Abstract
AIM:
To study and compare the incidence and time of occurrence of cytomegalovirus (CMV) infection in the posttransplant period in adult and pediatric liver transplant recipients.
MATERIALS AND METHODS:
Consecutive live donor liver transplant recipients not on CMV prophylaxis, were prospectively enrolled from March 2012 to September 2015 and followed up for 1 year post transplant to look for CMV infection. For analysis, patients were divided into pediatric (up to 18 years) and adult (>18 years) age groups.
RESULTS:
The study population of 146 patients consisted of 132 adult and 14 pediatric patients. Overall CMV infection posttransplant was seen in 54/146 (36.98%) patients, and 16/54 (29.6%) patients developed CMV disease. Post-transplant CMV infection rate was significantly higher in pediatric patients(10/14 [71.4%]) as compared to adults (44/132 [33.4%]) (P = 0.004). Among adults, CMV infection was seen in 22 (50%) patients in the 1st month, 13 (29.5%) patients in the 2nd month, 5 (11.4%) patients in the 3rd month, 2 (4.5%) patients in the 4th month, and 1 (2.3%) patient each in the 5th and 6th month. However, in pediatric patients, all the patients having CMV infection had it in the 1st-month posttransplant (P = 0.003). The median time of occurrence of CMV infection was 11.5 (7.75–19.00) days in pediatric patients versus 30 (18.5–54.5) days in adult patients (P = 0.001).
CONCLUSIONS:
The results of this study show a clear difference in the incidence and timeline of posttransplant CMV infection in pediatric patients as compared to adults.
Keywords: Adults, cytomegalovirus infection, liver transplant, pediatric patients
Introduction
Cytomegalovirus (CMV) is a herpes virus causing infection in 40%–100% population worldwide.[1,2] Primary infection with CMV results in latency. CMV infection and disease remain a major health concern after liver transplantation.[3,4,5,6] The effects of CMV infection after solid organ transplantation may be serious ranging from tissue-invasive disease to graft rejection and mortality in some cases.[3]
The incidence of CMV infection in the posttransplant period is dependent upon factors such as lack of preexisting CMV-specific humoral/cell-mediated immunity, coinfections (HHV-6/HHV-7), immune deficiencies, immunosuppressive therapy, and prophylactic therapy among others.[6,7] CMV serostatus of the donor and recipient is the most important predictor of CMV infection and disease posttransplant.[6] Most of the CMV infection and disease in the posttransplant period is a result of CMV reactivation due to the immunosuppression in case of CMV-seropositive transplant recipients. In patients not receiving any prophylactic therapy, most of the clinical effects of CMV occur in the first 3 months posttransplant, and it is estimated that 18%–29% of liver transplant recipients might develop CMV disease within 12 months posttransplant.[3,8] However, in patients receiving prophylactic therapy, CMV disease occurs 3–6 months after completing the prophylactic therapy and is known as “late-onset CMV disease.”[3,9]
In the present era where modern surgical techniques and improved immunosuppressive regimens have increased the survival rates to >90% in children, more pediatric patients are undergoing liver transplantation than before.[10] In children undergoing solid organ transplant, various pretransplant factors such as age, exposure to infectious agents (CMV/Epstein–Barr virus), immunity, and vaccination status influence the type and severity of infections experienced after transplant.[10,11] Therefore, to provide better care to the pediatric liver transplant recipients, it is important to understand the kinetics of posttransplant CMV infection in these patients as compared to adults. The objective of this study was to study and compare the incidence and time of occurrence of CMV infection in the posttransplant period in adult and pediatric liver transplant recipients.
Materials and Methods
Study design
This prospective study was conducted in our center on liver transplant recipients. Consecutive patients undergoing live donor liver transplant at the institute were enrolled from March 2012 to September 2015 and followed up for 12 months posttransplant. Patients who were HIV coinfected, unwilling to give consent, received cadaveric liver transplant or another organ cotransplant along with liver were excluded from the study. Patients who died during the follow-up period were excluded from the analysis. Patients were divided into adults (>18 years) and pediatric (up to 18 years) for the purpose of analysis.
CMV infection and disease were diagnosed and defined as per the recommendations.[12] CMV-DNA positivity in the blood (DNAemia) was considered as the evidence of CMV infection. CMV disease, i.e., CMV infection with attributable symptoms, was further categorized as CMV syndrome (fever, malaise, leukopenia, and/or thrombocytopenia) and CMV tissue-invasive disease (detection of CMV in the tissue specimen).
Immunosuppression protocol
All patients received triple-regimen immunosuppression (tacrolimus + mycophenolate mofetil + steroid). The trough level of tacrolimus was maintained at 5–10 ng/mL. Steroids were tapered in majority by the end of 3 months.
Preemptive therapy
None of the liver transplant recipients was on anti-CMV prophylaxis. Patients were clinically examined for signs and symptoms of CMV infection every month. Patients were given preemptive treatment if the CMV viral load was >500 copies/ml (significant viremia), regardless of clinical manifestations as per the Institution Protocol. The patients received preemptive therapy consisting of intravenous ganciclovir (5 mg/kg, 12 hourly) or oral valganciclovir (900 mg twice daily) until the patient became negative for CMV infection. Therapy was stopped once two consecutive samples taken 2 weeks apart were negative for CMV DNA.
Sample collection
Pretransplant blood samples were collected in ethylenediaminetetraacetic acid vials from all transplant recipients and their respective donors for CMV IgG antibody detection and polymerase chain reaction (PCR) for CMV-DNA detection. Plasma levels of CMV-DNA were measured every week up to 1 month, every fortnight up to 2 months, and monthly up to 1-year posttransplant.
Cytomegalovirus IgG detection
Determination of IgG antibodies to CMV in plasma was done by the Architect CMV-IgG assay (Abbott Laboratories, Chicago, IL, USA) which is a chemiluminescent microparticle immunoassay as per the manufacturer's instructions. The cutoff values were calculated based on each individual run to determine each sample as reactive or nonreactive.
Cytomegalovirus real-time polymerase chain reaction
Plasma was separated from the blood collected from the patients, and viral DNA was extracted using High Pure Viral Nucleic Acid Kit (Roche Diagnostics GmbH, Roche Applied Science, Germany) as per the manufacturer's instructions. Quantitative Real-Time PCR for CMV was performed in plasma samples using the LightCycler® 480 II Real-Time PCR System (Roche Life Science, USA). This assay targets viral glycoprotein gene gpB (UL 55), 226 bp in size using Light DNA master hybridization probe. Results were expressed as copies of virus/ml plasma. The linear range of the test is 102–106 copies/mL with the lower limit of detection being 10 copies/mL.
Statistical analysis
Quantitative variables were expressed as median with range, and qualitative variables were expressed as percentage. Chi-square test was used to compare the data in two groups. A “P” < 0.05 was considered statistically significant. Statistical analysis was done using SPSS version 20 (SPSS Inc., Chicago, IL, USA).
Ethical considerations
The study design and methodology were approved by the Institutional Ethics Committee.
Results
Baseline characteristics of the study population
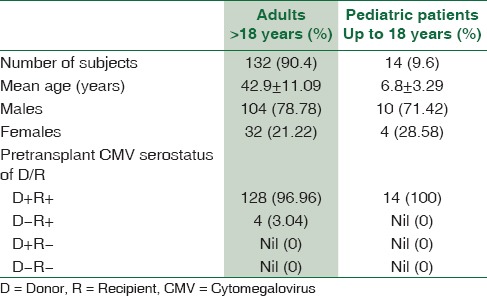
A total of 163 patients were screened. Nine patients were excluded from the study as they did not fulfill the inclusion criteria. Eight patients died during the follow-up period and hence excluded from the analysis. The final analysis included 146 patients [Figure 1] which consisted of 114 (78%) males and 32 (22%) females. There were 132 adult and 14 pediatric patients in the study population. The CMV serostatus in all 14 pediatric patients was D + R+ (D = donor, R = recipient), whereas among adults, 128 were D + R + and 4 were D − R + [Table 1].
Figure 1.
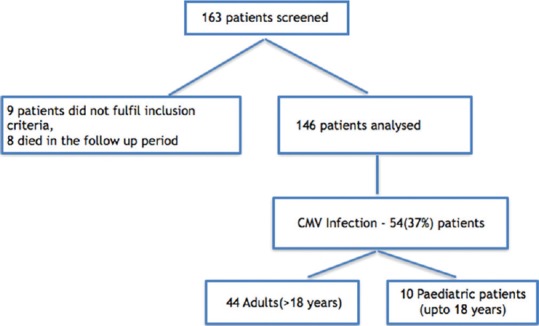
Flow diagram depicting the study population and number of patients with cytomegalovirus infection posttransplant
Table 1.
Baseline characteristics of the study population
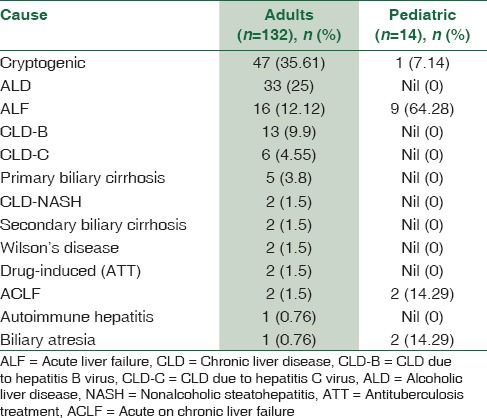
The most common indication for liver transplant was cryptogenic liver disease, followed by alcoholic liver disease, acute liver failure of unknown etiology, and chronic hepatitis B and C [Table 2].
Table 2.
End-stage liver disease in adult and pediatric live donor liver transplant recipients
Cytomegalovirus infection
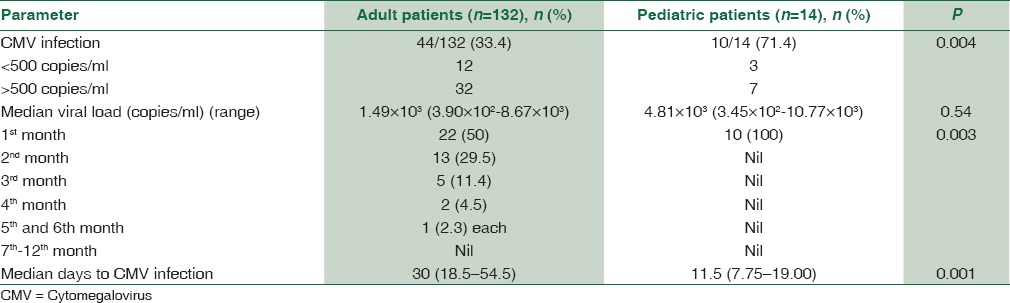
CMV-DNA was not detected by PCR in any patient in the pretransplant blood samples. Overall CMV infection was seen in 54/146 (36.98%) patients in the posttransplant period. Posttransplant CMV infection rate was significantly higher in pediatric 10/14 (71.4%) as compared to adult 44/132 (33.4%) patients (P = 0.004). Significant viremia (CMV viral load of >500 copies/ml) was seen in 32/44 adult and 7/10 pediatric patients showing CMV infection.
Timeline of CMV infection posttransplant is represented in Table 3. Majority of patients having CMV infection had it in the first 3 months posttransplant. Among adults, a total of 44 patients had CMV infection. Out of these, CMV infection was seen in 22 (50%) patients in the 1st month, 13 (29.5%) patients in the 2nd month, 5 (11.4%) patients in the 3rd month, 2 (4.5%) patients in the 4th month, and 1 (2.3%) patient each in the 5th and 6th month. No CMV infection was seen in any patient in the follow-up duration of 7th–12th month. In the pediatric age group, all 10 patients had CMV infection in the 1st-month posttransplant and none after that. This difference in the timeline of posttransplant CMV infection between adult and pediatric liver transplant recipients was found to be statistically significant (P = 0.003) [Table 3].
Table 3.
Comparison of cytomegalovirus infection between adult and pediatric liver transplant recipients
CMV infection was observed significantly earlier in pediatric patients as compared to adults. The median time of occurrence of CMV infection was 11.5 (7.75–19.00) days in pediatric patients versus 30 (18.5–54.5) days in adult patients (P = 0.001). The median viral load of CMV in adult patients was 1.49 × 103 (3.90 × 102–8.67 × 103) copies/ml as compared to 4.81 × 103 (3.45 × 102–10.77 × 103) copies/ml in pediatric patients. However, the difference was statistically insignificant [Figure 2].
Figure 2.
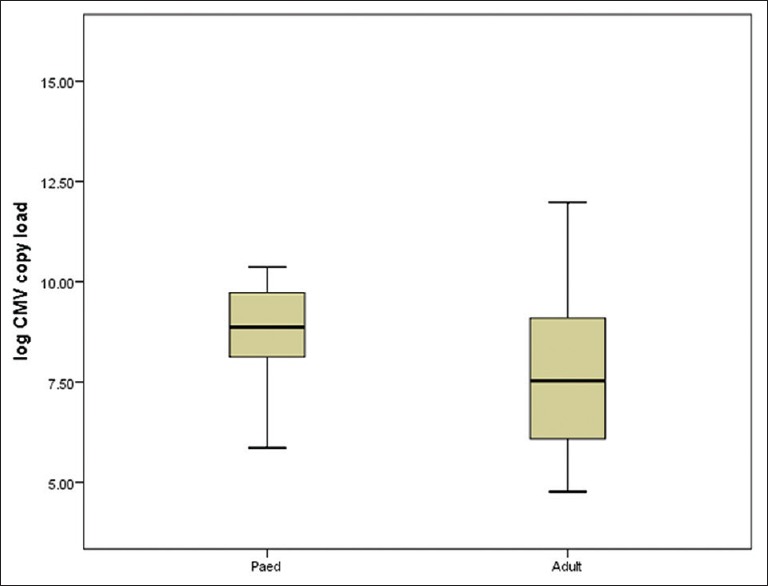
Box-plot representing median cytomegalovirus load (after log transformation) in adult and pediatric liver transplant recipients (P = 0.54)
CMV disease was seen in 16/54 (29.6%) patients (fever, malaise, abnormal leukocyte counts/thrombocytopenia) and no patient developed tissue-invasive disease. A total of 2/10 (20%) pediatric patients and 14/44 (31.8%) adults developed CMV disease. The median time of occurrence of CMV disease was 32 (8.4–76.5) days. The median viral load in patients who developed CMV disease was 1.74 × 103 (1.96 × 102–5.3 × 105) copies/ml as compared to 1.52 × 103 (1.18 × 102–6.8 × 104) copies/ml in patients with only CMV infection. This difference was statistically insignificant. All CMV infections were successfully treated with ganciclovir/valganciclovir as part of preemptive therapy as per the institute's protocol mentioned above. No difference in outcome of patients with CMV infection and CMV disease was found in this study.
Discussion
The rates of CMV infection and disease in the posttransplant period vary with the serostatus of donor and recipient and the administration of antiviral prophylaxis along with other factors.[3,6,8,13,14] This study dealt only with CMV-seropositive transplant recipients and none of them received antiviral prophylaxis. The overall rates of CMV infection and disease were found to be 36.9% and 10.95%, respectively. It has been estimated that in the absence of antiviral prophylaxis, the rate of CMV disease in the 1st-year posttransplant is between 8% and 19% in CMV-seropositive liver transplant recipients.[3,8] The results of the present study are concordant with the same.
Overall, highest rate of CMV infection was observed in the first 3 months with more than half of the infections occurring in the 1st-month posttransplant. The timeline is highly dependent on factors such as levels of immunosuppression and preventive strategy adopted.[8,9,15,16] Reports have appeared in literature in recent times stating that majority of the CMV infections take place in the first 3-month posttransplant.[3,6] However, there is an interesting trend to be noticed in the timeline of CMV infection in the present study as all the CMV infections in children and majority of infections in adults were seen in the 1st-month posttransplant. The pretransplant CMV-seropositive status of the transplant recipients and the absence of antiviral prophylaxis might have led to this finding.
If the CMV infection rates in adults and pediatric population are looked at separately, a significantly higher rate of CMV infection (71.4%) was observed in pediatric patients as compared to adults (33.4%) (P = 0.004). The median time to CMV infection was also significantly lesser in pediatric patients (11.5 days vs 30 days) (P = 0.001). There are no recent studies which compare the incidence of CMV infection in adult and pediatric liver transplant recipients. However, Breinig et al.[17] in 1987 reported high rates of CMV reactivation in children (88%) in the USA, which were similar to those in adults. Preventive and immunosuppressive strategies have evolved a lot since then and have reduced the incidence of CMV infection and disease. In a recent study from London, the authors have reported CMV infection rate of 66.7% in D + R + pediatric liver transplant recipients,[18] which is similar to the results of this study.
In our center, we mostly deal with CMV- seropositive patients and preemptive-preventive strategy is being followed in accordance with the guidelines by Kotton et al.[7] There are no clear guidelines stating the time when the monitoring should start posttransplant.[7,19] The results of the study suggest that it should be immediately after transplant, especially in children to avoid missing significant yet asymptomatic CMV infection. The small study population of the children is however a limiting factor in this study and the findings need to be established by conducting a similar study on a larger scale in seropositive population.
Future directions
The dynamics of CMV reactivation seem to be different in the pediatric population, and therefore separate guidelines might be needed to cater to such patients. Long-term prospective studies and randomized control trials need to be taken up in India to fine tune the existing preventive strategies.
Conclusions
The results of this study show a clear difference in the incidence and timeline of posttransplant CMV infection in pediatric patients as compared to adults. Larger studies should be done to establish this observation and decide upon the preventive strategies to be adopted (antiviral prophylaxis/preemptive therapy) in pediatric liver transplant recipients.
Financial support and sponsorship
Financial support for the study was provided by Indian Council of Medical Research. Grant number VIR/38/2011/ECD-1.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Mr. Gaurav Singh for his technical support.
References
- 1.Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bull World Health Organ. 1973;49:103–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Kothari A, Ramachandran VG, Gupta P, Singh B, Talwar V. Seroprevalence of cytomegalovirus among voluntary blood donors in Delhi, India. J Health Popul Nutr. 2002;20:348–51. [PubMed] [Google Scholar]
- 3.Razonable RR. Cytomegalovirus infection after liver transplantation: Current concepts and challenges. World J Gastroenterol. 2008;14:4849–60. doi: 10.3748/wjg.14.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013;85:893–8. doi: 10.1002/jmv.23539. [DOI] [PubMed] [Google Scholar]
- 5.Weigand K, Schnitzler P, Schmidt J, Chahoud F, Gotthardt D, Schemmer P, et al. Cytomegalovirus infection after liver transplantation incidence, risks, and benefits of prophylaxis. Transplant Proc. 2010;42:2634–41. doi: 10.1016/j.transproceed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: Updates on clinical management. World J Gastroenterol. 2014;20:10658–67. doi: 10.3748/wjg.v20.i31.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–60. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 8.Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol. 2010;2:325–36. doi: 10.4254/wjh.v2.i9.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthurs SK, Eid AJ, Pedersen RA, Dierkhising RA, Kremers WK, Patel R, et al. Delayed-onset primary cytomegalovirus disease after liver transplantation. Liver Transpl. 2007;13:1703–9. doi: 10.1002/lt.21280. [DOI] [PubMed] [Google Scholar]
- 10.Halasa N, Green M. Immunizations and infectious diseases in pediatric liver transplantation. Liver Transpl. 2008;14:1389–99. doi: 10.1002/lt.21605. [DOI] [PubMed] [Google Scholar]
- 11.Green M, Michaels MG. Infections in pediatric solid organ transplant recipients. J Pediatric Infect Dis Soc. 2012;1:144–51. doi: 10.1093/jpids/pir001. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 13.Bruminhent J, Razonable RR. Management of cytomegalovirus infection and disease in liver transplant recipients. World J Hepatol. 2014;6:370–83. doi: 10.4254/wjh.v6.i6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin RH. Cytomegalovirus in solid organ transplantation. Transpl Infect Dis. 2001;3(Suppl 2):1–5. doi: 10.1034/j.1399-3062.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870–80. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TM, Campos SV, et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo) 2015;70:515–23. doi: 10.6061/clinics/2015(07)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breinig MK, Zitelli B, Starzl TE, Ho M. Epstein-barr virus, cytomegalovirus, and other viral infections in children after liver transplantation. J Infect Dis. 1987;156:273–9. doi: 10.1093/infdis/156.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma A, Palaniswamy K, Cremonini G, Heaton N, Dhawan A. Late cytomegalovirus infection in children: High incidence of allograft rejection and hepatitis in donor negative and seropositive liver transplant recipients. [Last accessed on 2017 Nov 11];Pediatr Transplant. 2017 21:e12879. doi: 10.1111/petr.12879. Available from: https://doi.org/10.1111/petr.12879 . [DOI] [PubMed] [Google Scholar]
- 19.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–95. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]


