Abstract
The unicellular green alga Haematococcus pluvialis Flotow is known for its massive accumulation of ketocarotenoids under various stress conditions. Therefore, this microalga is one of the favored organisms for biotechnological production of these antioxidative compounds. Astaxanthin makes up the main part of the secondary carotenoids and is accumulated mostly in an esterified form in extraplastidic lipid vesicles. We have studied phytoene desaturase, an early enzyme of the carotenoid biosynthetic pathway. The increase in the phytoene desaturase protein levels that occurs following induction is accompanied by a corresponding increase of its mRNA during the accumulation period, indicating that phytoene desaturase is regulated at the mRNA level. We also investigated the localization of the enzyme by western-blot analysis of cell fractions and by immunogold labeling of ultrathin sections for electron microscopy. In spite of the fact that secondary carotenoids accumulate outside the chloroplast, no extra pathway specific for secondary carotenoid biosynthesis in H. pluvialis was found, at least at this early stage in the biosynthesis. A transport process of carotenoids from the site of biosynthesis (chloroplast) to the site of accumulation (cytoplasmatic located lipid vesicles) is implicated.
During the last decade, much of the research on carotenoid biosynthesis has been at the genetic level, i.e. the elucidation of the respective genes and enzymes. Less is known about the regulation of the processes involved. The unicellular green alga Haematococcus pluvialis (Volvocales) is known for its massive accumulation of the ketocarotenoid astaxanthin in the esterified form in response to, for example, nutritional or light stress conditions. The increasing commercial interest in astaxanthin results from its antioxidative properties (Nishino, 1997), which are important for the pharmaceutical and cosmetic industries. It is also used in huge amounts as a nutritional supplement in the aquaculture of salmonoids (Wathne et al., 1998). Biological functions of so-called secondary carotenoids (SC) as sunshade (Hagen et al., 1994) and as protection against photodynamic damage (Hagen et al., 1993) have been elucidated in H. pluvialis.
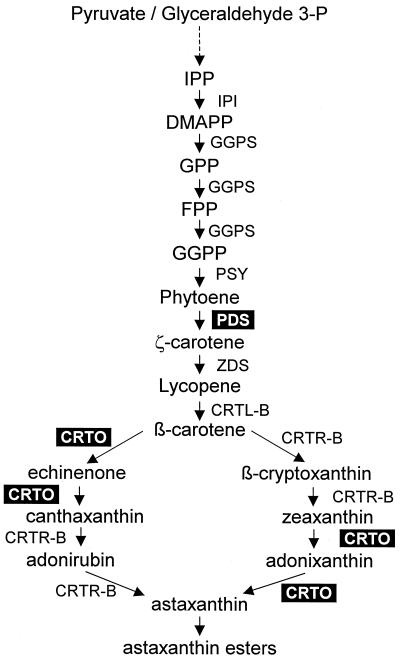
Carotenoids are synthesized from the universal isoprenoid precursor isopentenylpyrophosphate (IPP). The more recently discovered DOX-P-pathway for IPP biosynthesis, named after the first intermediate 1-deoxy-d-xylulose-5-phosphate (synonyms: non-mevalonate pathway), is located in the chloroplast and seems to be the favored, if not the only, pathway used for the synthesis of carotenoids in higher plants and green algae (Lichtenthaler et al., 1999). By head-to-tail additions and the subsequent action of the phytoene synthase, the first tetraterpene carotenoid, phytoene, is formed. The following desaturation steps leading to ζ-carotene are catalyzed by phytoene desaturase (PDS), the enzyme studied here. The subsequent biosynthetic steps resulting in the different carotenoids also occurring in H. pluvialis are summarized in Cunningham and Gantt (1998). A short scheme of the sequences leading to astaxanthin esters and indicating also the ketocarotenoid specific enzyme β-carotene oxygenase (CRTO; synonym: β-carotene ketolase) is given in Figure 1. The gene for this specific enzyme was cloned from two different strains of H. pluvialis by Lotan and Hirschberg (1995) and Kajiwara et al. (1995).
Figure 1.
Pathway of secondary carotenoid synthesis in H. pluvialis. Data are presented in this paper for the enzymes shown in black boxes. Enzyme designation is according to the corresponding gene: CRTL-B, lycopene β-cyclase; CRTO, β-carotene oxygenase; CRTR-B, β-ring hydroxylase; GGPS, geranylgeranyl diphosphate synthase; IPI, isopentenyl diphosphate isomerase; PDS, phytoene desaturase; PSY, phytoene synthase; ZDS, ζ-carotene desaturase.
According to hydropathy analysis, substrate hydrophobicity, and chloroplast subfractionation, the active plant PDS is assumed to be tightly bound or even intrinsic of membranes (Camara et al., 1982; Al-Babili et al., 1996). Linden et al. (1993) showed by immunogold labeling a thylakoid localization of the enzyme in chloroplasts isolated from spinach. The regulation of the enzyme was investigated for different organs and respective organelles in a number of carotenoid-accumulating plants such as tomato (Pecker et al., 1992; Giuliano et al., 1993), pepper (Römer et al., 1993), and narcissus (Al-Babili et al., 1996). In the green alga Dunaliella bardawil, both mRNA and protein level of PDS remained constant during the massive β-carotene synthesis on high-light conditions. In this uni-cellular alga the secondary β-carotene accumulation in intraplastidic lipid droplets is controlled by the formation of the sequestering structures (Rabbani et al., 1998).
In plants, carotenoids are reported to be synthesized exclusively within plastids (Lichtenthaler, 1999, and refs. cited therein). H. pluvialis is unique among all algae and plants that have been studied to date in that it accumulates huge amounts of carotenoids in lipid vesicles outside the plastid (Mesquita and Santos, 1984). This has given rise to speculations about the possible existence of a biosynthetic pathway specific for secondary carotenogenesis that is localized in the cytoplasm. This possibility is supported by the cytosolic accumulation of the final product and was addressed recently by Sun et al. (1998), who studied the function of two IPP isomerases found in H. pluvialis. However, data on the cellular localization of both isoenzymes were not presented by the authors.
We report on the regulation and compartmentation of PDS in flagellates of H. pluvialis grown in the cultivation system described previously (Grünewald et al., 1997).
MATERIALS AND METHODS
Organism and Growth Conditions
Haematococcus pluvialis Flotow (no. 192.80, culture collection of the University of Göttingen, Germany; synonym: Haematococcus lacustris [Girod] Rostafinski) was grown autotrophically in 100-mL Erlenmeyer flasks at 20°C (±2°C) in a medium described by Hedlich (1982) with the addition of 0.3 μm thiamine. The flasks containing 50 mL of algae suspension were neither shaken nor aerated. The accumulation of SC was achieved by nitrate deprivation of the medium to 5% and stronger light intensities (150 μmol photons m−2 s−1 of continuous white light) in the two-step batch cultivation system described in Grünewald et al. (1997).
Photon flux densities were measured using a photometer (model LI-189, LI-COR, Lincoln, NE). Cell number was determined using a cell counter (model Casy 1, Schärfe Systems, Reutlingen, Germany).
Preparation of Cell Fractions
Aliquots of cells were harvested by centrifugation at 1,400g for 2 min and re-suspended in break buffer consisting of 0.1 m Tris-HCl, pH 6.8, 5 mm MgCl2, 10 mm NaCl, 10 mm KCl, 5 mm Na2-EDTA, 0.3 m sorbitol, 1 mm aminobenzamidine, 1 mm aminohexanacid, and 0.1 mm phenylmethylsulfonyl fluoride (PMSF), and passed through a 10-μm isopore polycarbonate filter (Millipore, Eschborn, Germany). The filtrate was centrifuged for 10 min at 16,000g to yield a chloroplast and cell debris pellet. All supernatant was transferred to a fresh tube and centrifuged at 108,000g for 1 h. The resulting three fractions, the microsome pellet, the supernatant fraction, and the lipid vesicles floating on top, were separated and stored at −20°C.
Protein Analysis
Proteins were separated on 12% (w/v) SDS-PAGE gels. For western analysis, the gels were electrophoretically transferred semidry to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), and treated with Ponceau S for staining the protein ladder transiently. Primary antibodies were used at a dilution of the raw serum given in the text. Secondary antibody conjugates with alkaline phosphatase were used for immunostaining with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium chloride (NBT). Quantification of labeling was done using densitometry (Scanpack 3.0, Biometra, Göttingen, Germany). Total protein content was determined by means of the DC protein assay kit (Bio-Rad, Munich).
RNA Extraction and mRNA Measurement
Standard RNA/DNA techniques were carried out according to the standard methods described in Sambrook et al. (1989). RNA was isolated from aliquots of 107 frozen cells harvested at different stages of SC accumulation using the TriReagent (Sigma, St. Louis) according to the manufacturer's instructions. The concentration of total RNA was determined spectrophotometrically at 260 nm, and aliquots of the extracts were subjected to agarose gel electrophoresis to test for ethidium bromide staining intensity. The results of both methods were found to be identical. Reverse transcription followed by PCR (RT-PCR) was performed from 50 ng of RNA in a reaction buffer containing 1× first-strand buffer (GIBCO-BRL, Karlsruhe, Germany), 10 mm dithiothreitol (DTT), 1 mm dNTPs, 0.5 μm oligo-dT primer, 40 units of rRNasin (Promega, Madison, WI), and 200 units of Superscript II (GIBCO-BRL) in a total volume of 40 μL at 42°C for 45 min after 10 min at room temperature. The reaction was stopped by incubating the samples at 95°C for 5 min.
The PCR amplification was performed from 10 μL of the RT reaction mixture in a total volume of 50 μL with 0.03 μm of each primer, 2.5 μCi of 32P-dCTP, 1× Taq buffer, and 5 units of Taq polymerase. Primers were 5′-TCGCATCGGCCTGCTGC-3′ and 5′-GGCCAGGTGCTTGACGCT-3′ for yielding a 370-bp fragment for pds. The primers 5′-GCTGGTGAAGAGCCTGAC-3′ and 5′-TGGACCAGCTGCACTGGC-3′ were used for crtO, generating a 530-bp fragment. The PCR program was: 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C for 23 cycles. The PCR products were analyzed on 7% (w/v) polyacrylamide gels in 1× Tris-borate/EDTA (TBE) buffer. Dried gels were exposed to film and quantified using a phosphor imager and Macbas software (Fuji, Tokyo). To demonstrate linearity between the template mRNA and the final PCR product, different amounts of RNA were used.
Expression of H. pluvialis PDS in Escherichia coli and Enzyme Purification
PDS of H. pluvialis (GenBank accession no. X86783) was expressed in E. coli using the QIAexpress pQE 32 vector (Qiagen, Hilden, Germany) possessing a His-tag sequence. Plasmid ppdsHp was digested with BamHI-KpnI and the excised 2,137-bp fragment was ligated into the BamHI-KpnI site of the vector to yield plasmid pQE32-pdsHp. Correct ligation was confirmed by sequencing across the ligation sites using the QIAexpress primers (Qiagen). The plasmid was transfected into E. coli M15 cells carrying the lac repressor plasmid pREP4. Cells from a starter culture were grown in 2× YT medium at 37°C to an OD600 of 0.6. For induction, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 2 mm.
After 5 h of cultivation at 30°C, the cells were harvested by centrifugation at 4,000g for 20 min. Following cell lysis by sonication in binding buffer (0.1 m NaH2PO4 and 0.02 m Tris-HCl, pH 8.0), the homogenate was adjusted to 6 m guanidine-HCl in binding buffer, kept on ice for 1 h, and centrifuged at 15,000g for 30 min. The supernatant was loaded onto a Ni-NTA agarose column (Qiagen). After several washes, the protein was eluted with 50 mm imidazole. Aliquots of the fractions were analyzed for PDS by SDS-PAGE. Positive fractions were pooled and loaded onto a preparative SDS-PAGE. Gels were stained with Coomassie Brilliant Blue R250. The corresponding band of about 66 kD, verified by western blotting using a RGSHis antibody (Qiagen), was excised from the gel and eluted using Elucon (Biometra). The eluate was precipitated twice with ethanol, re-dissolved in water, dialyzed against 1 mm PBS, and stored lyophilized.
Antibody Preparation
Polyclonal antibodies against PDS overexpressed in E. coli and purified as described above were raised in rabbits. Immunization was as described in Eckert et al. (1996). The raw serum was used without further purification.
Electron Microscopy and Immunolocalization
For immunogold labeling, algal cells were harvested at 550g for 3 min, and then fixed with 4% (w/v) paraformaldehyde and 0.5% (w/v) glutaraldehyde in growth medium for 25 min. After several washes in distilled water, the specimens were dehydrated in a graded ethanol series with 3% (w/v) uranylacetate at 70% (v/v) ethanol for 10 min. Cells were embedded in LR White or LR Gold (London Resin, London) according to the manufacturer's instructions. Ultrathin sections were cut with a microtome (Ultrotome III, LKB, Stockholm) using a diamond knife and placed on Ni grids. Immunogold labeling of LR Gold-embedded sections was performed by floating the grids section side down on the following aqueous solutions: blocking on 5% (w/v) BSA in PBS for 1 h; primary antibody at 1:500 (if not otherwise indicated in the figures) in 1% (w/v) BSA in PBS for 10 h; five washes on 1% (w/v) BSA in PBS; 20 nm of gold-conjugated anti-rabbit-IgG 1:100 in 1% (w/v) BSA in PBS for 1 h; and five washes on distilled water. Sections were post-stained with 3% (w/v) aqueous uranylacetate for 5 min, 1% (w/v) aqueous lead citrate for 30 s, and were then examined in an electron microscope (model EM 900, Carl Zeiss, Oberkochen, Germany) at 80 kV.
RESULTS
Overexpression of PDS in E. coli and Antibody Generation
Antibodies directed against selected enzymes are indispensable tools for expression pattern and compartmentation studies in cell fractions and by cytoimmunological techniques. We generated antibodies against His-tagged PDS of H. pluvialis that was overexpressed in E. coli and purified subsequently by different steps including Ni-affinity chromatography. The plasmid ppdsHp, consisting of the full-length cDNA of H. pluvialis PDS (accession no. X86783) cloned in EcoRI-XhoI of pBluescript SK(+) (Stratagene, La Jolla, CA), was kindly provided by Tamar Lotan (Department of Genetics, Hebrew University of Jerusalem, Israel). The coding sequence of pds was expressed in E. coli, as described in “Materials and Methods.” Protein extracts from IPTG-induced E. coli cells were analyzed by SDS-PAGE. A prominent polypeptide of about 66 kD, which also showed immunoreactivity with an anti-His-tag antibody, appeared. After Ni-affinity chromatography, this protein was further purified on a 12% (w/v) SDS-PAGE. The protein from the excised gel band was electroeluted, dialyzed, mixed with Freund's complete adjuvants, and used for immunization of rabbits.
Abundance of PDS during SC Accumulation
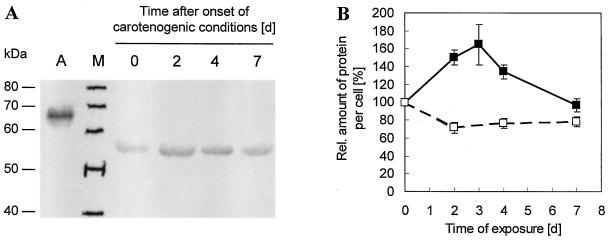
Accumulation of SC under stress conditions leads to a dramatic color shift of the flagellates from green to red. To investigate whether this process is accompanied by a parallel increase in enzyme we quantified the abundance of PDS by western analysis. Carotenogenic enzymes show high turnover rates, and their cellular protein levels are very low (Sandmann, 1997). The antibodies raised against the recombinant His-tagged PDS recognized traces of the antigen at very high dilution (Fig. 2A, lane A). We separated total protein extracts of start samples and of samples drawn 2, 4, and 7 d after the onset of conditions inductive for SC biosynthesis on SDS-PAGE and detected the amount of PDS protein immunologically (Fig. 2A, lanes 0, 2, 4, and 7). The antiserum recognized a single band at about 53 kD, representing the mature enzyme cleaved of its N-terminal transit sequence (Sandmann, 1994). This band appeared in all stages and showed typical induction kinetics in the time course of SC biosynthesis, being constitutively abundant in the green flagellates, reaching a maximum after about 3 to 4 d after the start of induction, and declining notably during the later SC synthesis period (Fig. 2B). The preimmune serum did not give any signal in the immunological analysis whether in the total extracts or with the antigen (data not shown).
Figure 2.
Induction of PDS during the synthesis of secondary carotenoids in H. pluvialis. A, Typical nitrocellulose blot probed with PDS antiserum (1:6,000). From left: 0.05 μg of the antigen (A); marker (M); total protein extracts from 105 flagellates, before (0), 2, 4, or 7 d after the onset of inductive conditions for secondary carotenoid biosynthesis. B, Densitometric results for the induction of PDS on a cell base (▪) and the drop of total protein content per cell (□). Bars indicate se of 17, 15, 2, 17, and 17 experiments for 0, 2, 3, 4, and 7 d after onset of induction, respectively, and of four experiments for total protein content.
Transcript Levels of PDS and CRTO during SC Accumulation
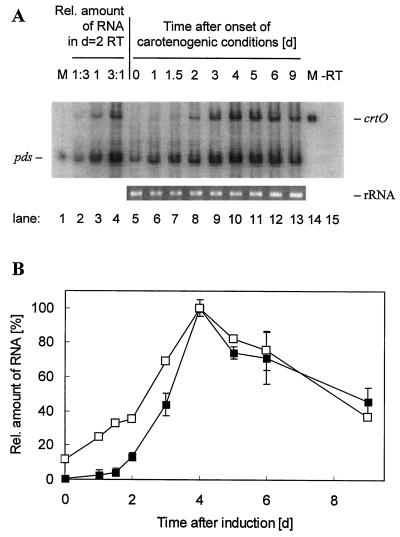
In addition to data on protein abundance, for further examination of the regulation of carotenoid genes during induction of SC synthesis in H. pluvialis, the changes of mRNA levels were investigated. Due to very low abundance of transcripts of carotenogenic genes (Cunningham and Gantt, 1998), the sensitive method of RT-PCR was used for quantification. We measured the steady-state levels of mRNA for PDS and CRTO by RT-PCR of total RNA. During the course of exposure, changes in total RNA per cell content did not exceed 10%. Co-amplification RT-PCR of the two transcripts was performed using four specific primers. As demonstrated for the RNA isolated from samples drawn 2 d after the onset of SC induction, the amount of amplification product was linearly correlated with the initial amount of total RNA used for the RT reaction (Fig. 3A, lanes 2–4). No amplification product appeared if the RT reaction prior to PCR was omitted (Fig. 3A, last lane). The steady-state PDS mRNA level was found to be linearly increased from the preexisting basal level immediately upon onset of inductive conditions, reaching its maximum after 4 d, and then decreasing slowly. In the case of CRTO, no mRNA was detected in the green flagellates of the start samples. After a delay of about 1.5 d of incubation the steady-state level of the CRTO transcripts increased rapidly. The maximal amount after 4 d matched the maximum observed for the PDS, and the subsequent decrease was comparable to that observed in PDS.
Figure 3.
Changes in the mRNA content of H. pluvialis PDS and CRTO during secondary carotenoid biosynthesis measured by RT-PCR. A, Typical autoradiograph of the RT-PCR products. The relative amount of RNA indicated as rRNA on an ethidium bromide-stained agarose gel is shown below. Lanes from left: M, PCR from pds in pBluescript (used as a marker); lanes 2 to 4, RT-PCR from 16.7, 50, and 150 ng total RNA of the extract 2 d after onset of inductive conditions; lanes 5 to 13, RT-PCR products from 50 ng total RNA for the different days after induction start; M, PCR from crtO in pBluescript (used as marker); lane 15, PCR from 50 ng total RNA as used in lane 2 (RT reaction omitted). B, Results from the phosphor imager quantification analyses of the RTR-PCR products for crtO (▪) and pds (□). Bars indicate the se of four parallels.
Immunogold Localization of PDS
We found that the best structural preservation and maintenance of antigenic structures was achieved when cells were fixed at increasing concentrations of paraformaldehyde and glutaraldehyde and embedded after dehydration in LR Gold resin. Accessibility of antigenic determinants was proven for membrane-bound antigens by probing with a anti-light-harvesting protein (LHC) antibody raised against spinach LHC, and for soluble proteins with the anti-Rubisco small subunit (data not shown). Both antibodies were kindly provided by U. Johanningmeier (Institute for Plant and Cell Physiology, University of Halle, Germany).
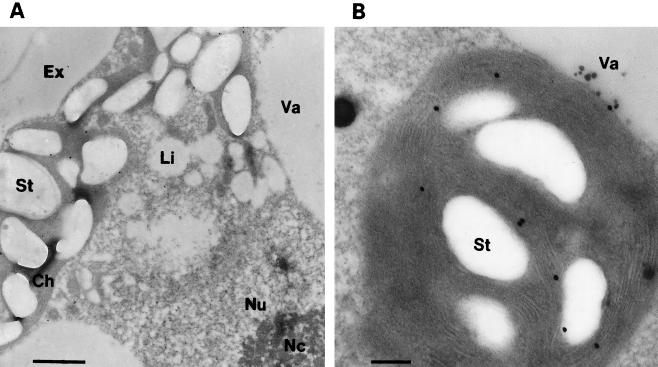
Probing the sections with the polyclonal antibodies raised against PDS resulted in a typical pattern of gold decoration (Fig. 4A; Table I). Two main parts of the cells were specifically labeled, the chloroplast and the outer paracrystalline layer of the extracellular matrix (Siegmund, 1999). No specific signal was obtained in or around the lipid vesicles or in the cytoplasm. Statistics of the distribution of the gold particles in the course of SC biosynthesis are presented in Table I. The signal in the extracellular matrix was evident at all stages and is interpreted as cross-reactivity due to a antigenic determinant occurring in PDS and a structural protein of the extracellular matrix. Interestingly, western-blot analysis did not reveal any crossreactivity of such proteins. No specific labeling was observed when sections were probed with preimmune serum (Table I). At higher magnifications, the chloroplast labeling proved to be specific to the thylakoids (Fig. 4B).
Figure 4.
Immunogold labeling of PDS in a flagellate of H. pluvialis exposed for 2 d to SC-inducing conditions. A, Typical part of a flagellate showing all major cell compartments. Bar represents 1 μm. B, Magnification of the chloroplast. Bar represents 0.2 μm. Ch, Chloroplast; Ex, extracellular matrix; Li, lipid vesicles; Nc, nucleolus; Nu, nucleus; St, starch; Va, vacuole.
Table I.
Distribution of PDS immunogold labeling in the major compartments of the flagellates at different stages of SC biosynthesis
| Time after Onset of Induction | Primary Antibody | Relative Immunogold Labeling
|
Cells, Labeling Counted for | Mean of Gold Particles per Cell | |||
|---|---|---|---|---|---|---|---|
| Chloroplast | Extracellular matrix | Cytoplasma | Nucleus | ||||
| d | % | ||||||
| 0 | Anti-PDS 1∶1,000 | 39.9 ± 2.2 | 51.9 ± 1.7 | 6.0 ± 0.6 | 2.2 ± 0.7 | 10 | 69 |
| 2 | Anti-PDS 1∶1,000 | 42.7 ± 4.0 | 47.7 ± 4.0 | 8.2 ± 1.7 | 1.3 ± 0.5 | 11 | 51 |
| 4 | Anti-PDS 1∶1,000 | 50.1 ± 4.0 | 41.1 ± 3.7 | 6.7 ± 0.7 | 2.1 ± 0.5 | 12 | 66 |
| 7 | Anti-PDS 1∶1,000 | 24.2 ± 1.4 | 68.9 ± 1.8 | 6.5 ± 0.7 | 0.5 ± 0.2 | 11 | 113 |
| 2 | Preimmune 1∶250 | 56.3 ± 2.9 | 17.2 ± 4.1 | 20.3 ± 1.7 | 6.2 ± 2.1 | 11 | 22 |
Includes lipid vacuoles.
Compartmentation of PDS
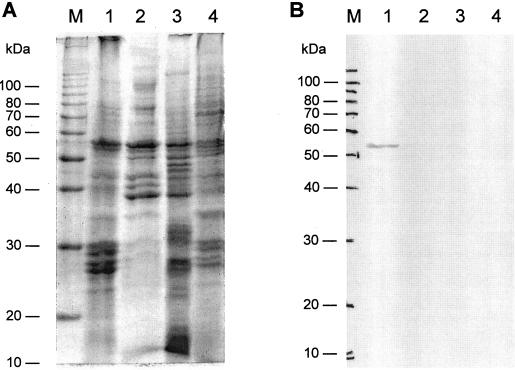
To prove the results from the immunogold localization experiments, we probed cell fractions with the anti-PDS antibodies. Following a gentle cell disruption by passing flagellates through polycarbonate isopore filters, fractions were isolated by differential centrifugation and phase separation. Four fractions were obtained (Fig. 5A, lanes 1–4). Light-microscopic observations identified them as: (a) a pellet of starch granules, cell debris, and larger cell organelles, mainly of the chloroplast; (b) a supernatant fraction; (c) a pellet of microsomes and cytoplasmic membranes; and (d) the lipid vesicle fraction. When probed with PDS antibodies, an immunoreactive band occurred only in the chloroplast membrane fraction (Fig. 5B). The results shown here are for fractions of flagellates incubated for 4 d under inductive conditions for SC synthesis, representing a state at which the amount of PDS in total extracts was elevated compared with the values before the onset of induction.
Figure 5.
Distribution of PDS in cellular fractions of H. pluvialis flagellates exposed for 4 d to N deprivation and high light. A, Coomassie-stained SDS-PAGE gel of chloroplastic (1), supernatant (2), microsomal (3), and lipid vesicle fraction proteins (4). Each lane was loaded with 15 μg of protein. B, Western-blot analysis with antibodies raised against recombinant PDS (1:4,000). Lanes 1 to 4 are as indicated in A, but were loaded with cell fraction aliquots of 105 cells.
DISCUSSION
In the present study we addressed the question of whether the biosynthesis of secondary carotenoids in H. pluvialis proceeds via an independent second pathway that operates outside the chloroplast, as the accumulation of the astaxanthin esters in cytosolic lipid vesicles might indicate. According to our data for PDS, we conclude that, at least for this relatively early biosynthetic step of carotenogenesis, no second cytosolic pathway exists and that higher biosynthetic activity is coupled to higher amounts of enzyme. Up-regulation on both the mRNA and protein levels was observed upon induction of SC synthesis. Because of slight variability in the extent of SC accumulation between parallels (Grünewald, 1997), we do not interpret the small difference between the maxima in PDS mRNA and protein levels as a sign for post-translational regulation events—at least the main part of up-regulation takes place at the mRNA level.
Bouvier et al. (1998) showed that pepper PDS mRNA increased under different stress conditions. During accumulation of capsanthin and capsorubin, Römer et al. (1993) observed after an increase between the mature green pepper fruit and the first defined ripening stage a constant level of PDS mRNA despite the massive increase of carotenoids occurring during the further ripening stages, and this resembles our findings here. Although SC continued to accumulate, PDS mRNA did not increase further and, moreover, even slightly decreased. Despite the delay in the increase and the absence in green, unstressed flagellates, an analogous pattern was observed for CRTO mRNA. In respect to the absence in green flagellates, our results are different from those reported by Sun et al. (1998), who detected mRNA of CRTO even in noninduced green cells. This might have been caused by different cultivation conditions used. Especially, the presence of acetate in the medium before induction could be responsible for the basic level of CRTO mRNA reported by these authors. Unfortunately, at the present time, antibodies directed specifically against the β-carotene oxygenase or other enzymes involved in the late steps of SC synthesis are not available.
The increase in the transcripts of carotenogenic genes, as shown here for β-carotene oxygenase and PDS, and the parallel increase in the protein level, observed at least for PDS, obviously enable the alga to maximize SC synthesis under stress conditions. Support for this point of view comes from Linden (1999), who cloned β-carotene hydroxylase from H. pluvialis and observed an increase of the corresponding mRNA in parallel to astaxanthin synthesis. Contrary findings have been reported in Dunaliella bardawil (Rabbani et al., 1998). On stress induction, this alga massively accumulates β-carotene in intraplastidic droplets. No significant parallel increases in PDS protein or mRNA levels were observed. One reason for this striking difference between the regulation patterns of carotenogenesis in these two Volvocales might be the different localization of the final product. The intraplastidic accumulation in D. bardawil seems to be regulated by the presence of the sequestering structures (Rabbani et al., 1998). In contrast, the extraplastidic accumulation in H. pluvialis might allow the common transcriptional control of biosynthesis of SC and cytoplasmic lipid vesicle material. This reflects the importance of the localization of the processes involved in terms of regulation.
Only very few immunogold localization experiments have been done for carotenogenic enzymes, and most have dealt with isolated and enriched organelles. Typical examples are geranylgeranylpyrophosphate synthase in pepper (Cheniclet et al., 1992) and PDS in isolated spinach chloroplasts (Linden et al., 1993). We present, for the first time to our knowledge, immunogold labeling data on the localization of PDS in algal cells. Most of the labeling was found within the chloroplast. Moreover, the absence of specific signals in the cytoplasm or inside or around the SC-containing lipid vesicles ruled out the possible occurrence of PDS there. Higher magnifications of chloroplast regions showed the signal predominantly located in close contact to the thylakoids. The non-thylakoidal-localized gold particles might indicate a soluble inactive population of PDS in the stroma (Al-Babili et al., 1996).
Our results suggest that PDS isoenzymes, possibly located in the cytoplasm, are not involved in the synthesis of SC (for IPP isomerase, compare with Sun et al., 1998). From the highly conserved structure of plant type PDS (Sandmann, 1994), and the polyclonal nature of the antibodies, we conclude that isoenzymes of PDS would have been recognized in our cytoimmunochemical experiments. Moreover, changes in the enzyme amount were in accordance with SC synthesis and the mRNA pattern.
Cell fractionation experiments supported results from immunogold cytochemistry. Fractionation of cells with increased PDS amounts resulted in four fractions, of which only the chloroplast membranes gave a signal in western-blot analysis. This confirms previous data on PDS localization in different plants and sequence information of a chloroplast import signal that is found in all PDS (Bonk et al., 1997).
We conclude that PDS in H. pluvialis is restricted to the chloroplast. We also demonstrated that the regulation of PDS occurs primarily at the mRNA level, most likely by transcriptional control, and that the enzyme is induced in response to stress conditions leading to SC biosynthesis. The localization results implicate for H. pluvialis a transport process of SC over compartment borders from the chloroplast as the site of synthesis to the lipid vesicles located in the cytoplasm as the site of accumulation. Further elucidation of this process and the intermediate step at which it occurs will help to complete our knowledge on the regulation of SC biosynthesis.
ACKNOWLEDGMENTS
We thank Prof. Dr. W. Braune who has suggested and encouraged us to work on the H. pluvialis carotenoid problem. We are indebted also to S. Schmidt for technical assistance and to M. Goldstein, V. Mann, M. Utting, and J. Müller for helpful comments during the course of this work, and especially to U. Johanningmeier (Institute for Plant and Cell Physiology, University of Halle, Germany) for kindly providing the antispinach LHC and the anti-Rubisco small subunit antibodies.
Footnotes
This study was supported in part by the Deutscher Akademischer Austauschdienst (short-term fellowship to K.G.), by the Thüringer Ministerium für Forschung, Wissenschaft und Kultur (grant no. B301–69013), and by a graduate fellowship from the Freistaat Thüringen for K.G.
This paper is dedicated to the occasion of the 65th birthday of Prof. Dr. Wolfram Braune.
LITERATURE CITED
- Al-Babili S, von Lintig J, Haubruck H, Beyer P. A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J. 1996;9:601–612. doi: 10.1046/j.1365-313x.1996.9050601.x. [DOI] [PubMed] [Google Scholar]
- Bonk M, Hoffmann B, Von Lintig J, Schledz M, Al-Babili S, Hobeika E, Kleinig H, Beyer P. Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem. 1997;247:942–950. doi: 10.1111/j.1432-1033.1997.00942.x. [DOI] [PubMed] [Google Scholar]
- Bouvier F, Backhaus RA, Camara B. Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem. 1998;273:30651–30659. doi: 10.1074/jbc.273.46.30651. [DOI] [PubMed] [Google Scholar]
- Camara B, Bardat F, Moneger R. Sites of biosynthesis of carotenoids in Capsicum chromoplasts. Eur J Biochem. 1982;127:255–258. doi: 10.1111/j.1432-1033.1982.tb06863.x. [DOI] [PubMed] [Google Scholar]
- Cheniclet C, Rafia F, Saint-Guily A, Verna A, Carde J-P. Localization of the enzyme geranylgeranylpyrophosphate synthase in Capsicum fruits by cytochemistry after conventional chemical fixation or quick-freezing followed by freeze-substitution: labelling evolution during fruit ripening. Biol Cell. 1992;75:145–154. [Google Scholar]
- Cunningham FX, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Eckert M, Gabriel J, Birkenbeil H, Greiner G, Rapus J, Gäde G. A comparative study using an antiserum against a synthetic analogue of the corpora cardiaca peptide Pea-CAH-I (MI, neurohormone D) of Periplaneta americana. Cell Tissue Res. 1996;284:401–413. doi: 10.1007/s004410050601. [DOI] [PubMed] [Google Scholar]
- Giuliano G, Bartley GE, Scolnik PA. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald K, Hagen C, Braune W. Secondary carotenoid accumulation in flagellates of the green alga Haematococcus lacustris. Eur J Phycol. 1997;32:387–392. [Google Scholar]
- Hagen C, Braune W, Björn LO. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales): III. Action as a “sunshade.”. J Phycol. 1994;30:241–248. [Google Scholar]
- Hagen C, Braune W, Greulich F. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales): IV. Protection from photodynamic damage. J Photochem Photobiol B Biol. 1993;20:153–160. [Google Scholar]
- Hedlich R. Haematococcustest. In: Breitig G, Tümpling W, editors. Ausgewählte Methoden der Wasseruntersuchung. II. Jena, Germany: Gustav Fischer; 1982. pp. 328–331. [Google Scholar]
- Kajiwara S, Kakizono T, Saito T, Kondo K, Ohtani T, Nishio N, Nagai S, Misawa N. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol Biol. 1995;29:343–352. doi: 10.1007/BF00043657. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Linden H. Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim Biophys Acta. 1999;1446:203–212. doi: 10.1016/s0167-4781(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Linden H, Lucas MM, De Felipe MR, Sandmann G. Immunogold localization of phytoene desaturase in higher plant chloroplasts. Physiol Plant. 1993;88:229–236. [Google Scholar]
- Lotan T, Hirschberg J. Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 1995;364:125–128. doi: 10.1016/0014-5793(95)00368-j. [DOI] [PubMed] [Google Scholar]
- Nishino H. Cancer prevention by natural carotenoids. J Cell Biochem (Suppl) 1997;27:86–91. [PubMed] [Google Scholar]
- Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci USA. 1992;89:4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani S, Beyer P, Vonlintig J, Hugueney P, Kleinig H. Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol. 1998;116:1239–1248. doi: 10.1104/pp.116.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer S, Huegeney P, Bouvier F, Camara B, Kuntz M. Expression of the genes encoding the early carotenoid biosynthetic enzymes in Capsicum annuum. Biochem Biophys Res Commun. 1993;196:1414–1421. doi: 10.1006/bbrc.1993.2410. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sandmann G. Phytoene desaturase: Genes, enzymes and phylogenetic aspects. J Plant Physiol. 1994;143:444–447. [Google Scholar]
- Sandmann G. High level expression of carotenogenic genes for enzyme purification and biochemical characterization. Pure Appl Chem. 1997;69:2163–2168. [Google Scholar]
- Santos F, Mesquita JF. Ultrastructural study of Haematococcus lacustris: I. Some aspects of carotenogenesis. Cytologia. 1984;49:215–228. [Google Scholar]
- Siegmund S. Die Zellhüllen der Grünalge Haematococcus pluvialis Flotow. Strukturelle und cytochemische Veränderungen während der Morphogenese vom Flagellaten zur Aplanospore. PhD thesis. Germany: Friedrich Schiller University Jena; 1999. [Google Scholar]
- Sun Z, Cunningham FX, Gantt E. Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc Natl Acad Sci USA. 1998;95:11482–11488. doi: 10.1073/pnas.95.19.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathne E, Bjerkeng B, Storebakken T, Vassvik V, Odland AB. Pigmentation of Atlantic salmon (Salmo salar) fed astaxanthin in all meals or in alternating meals. Aquaculture. 1998;159:217–231. [Google Scholar]