLD response to mPFC stimulation, factorial design.
| Contrast | Cluster | Local Maxima | MNI coordinates | Description | |||
|---|---|---|---|---|---|---|---|
| p-value& | size | Z | x | y | z | Label^ | |
| Brain Regions with lower BOLD signal to following Real (versus Sham) cTBS # | |||||||
| <0.001 | 4198 | 3.50 | −1 | 17 | −7 | Caudate, Accumbens, Cingulate(Anterior)* | |
| 3.39 | 15 | −73 | 50 | Precuneus, Lateral Occipital | |||
| 3.36 | −45 | −46 | 53 | Posterior Parietal (Inferior) | |||
|
| |||||||
| Brain Regions with higher BOLD signal to following Real (versus Sham) cTBS% | |||||||
| No significant clusters | |||||||
These data reflect the results of the positive (#) and negative (%) interaction terms of the factorial design {Real versus Sham for the “Before cTBS” greater than “After cTBS” contrast}.
Clusters displayed that meet Family Wise Error multiple comparison correction p<0.05.
Labels reflect consensus results from 6mm sphere around local maxima, Harvard-Oxford Atlas and Automated Anatomical Labeling atlas.
Note: while this local maxima is not located within the orbitofrontal cortex, the spatial distribution of the cluster extends to the OFC (Figure 2).
Figure 2.
Cocaine Users.
The effect of LTD-like theta burst stimulation on the evoked BOLD response to MPFC stimulation: Clusters shown reflect the results of the positive interaction terms of the factorial design {Real versus Sham for the “Before cTBS” greater than “After cTBS” contrast}. Family Wise Error multiple comparison correction p<0.05. Color bar contains T-values 0–5.
3.1.2 Alcohol users
As with the cocaine users, at baseline, the frontal pole stimulation (single pulse) led to elevated BOLD signal in multiple large clusters, including the temporal cortex and insula bilaterally, and the rostromedial cingulate cortex (Supplementary Table 34).
Following real cTBS alcohol users had significantly lower activity in 1 large cluster (Table 4) that contained local maxima in the parahippocampal gyrus, anterior insula, orbitofrontal cortex and the temporal pole. There was also an increase in TMS-evoked BOLD signal following real cTBS in the left dorsal striatum. Relative to sham stimulation, real cTBS led to significantly lower BOLD signal (in response to single TMS pulses) in 1 large cluster (Positive interaction of time x treatment; FDR corrected p-value< 0.001, 1139 voxels) which contained local maxima in several discrete areas: 1) the left postcentral gyrus and posterior insula, 2) the orbitofrontal cortex and anterior insula, and 3) the left inferior frontal gyrus (Table 3, Figure 3). Sham cTBS led to a significant decrease in 1 large cluster located in the cingulate cortex. There were no regions with elevated BOLD signal following sham cTBS. There was also no difference in the primary auditory cortex response to TMS pulses (a positive control region for interleaved TMS/BOLD studies).
Table 4.
Alcohol Users: The effect of left FP cTBS treatment, by condition.
| Contrast | Cluster | Maxima | MNI coordinates | Description | |||
|---|---|---|---|---|---|---|---|
| p-value& | size | Z | x | y | z | Label^ | |
| Real cTBS, Before greater than After | |||||||
| <0.001 | 2458 | 3.94 | 30 | −34 | −10 | Parahippocampal Gyrus, Hippocampus | |
| 3.84 | −42 | 11 | −25 | Insula (Anterior) Frontal Orbital, Temporal | |||
| 3.79 | −33 | 17 | −25 | pole | |||
|
| |||||||
| Real cTBS, Before less than After | |||||||
| <0.001 | 3410 | 4.04 | 27 | −10 | 14 | Putamen | |
| 3.92 | 27 | −40 | 20 | Pallidum | |||
| 3.91 | 27 | −22 | 17 | Putamen | |||
|
| |||||||
| Sham cTBS, Before greater than After | |||||||
| 0.008 | 1360 | 3.94 | 3 | 14 | 26 | Temporal Pole | |
| 3.41 | 0 | −1 | 29 | Subcallosal Cingulate | |||
| 3.29 | 66 | −34 | 44 | Cingulate | |||
|
| |||||||
| Sham cTBS, Before less than After | |||||||
| no significant clusters | |||||||
These data reflect the results of secondary T-tests within the factorial design {Treatment x Time}.
Clusters displayed meet Family Wise Error multiple comparison correction p<0.05.
Labels reflect consensus results from 6mm sphere around local maxima, Harvard-Oxford Atlas and Automated Anatomical Labeling atlas
Table 3.
Alcohol Users: The effect of left FP cTBS treatment (LTD-like) on the BOLD response to mPFC stimulation, factorial design.
| Contrast | Cluster | Local Maxima | MNI coordinates | Description | |||
|---|---|---|---|---|---|---|---|
| p-value& | size | Z | x | y | z | Label^ | |
| Brain Regions with lower BOLD signal to following Real (versus Sham) cTBS # | |||||||
| <0.001 | 1139 | 3.29 | −60 | −10 | 29 | Postcentral Insula (Posterior) | |
| 3.28 | −36 | 32 | 2 | Frontal Orbital, Insula (Anterior) | |||
| 3.05 | −51 | 2 | 38 | Precentral, Inferior Frontal | |||
|
| |||||||
| Brain Regions with higher BOLD signal to following Real (versus Sham) cTBS% | |||||||
| No significant clusters | |||||||
These data reflect the results of the positive (#) and negative (%) interaction terms of the factorial design {Real versus Sham for the “Before cTBS” greater than “After cTBS” contrast}.
Clusters displayed that meet Family Wise Error multiple comparison correction p<0.05.
Labels reflect consensus results from 6mm sphere around local maxima, Harvard-Oxford Atlas and Automated Anatomical Labeling atlas
Figure 3.
Alcohol Users.
The effect of LTD-like theta burst stimulation on the evoked BOLD response to MPFC stimulation: Clusters shown reflect the results of the positive interaction terms of the factorial design {Real versus Sham for the “Before cTBS” greater than “After cTBS” contrast}. Family Wise Error multiple comparison correction p<0.05. Color bar contains T-values 0–5.
3.2 Self-reported craving
Within the cocaine users there was a significant main effect of time on self-reported craving ratings (F(2,138)=4.91), but there was no interaction, nor main effect of condition (real versus sham) (Supplementary Figure 1 5). The average craving rating before cTBS (Real: 3.66±2.91; Sham:3.33±2.65) was numerically (but not significantly) higher than the craving rating immediately after cTBS (Real: 2.93±2.78; Sham:2.90±2.25) and at the conclusion of the MRI scanning session (approximately 60 minutes after cTBS treatment)(Real: 2.60±2.25; Sham: 2.47±2.54).
Within the alcohol drinkers there was also a significant main effect of time (F(2,132)=3.62), but no interaction nor effect of condition. The average craving rating before cTBS (Real: 1.52±1.53; Sham: 1.39±1.64) was relatively low and remained low immediately after cTBS (Real: 1.66±1.53; Sham: 1.39±1.64) and at the conclusion of the MRI scanning session (Real: 1.36±1.18; Sham: 1.56±1.68).
4. Discussion
The data from the present study demonstrated that: 1) six sessions of cTBS to the frontal pole induces a significant decrease in orbitofrontal activity, as well as indirectly attenuates activity in several functionally-related nodes in the salience network, including the anterior insula, and anterior cingulate, and 2) 6 sessions of LTD-like cTBS delivered in a single day is feasible and tolerable for a cohort of cocaine users and alcohol users. It is important to note that while a reduction in BOLD activity within the OFC is presumably the result of direct cTBS, remote regions within the salience network have reduced BOLD due to their functional relationship to the OFC. Additionally, while cTBS appears to decrease the orbitofrontal cortex and insula in both cocaine users and alcohol dependent individuals, cocaine users showing a decrease in ventral striatal BOLD signal, while alcohol users have a corresponding increase in dorsal striatal BOLD signal. These data extend previous work by our group which demonstrated the FP cTBS decreases the OFC and ventral striatum, and complements a growing body of preclinical research which demonstrated the importance of these mesolimbic frontal-striatal areas in cocaine and alcohol use. As with all rTMS work to date, a single day of cTBS administration, as performed in this study, is not likely to have a sustainable effect on drug use behavior or craving. These data however, provide a critical proof of principle that FP cTBS can change baseline reactivity of the OFC, insula and cingulate – a known network of brain regions that are altered in cocaine and alcohol use disorder. This is a biological foundation upon which future studies can build - exploring the sustainability and feasibility of inducing behavioral change following multiple days of FP cTBS in cocaine and alcohol use disorder.
In aggregate, the results of this investigation suggest that this novel brain stimulation protocol (6 trains of FP cTBS) may effectively attenuate multiple areas of the salience network (typically engaged by drug cues) in both cocaine dependent and alcohol dependent individuals- a promising step towards developing a neural circuit based treatment intervention for these individuals. The use of non-invasive brain stimulation techniques, such as rTMS, to modulate frontal-striatal circuits typically engaged by drug cues, therefore might be a powerful new adjuvant to behavioral treatment in addiction, if the circuits could be imaged and then modulated in a therapeutically appropriate direction, and the modulation resulted in behavioral changes. This is particularly true for cocaine dependence in which there is currently no FDA-approved pharmacotherapy.
4.1 Frontal Pole as a candidate target for treatment development
Theoretically, there are at least two primary methods for developing brain stimulation as a treatment for addiction. One is to dampen the frontal-striatal circuit involved in craving by down-regulating the mPFC, and the other is to amplify the frontal-parietal circuits involved in executive control by up-regulating the dlPFC (reviews: (Dunlop et al., 2016; Hanlon et al., 2015). Amplifying executive control circuitry is certainly a very reasonable target and has been the primary target of choice in most TMS studies in substance dependent populations (reviews:(Barr et al., 2011; Bellamoli et al., 2014; Gorelick et al., 2014; Wing et al., 2013)). From a craving and relapse perspective however, the majority of clinical neuroimaging studies demonstrate that the OFC, anterior cingulate cortex, orbitofrontal cortex, and even the insula are regions that are more directly involved in craving, and resting state connectivity among these regions is related to relapse(Camchong et al., 2014; Garavan et al., 2000; Hanlon et al., 2016a; McHugh et al., 2014; Schacht et al., 2013; Sutherland et al., 2012). Consequently, applying an LTD-like dampening strategy to the MPFC might be an equally or even more efficient means of reaching a clinically-meaningful endpoint.
In this manuscript, we present the first study in a relatively large substance-dependent sample which examines the effect of an LTD-like rTMS protocol (cTBS) on baseline frontal-striatal connectivity (as measured by interleaved TMS/BOLD imaging) involved in craving. We demonstrate that, in both cocaine and alcohol users, relative to sham stimulation, FP cTBS decreases activity in salience-related regions, including ACC and insula. These regions have been postulated (or established) to be part of a salience related network. Although it is not clear from this data set if this attenuating effect of cTBS on these regions will generalize to an attenuation of drug-cue induced craving, these results demonstrate a critical and robust “proof of principle” that FP cTBS can, in fact, decrease activity (as measured with interleaved TMS/BOLD) among brain regions involved in salience and reward in both cocaine and alcohol dependent individuals. Assuming these results can be reliably replicated by other groups, these data can serve as a foundation for future clinical research studies in addiction.
4.2 Scientific rationale for 6 sessions of mPFC cTBS at 110% rMT per day
In 2015, our group presented data which demonstrated that 3600 pulses of cTBS to the left frontal pole reduced TMS-evoked BOLD signal in the OFC and ventral striatum, including the nucleus accumbens (Hanlon et al., 2015). At the time, this was the highest number of cTBS pulses reported to have been delivered to the brain in a single session, and one of the few protocols that had ever applied cTBS to the frontal pole. The present manuscript extends these findings to a larger group of cocaine-dependent participants and alcohol-dependent participants. Here we demonstrate that 3600 pulses (6 times the standard ‘dose’ of cTBS given to the motor cortex) has significant effects on the neural response to frontal pole stimulation. While the field of developing rTMS as a treatment for addiction is still in its infancy, and the parameter space is very large (e.g., “How many sessions are needed? What is the best site of stimulation? What intensity should be used?”), it is important to note that the parameters chosen for this protocol were logically chosen based on prior research in clinical depression treatment and studies on the motor control system.
Specifically, while 80% of the active motor threshold is typically chosen for TBS protocols over the motor cortex, for this study and our previous study (Hanlon et al., 2013), 110% rMT was chosen because 1) the scalp-to-cortex distance from the frontal pole is greater than from the motor cortex (Stokes et al., 2013) and 2) this is the dose chosen for clinical treatment of depression over the dlPFC. The number of pulses used (3600 per day; 6 sessions of traditional cTBS (Huang et al., 2005)) was chosen to 1) keep the total number of pulses similar to those given for clinical treatment of depression (3000), 2) maintain the theta burst rhythm (600 pulses), yet 3) enabling participants to receive a full course of clinical rTMS as given for depression (30 sessions) in a five day span (a target for future cTBS studies in addiction). Having stated this, since the original conception of this study, data has emerged suggesting that, while multiple sessions of TBS in a single day are effective, intersession intervals of 15 minutes or greater may be necessary for dose-dependent effects on c-Fos and synaptic activity markers to be observed (Volz et al., 2013). Consequently, while we observed significant effects of cTBS in this study, future studies including a 15 minute break between 600 pulse sessions of cTBS, may have amplified effects.
4.3 Using interleaved TMS/BOLD imaging as a neurobiological “Proof of Principle”
As the momentum for developing rTMS as a treatment for addiction grows, it is important to be mindful about what we are actually doing to the brain. The present study is the first study that has used an integrated neuroimaging and brain stimulation approach to investigate the effect of LTD-like brain stimulation on neural circuitry which governs craving in both cocaine and alcohol dependent individuals. By measuring functional connectivity with interleaved TMS/BOLD immediately before and after the real or sham cTBS session, it is possible to directly investigate the causal effects of this stimulation intervention on frontal-striatal circuit activity.
In a similar manner, Chen and colleagues (2013)(Chen et al., 2013) demonstrated that an LTD-like rTMS protocol to the lateral prefrontal cortex amplified BOLD activity in the central executive network and dampened activity in the medial prefrontal aspects of the salience network in healthy controls. Additionally, Liston et al. (2014) (Liston et al., 2014)showed that a 5-week course of LTP-like stimulation (10 Hz) to the DLPFC in 17 patients with major depressive disorder normalized previously high connectivity within the default mode network (which includes the MPFC). They also demonstrated that activity in the DLPFC was inversely related to activity in the MPFC. However, our group has previously shown that the reciprocal relationship found between executive control and default mode networks in healthy controls is not present in cocaine users (Hanlon et al., 2016b). That is, stimulating the DLPFC did not have a significant attenuating effect on MPFC activity in cocaine users. This suggests that, it may be more efficient to attenuate activity in the FP directly rather than to amplify DLPFC activity and indirectly attenuate activity in the mPFC.
4.4 Caveats and limitations
While the data from the present study suggest that the FP may be a strong target for modulating limbic circuit activity in cocaine abusers and heavy alcohol users, there was no significant difference in the effect of real versus sham cTBS on self-reported craving. Individuals reported a decrease in craving over the course of the experimental day that was no difference for real cTBS and for sham cTBS. This was true for both the cocaine users and the alcohol users. These data demonstrate the importance of having a well-designed active sham control for all rTMS studies – especially studies that rely on subjective measures, such as self-reported craving – as dependent variables. Future studies with multiple days of TMS intervention using traditional functional neuroimaging protocols of cue-induced craving are necessary in order to evaluate the generalizability of these findings to cue-associated craving.
Other limitations of this study include the fact that it was single-blind, and that we do not know if the attenuating effects of cTBS will be sustainable beyond the stimulation day (Di Lazzaro et al., 2005; Huang et al., 2005; Huang et al., 2009). In order for this to be a viable treatment adjuvant for patients, multiple days of cTBS sessions are likely required in order to achieve a sustainable effect. For example, in the FDA-approved treatment protocol for depression, several weeks of 10Hz TMS sessions are given in order to achieve a clinical effect that lasts for several months beyond the cTBS session itself. Yet, it is not currently known whether LTP-like effects would be achieved faster or slower with a bursting pattern protocol (as in TBS) rather than a single frequency (as in traditional rTMS).
As the field moves forward with pursuing rTMS as a potential treatment tool for substance-dependent populations there are a few primary things to consider: 1) what is the best target?, 2) what is the best frequency and pattern to use?, 3) which patients are likely to benefit the most? 4) do individuals with different substance abuse patterns need different treatments or length of treatments? 5) will multiple days of treatment be feasible and tolerable and most importantly “safe” in this patient population. The data from the present study demonstrate a crucial first step - that decreasing activity in the mPFC and ventral striatum with TMS is feasible and that it may be a promising target. Before moving forward with slow and expensive clinical trials, however, it is important to have a comprehensive understanding of limbic and executive circuit functioning in substance use populations. With this knowledge we will hopefully be able to develop circuit-specific treatment strategies across different substance use disorders.
Supplementary Material
Highlights.
Theta burst stimulation (TBS) was delivered to the ventromedial prefrontal cortex
TBS decreased mesolimbic brain reactivity in both cocaine users and alcohol users
Orbitofrontal cortex, insula, cingulate, and striatum were significantly decreased
There were no significant differences between cocaine and alcohol users
One visit of real cTBS did not decrease craving more than sham cTBS
Acknowledgments
Role of Funding Source:
This work was supported by R01DA0036617 (Hanlon), T32DA007288 (McGinty), P50 DA015369 (Kalivas), P50 AA010761 (Becker), K05 AA017435 (Anton). Additional assistance was given by the South Carolina Translational Research Institute UL1 TR000062.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors:
Logan T Dowdle and Brittany Correia performed analysis on data. Oliver Mithoefer collected the data. Mark George and Raymond Anton contributed to the initial design and final interpretation of the data. Colleen A. Hanlon designed experimental procedures, and write the primary drafts of the manuscript. All authors approved of the final manuscript before submission.
Conflict of Interest:
The authors have no personal or professional scientific conflicts of interest to disclose related to the content or equipment used in this investigation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbas H. Pattern in the cortical distribution of prefrontally directed neurons with divergent axons in the rhesus monkey. Cereb Cortex. 1995;5:158–65. doi: 10.1093/cercor/5.2.158. [DOI] [PubMed] [Google Scholar]
- Barbas H. Complementary roles of prefrontal cortical regions in cognition, memory, and emotion in primates. Adv Neurol. 2000;84:87–110. [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23:454–66. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: An update about human studies. Behav Neurol. 2014;2014:815215. doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, Teneback C, Vincent DJ, George MS. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–94. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Camchong J, Macdonald AW, 3rd, Mueller BA, Nelson B, Specker S, Slaymaker V, Lim KO. Changes in resting functional connectivity during abstinence in stimulant use disorder: A preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 2014;139:145–51. doi: 10.1016/j.drugalcdep.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZF, Burdakov D, Sarnyai Z. Optogenetics: Potentials for addiction research. Addict Biol. 2011;16:519–31. doi: 10.1111/j.1369-1600.2011.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, Sjulson L. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology. 2014;39:283–90. doi: 10.1038/npp.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110:19944–9. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–64. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–50. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Ann NY Acad Sci. 2016;1394:31–54. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Neumaier JF. Grateful DREADDs: Engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology. 2012;37:296–7. doi: 10.1038/npp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann NY Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Jones JL. Biomarkers for success: Using neuroimaging to predict relapse and develop brain stimulation treatments for cocaine-dependent individuals. Int Rev Neurobiol. 2016a;129:125–56. doi: 10.1016/bs.irn.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Moss H, Canterberry M, George MS. Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: An interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology. 2016b;41:3032–3041. doi: 10.1038/npp.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W, Classen J, Peterchev AV, Zangen A, Ugawa Y. Consensus: New methodologies for brain stimulation. Brain Stimul. 2009;2:2–13. doi: 10.1016/j.brs.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, 3rd, Rao H, Deng ZD, Peterchev AV, Sommer MA, Egner T, Platt ML, Grill WM. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci. 2014;17:1130–6. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology (Berl) 2013;227:627–37. doi: 10.1007/s00213-013-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C, Pascual-Leone A, Huber R, Taylor PC, Ilmoniemi RJ, De Gennaro L, Strafella AP, Kahkonen S, Kloppel S, Frisoni GB, George MS, Hallett M, Brandt SA, Rushworth MF, Ziemann U, Rothwell JC, Ward N, Cohen LG, Baudewig J, Paus T, Ugawa Y, Rossini PM. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Janak PH. Establishing causality for dopamine in neural function and behavior with optogenetics. Brain Res. 2013;1511:46–64. doi: 10.1016/j.brainres.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Barker AT, Dervinis M, Verbruggen F, Maizey L, Adams RC, Chambers CD. Biophysical determinants of transcranial magnetic stimulation: Effects of excitability and depth of targeted area. J Neurophysiol. 2013;109:437–44. doi: 10.1152/jn.00510.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–95. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain. 2012;153:1219–25. doi: 10.1016/j.pain.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, George MS. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology. 2013;38:1189–97. doi: 10.1038/npp.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Benali A, Mix A, Neubacher U, Funke K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul. 2013;6:598–606. doi: 10.1016/j.brs.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–30. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.