Abstract
Regulatory guidance documents stress the value of assessing multiple tissues and the most appropriate endpoints when evaluating chemicals for in vivo genotoxic potential. However, conducting several independent studies to consider multiple endpoints and/or tissue compartments is resource intensive. Furthermore, conventional approaches for scoring genotoxicity endpoints are slow, tedious, and less objective than what would be considered ideal. In an effort to address these issues with current practices, we attempted to i) employ flow cytometry-based methods to score liver micronuclei, blood micronuclei, and blood Pig-a gene mutation, and ii) integrate the endpoints into a common general toxicology study design—the rat 28-day repeat dose study. A proof-of-principle experiment was performed with 6-week old male Crl:CD(SD) rats exposed to diethylnitrosamine (DEN) for 28 consecutive days. One day later blood was collected for micronucleated reticulocyte (MN-RET) and Pig-a mutation assays, and liver tissue was obtained for micronucleated hepatocyte (MNHEP) scoring. MN-RET frequencies were not affected by DEN exposure, and mean Pig-a mutant cell frequencies were only slightly elevated. On the other hand, %MNHEP showed marked, dose-related increases (2.2, 7.2, and 9.1 mean fold-increase for 5, 10, 15 mg DEN/kg/day, respectively). Concurrent with MNHEP analyses, assessments of Ki-67-positive events and the proportion of 8n nuclei provided evidence for treatment-related changes to hepatocyte proliferation. Collectively, these results reinforce the importance of evaluating chemicals’ genotoxic potential in liver in addition to hematopoietic cells, and suggest that several automated measurements can be successfully integrated into repeat-dose studies for higher efficiencies and better utilization of fewer animals.
Keywords: micronuclei, hepatocytes, blood, Pig-a gene mutation, flow cytometry
Introduction
The in vivo micronucleus test is most often conducted with immature erythrocytes (i.e., reticulocytes, or RET) obtained from bone marrow or peripheral blood [1–3]. Indeed, for the last several decades, the micronucleated reticulocyte (MN-RET) based assay has been a key component of regulatory safety assessment packages [4–5]. Even so, recent guidance documents stress the importance of considering other tissues in addition to those of the hematopoietic compartment. For example, the International Conference on Harmonisation S2(R1) guidance describes the importance of evaluating a second tissue to guard against negative MN-RET results being due to lack of adequate bone marrow exposure to a drug and/or its metabolites [4].
To address exposure and metabolite concerns, contemporary in vivo genotoxicity studies often supplement MN-RET analyses with a liver-based assay [6–8]. The liver is the main site of drug metabolism, and the concentration of genotoxic intermediates can therefore be highest in this tissue. However, the most commonly utilized approach, a liver tissue comet assay, is not sensitive to aneugens, and owing to fast repair kinetics, it is not easily integrated into standard repeat-dose toxicology studies. Industrial genetic toxicologists, regulatory officials, and other stake-holders are therefore in need of more efficient, 3Rs friendly methods for measuring in vivo DNA damage in non-hematopoietic tissues, especially the liver.
One potential solution that has been described by the JEMS/MMS work group is to treat 6-week old rats for 28 consecutive days, and one day later harvest liver tissue for micronucleated hepatocyte (MNHEP) measurements. This approach takes advantage of the fact that at this age the rate of rat hepatocyte proliferation has slowed but not ceased, and genotoxicant-induced MNHEP accumulate with repeat treatments [9–10]. Data collected to date are encouraging, as they suggest that as with MN-RET and Pig-a gene mutation assays [11–13], MNHEP analyses can be integrated into ongoing 28-day general toxicology studies [10, 14–16].
The present report describes a proof-of-principle experiment aimed at enhancing the throughput and objectivity by which several genetic toxicology endpoints are made through the use of flow cytometry, while simultaneously addressing animal and other resource requirements by utilizing a 28-day integrated study design. The experiment was conducted with diethylnitrosamine (DEN), a potent hepatocarcinogen that has generated mixed results in the blood-based Pig-a mutation assay [17–19], and consistently negative results in MN-RET assays [17–18, 20]. Thus, while many hepatocarcinogens that require enzymatic bioactivation in order to form proximate genotoxic and tumorigenic metabolites are positive in Pig-a and MN-RET assays [13, 21], we anticipated DEN would highlight the desirability of investigating hepatocytes in addition to hematopoietic cells whenever adequate systemic exposure is not anticipated. The genotoxicity results, all generated via flow cytometric analyses, are discussed in terms of the desirability of higher data acquisition efficiencies, as well as the animal reduction opportunities afforded by commonly employed repeat-treatment study designs.
Materials and Methods
Reagents, Miscellaneous Supplies
DEN (CAS No. 55-18-5) and dimethyl sulfoxide (CAS No. 67-68-5) were purchased from Sigma-Aldrich, St. Louis, MO. Heat-inactivated fetal bovine serum (FBS; cat. no. 89510-186) was from VWR, Radnor, PA. Reagents used for flow cytometric MN-RET scoring (Anticoagulant Solution, Buffer Solution, DNA Stain, Anti-CD71-FITC and Anti-CD61-PE Antibodies, RNase Solution, and Malaria Biostandards) were from In Vivo MicroFlow® PLUS R Kits, Litron Laboratories, Rochester, NY. Reagents used for flow cytometric enumeration of mutant erythrocytes (RBCCD59−) and mutant reticulocytes (RETCD59−) were from Rat MutaFlow® Kits, Litron Laboratories, and included Anticoagulant Solution, Buffer Solution, Nucleic Acid Dye Solution (contains SYTO® 13), Anti-CD59-PE, and Anti-CD61-PE. Reagents used for flow cytometric MNHEP scoring (Liver Preservation Buffer, Buffer Solution, Erythrocyte Clearing Solution, Collagenase Solution, Lysis Solution 1, Lysis Solution 2, Anti-Ki-67-eFluor® 660, DNA Stain (contains SYTOX® Green), and RNase Solution) were from Prototype In Vivo MicroFlow® PLUS RL Kits, Litron Laboratories. Additional supplies included Lympholyte®-Mammal cell separation reagent from CedarLane, Burlington, NC; Anti-PE MicroBeads, LS Columns, and a QuadroMACS™ Separator from Miltenyi Biotec, Bergisch Gladbach, Germany; CountBright™ Absolute Count Beads and fetal bovine serum (FBS) from Invitrogen, Carlsbad, CA; Falcon-brand 35 micron cell strainers (cat. no. 352235) from Corning, Corning, NY; and heparinized capillary tubes from Fisher Scientific, Pittsburg, PA (cat. no. 22-260-950).
Animals, Treatments
Experiments were conducted with the oversight of the University of Rochester’s Institutional Animal Care and Use Committee. Male Crl:CD(SD) rats were purchased from Charles River Laboratories, Wilmington, MA. Rodents were allowed to acclimate for approximately one week. Water and food were available ad libitum throughout the acclimation and experimental periods.
Age at the start of treatment was 6 weeks, n = 5 per group. Six-week old rats were chosen to match the JEMS/MMS group’s work that has recently focused on this age [10]. DEN was prepared in water at 10× concentrations, and aliquots were frozen at −20°C until use. On each day of administration, aliquots were thawed and added to water to prepare working solutions of 0, 0.5, 1, and 1.5 mg/mL. Administration was by oral gavage at 10 mL/kg body weight, for final dose levels of 0, 5, 10, or 15 mg/kg/day. The 15 mg/kg/day top dose was based on the report of Narumi and colleagues, who also studied male Crl:CD(SD) rats and characterized 12.5 mg/kg/day for 28 days as “about or lower than the maximum tolerated dose” [22].
Blood Harvest
Day 29 blood specimens were collected into MicroFlow kit-supplied Anticoagulant Solution-coated needles and syringes via heart puncture exsanguination (typically 6–9 mL blood were collected per rat). For the MicroFlow assay, 50 µL aliquots of each whole blood sample were transferred to tubes containing 175 µL Anticoagulant Solution, and they were maintained at room temperature for less than 3 hrs until fixation with ultracold methanol as described by Torous and colleagues [23]. For Pig-a analyses, 80 µL of each blood sample were transferred to tubes containing 100 µL kit-supplied Anticoagulant Solution where they remained at room temperature for less than 3 hr until leukodepletion as described previously [10].
Micronucleated Reticulocyte Assay: Sample Preparation, Data Acquisition
MN-RET and reticulocyte (RET) frequencies were determined for each of 20 blood samples via flow cytometry according to the In Vivo MicroFlow PLUS-R Kit manual, v170503 (www.litronlabs.com). These procedures have been described in detail [23–24]. MN-RET frequency measurements were based on the acquisition of approximately 20,000 high CD71-positive RET per blood sample. Instrument setup and calibration was performed using kit-supplied biological standards (P. berghei-infected blood cells) [25–26]. A BD FACSCalibur™ flow cytometer running CellQuest™ Pro v5.2 software was used for data acquisition and analysis.
Pig-a Gene Mutation Assay: Sample Preparation, Data Acquisition
RETCD59− and RBCCD59− frequencies were determined for each blood sample via immunomagnetic depletion of wild-type erythrocytes and flow cytometric analysis, as described previously [13, 27]. In addition to reducing analysis times to 4 minutes per sample, immunomagnetic depletion made it practical to evaluate many times more cells than is otherwise feasible. For instance, an average of 200 × 106 erythrocytes and 5.4 × 106 RET per sample were evaluated for the CD59-negative phenotype.
Pig-a sample labeling and washing steps utilized deep-well 96 well plates from Axygen Scientific (cat. no. P-DW-20-C) that facilitated efficient, parallel processing. Flow cytometric analyses were also conducted using 96 well plates (U-bottom, Corning, cat. no. 3799) and the BD High Throughput Sampler (HTS) provided automated, walk-away flow cytometric analysis. These variations are described in the Rat MutaFlow Instruction Manual, v140403 (www.litronlabs.com).
An Instrument Calibration Standard was created with approximately 50% wild-type and 50% mutant-mimic erythrocytes, and as described previously, it provided a means to rationally and consistently define the location of CD59-negative cells [13, 27]. A BD FACSCanto II flow cytometer running Diva v6.1.2 software was used for data acquisition and analysis.
Micronucleated Hepatocyte Assay: Sample Preparation, Data Acquisition
Rats were anesthetized via CO2 overdose, exsanguination occurred by heart puncture, and then livers were immediately excised. Wet weights were recorded, and left lateral lobes were transferred to 50 mL tubes that contained ice-cold kit-supplied Liver Preservation Buffer (includes 10% v/v dimethyl sulfoxide, added same day as use).
Approximately 1 g of each left lateral lobe was processed for flow cytometric analysis according to procedures described previously [28]. Briefly, following collagenase treatment and centrifugation/washing steps, cells were passed through a 35 micron cell strainer and stored overnight in a 4 °C refrigerator. On the day of flow cytometric analysis, cells were washed again via centrifugation, and 100 µL aliquots were resuspended with 400 µL kit-provided Lysis Solution 1 followed by 400 µL Lysis Solution 2. Samples were analyzed with a FACSCanto™ II flow cytometer equipped with 488 nM and 633 nM excitation (BD Biosciences, San Jose, CA).
MNHEP were also scored via microscopy, whereby 10 µL of strained cell preparations were applied to acridine orange-coated slides prepared as described in Hayashi and colleagues [29]. Slides were coded before analyses that were accomplished with an Olympus BH-2 microscope (10× eyepiece, 40× objective) outfitted for fluorescence using blue light excitation via a mercury lamp.
Data Analysis
The incidence of MNHEP, MN-RET, and RET are expressed as frequency percent. The formulas used to calculate RBCCD59− and RETCD59− frequencies based on pre- and post-immunomagnetic column data are described in Dertinger et al. [13] and the MutaFlow manual (www.litronlabs.com).
To evaluate the effect that DEN treatment may have had on %MNHEP, %MN-RET, and RBCCD59− and RETCD59− frequencies relative to concurrent vehicle control rats, two approaches were utilized. Dunnett’s multiple comparison t-tests were performed using JMP software’s one-way ANOVA platform (v12.0.1, SAS Institute Inc., Cary, NC). These tests were performed at the 5% level using a one-tailed test to identify significant increases relative to vehicle control. Second, the presence of a dose-related trend was evaluated, where a statistically significant regression effect required p < 0.025. Additionally, each of the genotoxicity endpoints generated in the current DEN study was considered against the distribution of lab-specific historical controls. Specifically, the number of animals in each treatment group that exceeded historical negative control rats’ upper bounds 95% tolerance interval, alpha 0.05, was determined (historical control n = 156 rats for the MN-RET endpoint, 756 for RBCCD59− and RETCD59− frequencies, and 64 for MNHEP).
Along with MNHEP assessments, several indices of hepatocyte growth and proliferation were collected, including %Ki-67-positive nuclei, proportion of nuclei with 8n and greater DNA content (%8n+), hepatocyte proliferation index (HPI), and normalized liver weight (g liver weight / kg body weight) [28]. These data, along with %RET, were evaluated for treatment-related effects using Dunnett’s multiple comparison t-tests. In each of these cases, the tests were two-tailed in order to identify significant increases or decreases compared to concurrent vehicle controls.
Note that for the parametric tests describe above, Levene’s tests were used to verify the equality of variances in the samples (p > 0.05; JMP’s ANOVA platform). The following endpoints were log10 transformed in order to fulfill this requirement: RBCCD59− and RETCD59− frequencies, %MNHEP, and %Ki-67+ events. Since zero RETCD59− values were occasionally observed, a 0.1 offset was added to each RETCD59− per 106 number prior to log transformation.
Results
Signs of Toxicity
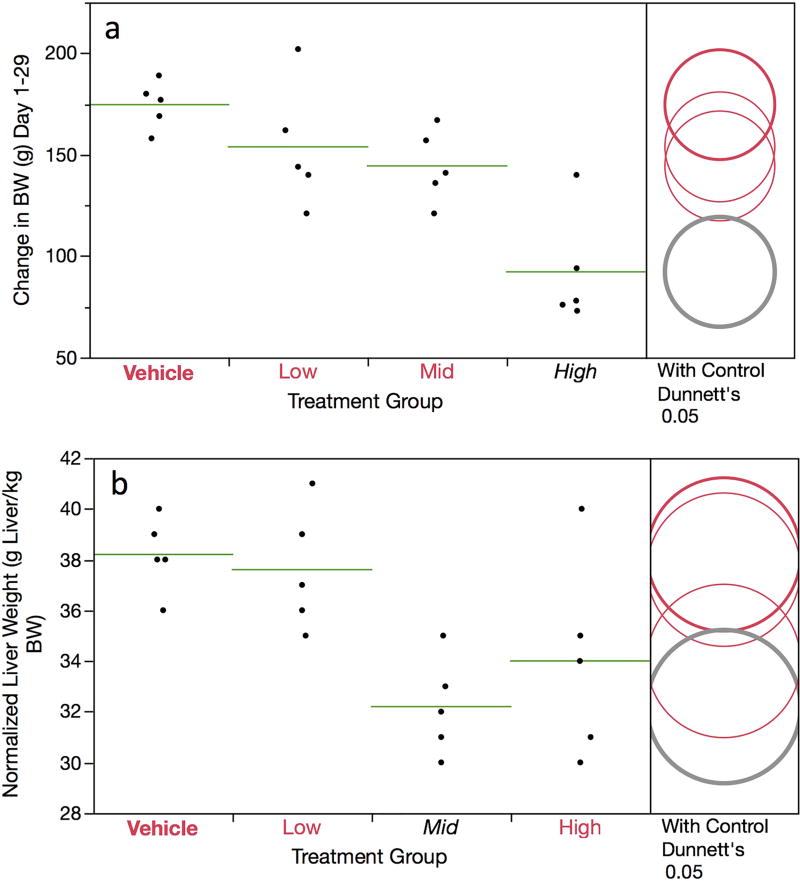
DEN treatment led to decreased mean body weight gains: 182, 156, 143, and 90 g for the 0, 5, 10 and 15 mg/kg/day dose groups, respectively (Figure 1a). The high dose group attained statistical significance. Additionally, mean normalized liver weights were lower in each DEN treatment group compared to vehicle control, and the mid dose group attained statistical significance (Figure 1b). As there were no deaths or other outward signs of toxicity beyond the reduced body weight gain noted above, the DEN doses used in this study appeared to be well tolerated over the 4-week treatment period.
Figure 1.
Change in rat weight data (g) (panel a) and normalized liver weights (panel b) are graphed for each of four DEN treatment groups. Data for individual rats are shown, and group means appear as horizontal green lines. Dunnett’s test results are shown to the far right of each graph, where statistically significant differences relative to the concurrent vehicle control group appear as italicized black text as opposed to red text, and by a grey circle as opposed to a red circle. Circles’ diameters represent 95% confidence intervals.
Micronucleated Reticulocytes
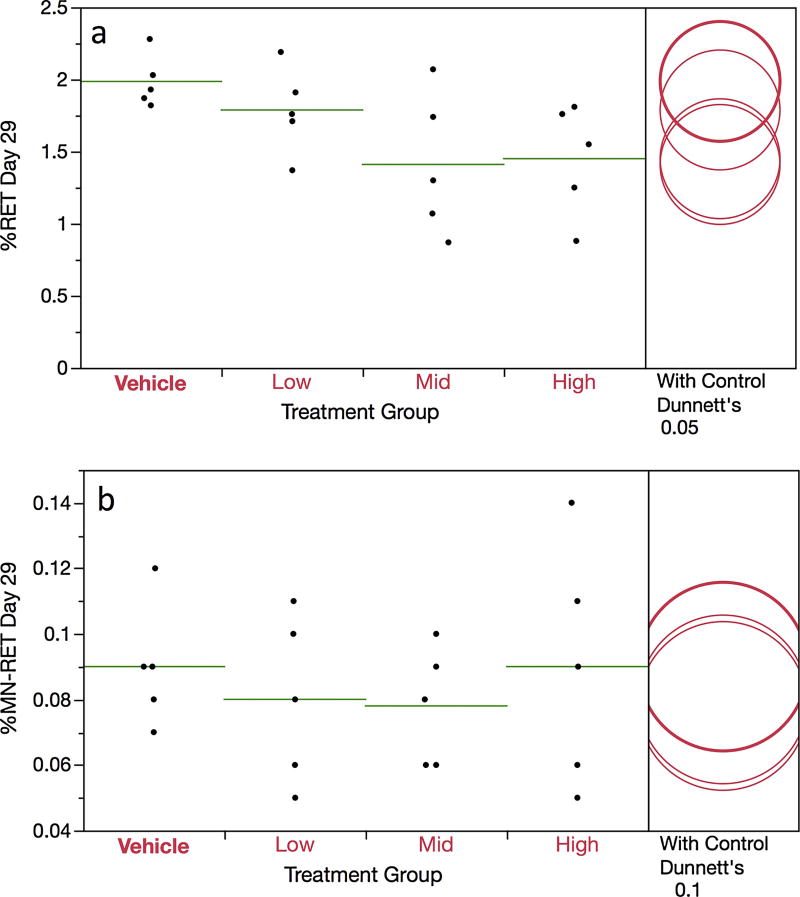
The mean frequency of CD71-positive RET, as measured in the In Vivo MicroFlow assay, were somewhat lower in each DEN-treated group compared to concurrent vehicle controls, although none of these differences reached statistical significance (Figure 2a). There were no indications that DEN affected %MN-RET (Figure 2b). This negative finding is supported in three manners: pair-wise and trend tests did not attain statistical significance, and none of the 15 DEN-treated rats exhibited %MN-RET that exceeded the historical negative control distribution’s 95% upper bound tolerance interval (i.e., > 0.19%).
Figure 2.
Frequency of day 29 blood reticulocytes (panel a) and micronucleated reticulocytes (panel b) are graphed for each of four DEN treatment groups. Data for individual rats are shown, and group means appear as horizontal green lines. Dunnett’s test results are shown to the far right of each graph, where statistically significant differences relative to the concurrent vehicle control group appear as italicized black text as opposed to red text, and by a grey circle as opposed to a red circle. Circles’ diameters represent 95% confidence intervals.
Pig-a Gene Mutation
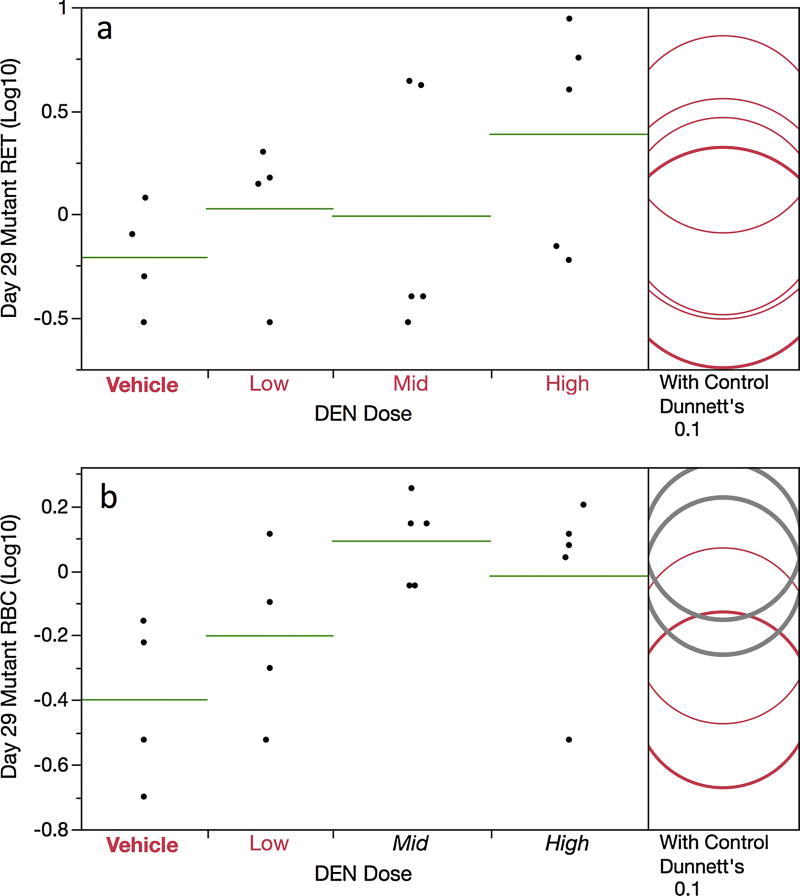
Unlike most other Pig-a studies we have performed to date, pre-treatment blood samples were not collected in order to deselect rats with unacceptably high RETCD59− and/or RBCCD59− frequencies. This led to 2 of 20 rats exhibiting unusually high frequencies (i.e., ≥ 32 × 10−6 RETCD59− ). Note that one of the high values occurred in the vehicle control group, and both of the outliers were excluded from the RETCD59− and/or RBCCD59− statistical analyses that follow.
While mean RETCD59− frequencies were moderately elevated in the high dose treatment group compared to controls (0.6 × 10−6 versus 3.9 × 10−6, respectively), a pair-wise test did not attain statistical significance (Figure 3a). Even so, we consider these results, in conjunction with three additional analyses, indicative of a weak mutagenic effect. First, 3 of 5 rats in the high dose group exhibited RETCD59− frequencies that exceeded the historical negative control distribution’s 95% upper bound tolerance interval (i.e., > 2.9 × 10−6). Second, mean RBCCD59− values were significantly increased in mid and high dose groups relative to concurrent controls (Figure 3b). Finally, RBCCD59− frequencies showed a significant dose-related (positive) trend.
Figure 3.
Frequency of day 29 mutant reticulocytes (panel a) and mutant erythrocytes (panel b) are graphed for each of four DEN treatment groups. Data for individual rats are shown, and group means appear as horizontal green lines. Note that the mutant cell frequencies (per million total cells) have been log transformed. Dunnett’s test results are shown to the far right of each graph, where statistically significant differences relative to the concurrent vehicle control group appear as italicized black text as opposed to red text, and by a grey circle as opposed to a red circle. Circles’ diameters represent 95% confidence intervals.
Micronucleated Hepatocytes
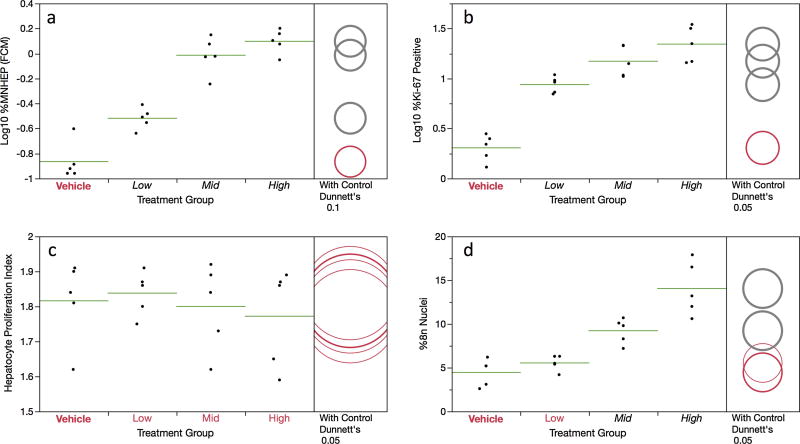
As shown by Figure 4a, %MNHEP values were observed to increase in a dose-dependent manner. Whereas the vehicle control rats averaged 0.14% MNHEP, animals in the top dose group exhibited a mean frequency of 1.28%, or a 9.1-fold increase. A positive dose-related trend test is supportive of the positive findings, as well as a comparison to the historical negative control distribution (i.e., 15/15 DEN-treated rats exhibited %MNHEP that exceeded the 95% upper bound tolerance interval, 0.31%). Note that in parallel to flow cytometric MNHEP frequencies presented in Figure 4, hepatocytes were also scored via conventional fluorescence microscopy using acridine orange-coated slides, and the results across methods are in good agreement; R2 = 0.89; see Supplemental file S1.
Figure 4.
Flow cytometric data for the 28 day are shown for each of four DEN treatment groups. Panel a = Log transformed MNHEP frequencies; panel b = Log transformed %Ki-67-positive nuclei; panel c = hepatocyte proliferation index; and panel d = %8n+ nuclei. For every graph, data for individual rats are shown, and group means appear as horizontal green lines. Dunnett’s test results are shown to the far right of each graph, where statistically significant differences relative to the concurrent vehicle control group appear as italicized black text as opposed to red text, and by a grey circle as opposed to a red circle. Circles’ diameters represent 95% confidence intervals.
Simultaneous with MNHEP frequencies, several measurements related to hepatocyte proliferation were collected. The frequency of Ki-67-positive nuclei showed a significant increase in each of the DEN treatment groups (Figure 4b). While the HPI metric was not affected by DEN treatment, the proportion of 8n+ nuclei were significantly elevated in the 10 and 15 mg/kg/day treatment groups (Figure 4c,d). Together with the normalized liver weight data presented in Figure 1b, these results suggest reactive DEN metabolites significantly affected the liver compartment to an extent that a regenerative proliferation response was induced.
Discussion
Treatment with DEN for 28 days did not affect blood MN-RET frequencies, and at best it was a very weak inducer of Pig-a gene mutation in hematopoieic cells. On the other hand, we observed substantial increases in %MNHEP. These responses were accompanied by remarkable increases in liver proliferation. For example, anti-Ki-67 labeling showed a mean 11-fold increase for rats treated with 15 mg/kg/day. The multiparametric capabilities of flow cytometry allowed us to acquire these proliferation data simultaneously with MNHEP scoring, an advantageous feature of the current method.
The results presented herein, including the liver proliferation response, agree with reports by JEMS/MMS and other groups that have investigated the genotoxic potential of this metabolically activated hepatocarcinogen. In those studies, hematopoietic cells showed slight or no genotoxic responses, whereas remarkable effects to liver tissue were observed, including micronucleus formation [10, 17–20, 22].
The JEMS/MMS group has advocated the use of 6 week old rats as a means to integrate liver MNHEP assessments into 28-day repeat dose toxicology studies [10]. This represents an important animal and resource-sparing design, one that would be expected to enhance our ability to interpret resulting genotoxicity data given the wealth of information that is collected in conjunction with 28-day studies. The experiment with DEN described herein extends this concept further by demonstrating the feasibility of collecting additional genotoxicity data in the same one study—MN-RET and Pig-a gene mutation in addition to MNHEP. These several assays provide a more comprehensive assessment of a chemical’s genotoxic potential, with the added benefit of considering two key tissue compartments. According to the OECD Repeat Dose 28-Day Oral Toxicity Study in Rodents guideline (no. 407) [30], 6 week-old rats are within the acceptable age range, and therefore no experimental design obstacles stand in the way of instituting approaches such as the one described herein.
Ongoing work by JEMS/MMS as well as this laboratory is being conducted to better understand the types of genotoxicants that are detected in the rat liver MNHEP assay. The highly promising data generated to date suggest that this endpoint and tissue has the potential to add considerable value to both short-term genotoxicity studies that combine more than one endpoint, as well as general toxicology studies with integrated genotoxicity assays. The additional value of automating genotoxicity measurements includes more objective scoring, higher throughput capacity, and likely greater sensitivity owing to the larger number of cells evaluated via flow cytometry.
Supplementary Material
Highlights.
Regulatory guidances stress the value of studying multiple tissues for genotoxicity.
A proof-of-principle study was conducted with rats exposed to diethylnitrosamine.
MN-RET, MNHEP, and Pig-a gene mutation were scored via flow cytometry.
Effects on hematopoietic cells were slight or nil; %MNHEP were clearly increased.
Multiple tissues and endpoints can be readily integrated into a 28-day study design.
Acknowledgments
This work was funded by a grant from the National Institute of Health/National Institute of Environmental Health Sciences (NIEHS; grant no. R44ES026464). The contents are solely the responsibility of the authors, and do not necessarily represent the official views of the NIEHS. We are especially grateful to Dr. Shuichi Hamada for this encouragement and advice over the past several years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors are employees of Litron Laboratories. Litron holds patents covering flow cytometric methods for scoring micronucleated erythrocytes and sells kits based on this technology (In Vivo MicroFlow®); Litron hold patents for scoring GPI anchor-deficient erythrocytes and sells kits based on this technology (In Vivo MutaFlow®); Litron has filed a patent covering flow cytometric methods for scoring micronucleated hepatocytes and plans to sell kits based on this technology (In Vivo MicroFlow® PLUS RL Kits).
Author contributions
S.D.D. and J.C.B. primarily designed these studies. S.K., P.S., S.L.A., and D.K.T. executed various aspects of the experiments. S.D.D. primarily wrote the manuscript, with significant input from all authors.
References
- 1.Matter B, Schmid W. Trenimon-induced chromosomal damage in bone marrow cells of six mammalian species, evaluated by the micronucleus test. Mutat. Res. 1971;12:417–425. doi: 10.1016/0027-5107(71)90092-3. [DOI] [PubMed] [Google Scholar]
- 2.Heddle J. A rapid in vivo test for chromosome damage. Mutat. Res. 1973;18:187–190. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor JT, Wehr CM, Gould DH. Clastogen-induced micronuclei in peripheral blood erythrocytes: The basis of an improved micronucleus test. Environ. Mol. Mutagen. 1980;2:509–514. doi: 10.1002/em.2860020408. [DOI] [PubMed] [Google Scholar]
- 4.ICH, S2(R1) Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use; International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline; Nov 9, 2011. Step 4. [Google Scholar]
- 5.Hayashi M, MacGregor JT, Gatehouse DG, Blakey DH, Dertinger SD, Abramsson-Zetterberg L, Krishna G, Morita T, Russo A, Asano N, Suzuki H, Ohyama W, Gibson D. In vivo erythrocyte micronucleus assay: III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussion of non-hematopoietic target cells and a single dose-level limit test. Mutat. Res. 2007;627:10–30. doi: 10.1016/j.mrgentox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Vasquez MZ. Combining the in vivo comet and micronucleus assays: a practical approach to genotoxicity testing and data interpretation. Mutagenesis. 2010;25:187–199. doi: 10.1093/mutage/gep060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speit G, Kojima H, Burlingson B, Collins AR, Kasper P, Plappert-Helbig U, Uno Y, Vasquez M, Beevers C, De Boeck M, Escobar PA, Kitamoto S, Pant K, Pfuhler S, Tanaka J, Levy DD. Critical issues with the in vivo comet assay: A report of the comet assay working group in the 6th International Workshop on Genotoxicity Testing (IWGT) Mutat. Res. 2015;783:6–12. doi: 10.1016/j.mrgentox.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Kraynak AR, Barnum JE, Cunningham CL, Ng A, Ykoruk BA, Bennet B, Stoffregen D, Merschman M, Freeland E, Galloway SM. Alkaline comet assay in liver and stomach, and micronucleus assay in bone marrow, from rats treated with 2-acetylaminofluorene, azidothymidine, cisplatin, or isobutyraldehyde. Mutat. Res. 2015;786–788:77–86. doi: 10.1016/j.mrgentox.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Higami Y, Shimokawa I, Okimoto T, Tomita M, You T, Ikeda T. Effect of aging and dietary restriction on hepatocyte proliferation and death in male F344 rats. Cell and Tissue Research. 1997;288:69–77. doi: 10.1007/s004410050793. [DOI] [PubMed] [Google Scholar]
- 10.Hamada S, Ohyama W, Takashima R, Shimada K, Matsumoto K, Kawakami S, Uno F, Sui H, Shimada Y, Imamura T, Matsumura S, Sanada H, Inoue K, Muto S, Ogawa I, Hayashi A, Takayanagi T, Ogiwara Y, Maeda A, Okada E, Terashima Y, Takasawa H, Narumi K, Wako Y, Kawasako K, Sano M, Ohashi N, Morita T, Kojima H, Honma M, Hayashi M. Evaluation of the repeated-dose liver and gastrointestinal tract micronucleus assays with 22 chemicals using young adult rats: Summary of the collaborative study by the Collaborative Study Group for the Micronucleus Test (CSGMT)/The Japanese Environmental Mutagen Society (JEMS) Mammalian Mutagenicity Study Group (MMS) Mutat. Res. 2015;780–781:2–17. doi: 10.1016/j.mrgentox.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Dertinger SD, Phonethepswath S, Franklin D, Weller P, Torous DK, Bryce SM, Avlasevich S, Bemis JC, Hyrien O, Palis J, MacGregor JT. Integration of mutation and chromosomal damage endpoints into 28-day repeat dose toxicology studies. Toxicol. Sci. 2010;115:401–411. doi: 10.1093/toxsci/kfq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stankowski LF, Jr, Roberts DJ, Chen H, Lawlor T, McKeon M, Murli H, Thakur A, Xu Y. Integration of Pig-a, micronucleus, chromosome aberration, and comet assay endpoints in a 28-day rodent toxicity study with 4-nitroquinoline-1-oxide. Environ. Mol. Mutagen. 2011;52:738–747. doi: 10.1002/em.20692. [DOI] [PubMed] [Google Scholar]
- 13.Dertinger SD, Phonethepswath S, Avlasevich SL, Torous DK, Mereness J, Bryce SM, Bemis JC, Bell S, Weller P, MacGregor JT. Efficient monitoring of in vivo Pig-a gene mutation and chromosomal damage: Summary of 7 published studies and results from 11 new reference compounds. Toxicol. Sci. 2012;130:328–348. doi: 10.1093/toxsci/kfs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uno F, Tanaka J, Ueda M, Nagai M, Fukumuro M, Natsume M, Oba M, Akahori A, Masumori S, Takami S, Wako Y, Kawasako K, Kougo Y, Ohyama W, Narumi K, Fujiishi Y, Okada E, Hayashi M. Repeat-dose liver and gastrointestinal tract micronucleus assays for quinoline in rats. Mutat. Res. 2015;780–781:51–55. doi: 10.1016/j.mrgentox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi A, Kosaka M, Kimura A, Wako Y, Kawasako K, Hamada S. Evaluation of the repeated-dose liver micronucleus assay using N-nitrosomorpholine in young adult rats: report on collaborative study by the Collaborative Study Group for the Micronucleus Test (CSGMT)/Japanese Environmental Mutagen Society (JEMS)-Mammalian Mutagenicity Study (MMS) Group. Mutat. Res. 2015;780–781:71–75. doi: 10.1016/j.mrgentox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Maeda A, Tsuchiyama H, Asaoka Y, Hirakata M, Miyoshi T, Oshida K, Miyamoto Y. Evaluation of the repeat-dose liver micronucleus assay using 2,4-dinitrotoluene: a report of a collaborative study by GSGMT/JEMS.MMS. Mutat. Res. 2015;780–781:41–45. doi: 10.1016/j.mrgentox.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Krsmanovic L, Bruce S, Kelly T, Paranipe M, Szabo K, Arevalo M, Atta-Safoh S, Debelie F, LaForce MK, Sly J, Springer S. Assessment of genotoxicity induced by 7,12-dimethylbenz(a)anthracene or diethylnitrosamine in the Pig-a, micronucleus and Comet assays integrated into 28-day repeat dose studies. Environ. Mol. Mutagen. 2011;52:711–720. doi: 10.1002/em.20678. [DOI] [PubMed] [Google Scholar]
- 18.Avlasevich SL, Phonethepswath S, Labash C, Carlson K, Torous DK, Cottom J, Bemis JC, MacGregor JT, Dertinger SD. Diethylnitrosamine genotoxicity evaluated in Sprague Dawley rats using Pig-a mutation and reticulocyte micronucleus assays. Environ. Mol. Mutagen. 2014;55:400–406. doi: 10.1002/em.21862. [DOI] [PubMed] [Google Scholar]
- 19.Wada K, Nishino R, Fukuyama T, Matsumoto K. Evaluation of the PIGRET assay as a short-term test using a single dose of diethylnitrosamine. Mutat. Res. 2016;811:70–74. doi: 10.1016/j.mrgentox.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi M, Shimada H. An improved method for the mouse liver micronucleus test. Mutat. Res. 1997;391:49–55. doi: 10.1016/s0165-1218(97)00031-1. [DOI] [PubMed] [Google Scholar]
- 21.Gollapudi BB, Lynch AM, Heflich RH, Dertinger SD, Dobrovolsky VN, Froetschl R, Horibata K, Kenyon MO, Kimoto T, Lovell DP, Stankowski LF, Jr, White PA, Witt KL, Tanir JY. The in vivo Pig-a assay: A report of the International Workshop on Genotoxicity Testing (IWGT) Workgroup. Mutat. Res. 2015;783:23–35. doi: 10.1016/j.mrgentox.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Narumi K, Ashizawa K, Takashima R, Takasawa H, Katayama S, Tsuzuki Y, Tatemoto H, Morita T, Hayashi M, Hamada S. Development of a repeat-dose liver micronucleus assay using adult rats: An investigation of diethylnitrosamine and 2,4-diaminotoluene. Mutat. Res. 2012;747:234–239. doi: 10.1016/j.mrgentox.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Torous DK, Hall NE, Murante FG, Gleason SE, Tometsko CR, Dertinger SD. Comparative scoring of micronucleated reticulocytes in rat peripheral blood by flow cytometry and microscopy. Toxicol. Sci. 2003;74:309–314. doi: 10.1093/toxsci/kfg143. [DOI] [PubMed] [Google Scholar]
- 24.Dertinger SD, Camphausen K, MacGregor JT, Bishop ME, Torous DK, Avlasevich S, Cairns S, Tometsko CR, Menard C, Muanza T, Chen Y, Miller RK, Cederbrant K, Sandelin K, Pontén I, Bolcsfoldi G. Three-color labeling method for flow cytometric measurement of cytogenetic damage in rodent and human blood. Environ. Mol. Mutagen. 2004;4:427–35. doi: 10.1002/em.20075. [DOI] [PubMed] [Google Scholar]
- 25.Tometsko AM, Torous DK, Dertinger SD. Analysis of micronucleated cells by flow cytometry. 1. Achieving high resolution with a malaria model. Mutat. Res. 1993;292:129–135. doi: 10.1016/0165-1161(93)90140-u. [DOI] [PubMed] [Google Scholar]
- 26.Dertinger SD, Torous DK, Hall NE, Tometsko CR, Gasiewicz TA. Malaria-infected erythrocytes serve as biological standards to ensure reliable and consistent scoring of micronucleated erythrocytes by flow cytometry. Mutat. Res. 2000;464:195–200. doi: 10.1016/s1383-5718(99)00183-7. [DOI] [PubMed] [Google Scholar]
- 27.Dertinger SD, Bryce SM, Phonethepswath S, Avlasevich SL. When pigs fly: immunomagnetic separation facilitates rapid determination of Pig-a mutant frequencies by flow cytometric analysis. Mutat. Res. 2011;72:163–170. doi: 10.1016/j.mrgentox.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avlasevich SL, Khanal S, Singh P, Torous DK, Bemis JC, Dertinger SD. Flow cytometric method for scoring rat liver micronuclei with simultaneous assessments of hepatocyte proliferation. Environ. Mol. Mutagen. doi: 10.1002/em.22168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate M., Jr The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. 1990;245:245–249. doi: 10.1016/0165-7992(90)90153-b. [DOI] [PubMed] [Google Scholar]
- 30.OECD Guidelines for the Testing of Chemicals: Repeat Dose 28-Day Oral Toxicity Study in Rodents, No. 407. 2008 available at: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg407-2008.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.