Abstract
Gut microbiota (GM) plays several crucial roles in host physiology and influences several relevant functions. In more than one respect, it can be said that you “feed your microbiota and are fed by it.” GM diversity is affected by diet and influences metabolic and immune functions of the host's physiology. Consequently, an imbalance of GM, or dysbiosis, may be the cause or at least may lead to the progression of various pathologies such as infectious diseases, gastrointestinal cancers, inflammatory bowel disease, and even obesity and diabetes. Therefore, GM is an appropriate target for nutritional interventions to improve health. For this reason, phytochemicals that can influence GM have recently been studied as adjuvants for the treatment of obesity and inflammatory diseases. Phytochemicals include prebiotics and probiotics, as well as several chemical compounds such as polyphenols and derivatives, carotenoids, and thiosulfates. The largest group of these comprises polyphenols, which can be subclassified into four main groups: flavonoids (including eight subgroups), phenolic acids (such as curcumin), stilbenoids (such as resveratrol), and lignans. Consequently, in this review, we will present, organize, and discuss the most recent evidence indicating a relationship between the effects of different phytochemicals on GM that affect obesity and/or inflammation, focusing on the effect of approximately 40 different phytochemical compounds that have been chemically identified and that constitute some natural reservoir, such as potential prophylactics, as candidates for the treatment of obesity and inflammatory diseases.
1. Introduction
Obesity is a chronic state of low-grade inflammation constituting a well-known risk factor for multiple pathological conditions, including metabolic syndrome and insulin resistance [1], and it has also been implicated as a proactive factor and associated with a nonfavorable disease course of chronic autoimmune inflammatory disorders, such as multiple sclerosis (MS) [2]. Several studies over the last decade report interest in fermentation products from gut microbiota (GM) in the control of obesity and related metabolic disorders [3]. GM denotes an entire ecosystem inhabiting each organism, thus constituting a “superorganism” [4]. GM plays several crucial roles in host physiology and influences several relevant functions: it harvests energy from indigestible food, influences fatty acid oxidation, fasting, bile acid production, satiety, and lipogenesis, and even influences innate immunity (reviewed in [3]). In more than one respect, we are able to establish that you “feed your microbiota and are fed by it.” GM provides signals that promote the production of cytokines, leading to the maturation of immune cells modulating the normal development of immune functions of the host immune system [5, 6]. Consequently, an imbalance of GM, or dysbiosis, can be the cause or at least lead to the progression of several pathologies such as infectious diseases, gastrointestinal cancers, cardiovascular disease, inflammatory bowel disease, and even obesity and diabetes [7, 8]. Additionally, a pathological state can cause an imbalance in this microbial ecosystem. For instance, a dysfunction of the innate immune system may be one of the factors that favor metabolic diseases through alteration of the GM [9].
In terms of immune response, the immune system recognizes conserved structural motifs of microbes, called PAMPs (pathogen-associated molecular patterns), by mean of toll-like receptors (TLR), which are expressed in the membrane of sentinel cells [10]. This interaction induces immune responses against microbes through the activation of inflammatory signaling pathways. Therefore, GM, which interacts with epithelial TLR, critically influences immune homeostasis [9]. Although the complete etiology of inflammatory diseases remains unknown, intestinal gut dysbiosis has been associated with a variety of neonatal and children's diseases [4], in which chronic intestinal inflammation and mucosal damage derives from alteration of GM [11].
Diet provides the nutritional supplies for life and growth, and some components exert valuable effects when consumed regularly. These components are called “functional foods” or “nutraceuticals” [12]. Consequently, functional foods contain bioactive substances, nutraceutics, which can be classified as micronutrients (vitamins and fatty acids) and nonnutrients (phytochemicals and probiotics) (see Table 1 in [13]). These components, with a wide range of chemical structures and functionality, provide different beneficial effects beyond simple nutrition, resulting in improved health.
Table 1.
Effects of different phytochemicals on GM and/or obesity with anti-inflammatory actions.
| Phytochemicals | Compound | Model | Effect on gut microbiota | Antioxidant and anti-inflammatory effect | Effect on obesity | Ref |
|---|---|---|---|---|---|---|
| Polyphenols | C57BL/6 J ApcMin mice | Bacterial diversity was higher in the bilberry group than in the other groups | Attenuation of inflammation in cloudberry-fed mice | [183] | ||
| Flavonones | Baicalein | C57BL/6 J mice | Suppress activation of NF-κB and decrease expression of iNOS and TGF-β | Activation of AMPK pathway and suppression of fatty acid synthesis, gluconeogenesis, and increased mitochondrial oxidation | [184] | |
| Catechins | Epigallocatechin-3-gallate | C57BL/6 J mice | The Firmicutes/Bacteroidetes ratio is significantly lower in HFD + EGCG but higher in control diet + EGCG | Potential use for prevention, or therapy, for obesity-related and oxidative stress-induced health risks | [185] | |
| Epigallocatechin-3-gallate | C57BL/6 J mice | Regulates the dysbiosis and maintains the microbial ecology balance | Significant protective effect against obesity induced by high-fat diet (HFD) | [186] | ||
| Epigallocatechin-3-gallate | Wistar rats | EGCG affects the growth of certain species of GM | Weights of abdominal adipose tissues fed 0.6% EGCG diet were suppressed. Regulated energy metabolism in the body | [187] | ||
| Quercetin | C57BL/6 J mice | An increase in Firmicutes/Bacteroidetes ratio and in gram-negative bacteria and increased in Helicobacter by HFD. Quercetin treatment benefits GM balance | Quercetin reverted dysbiosis-mediated Toll-like receptor 4 (TLR-4) NF-κB signaling pathway activation and related endotoxemia, with subsequent inhibition of inflammasome response and reticulum stress pathway activation | Benefits gut-liver axis activation associated to obesity, leading to the blockage of lipid metabolism gene expression deregulation | [109] | |
| Quercetin | Wistar rats | Quercetin supplementation attenuates Firmicutes/Bacteroidetes ratio and inhibiting the growth of bacterial species previously associated to diet-induced obesity (Erysipelotrichaceae, Bacillus, Eubacterium cylindroides). Quercetin was effective in lessening high-fat sucrose diet-induced GM dysbiosis | [188] | |||
| Quercetin | Fischer 344 rats | Exerts prebiotic properties by decreased pH, increased butyrate production, and altered GM | Onion extract increased glutathione reductase (GR) and glutathione peroxidase (GPx1) activities in erythrocytes. In contrast, g-glutamate cysteine ligase catalytic subunit gene expression was upregulated | [189] | ||
| Kaempferol | 3 T3-L1 adipocytes | Kaempferol reduced LPS proinflammatory action. Demonstrating the anti-inflammatory and antioxidant effects | Concomitantly, polyphenols increased the production of adiponectin and PPARγ, known as key anti-inflammatory and insulin-sensitizing mediators | [110] | ||
| Anthocyanins | C57BL/6 J mice | Feces of GM-deficient mice showed an increase in anthocyanins and a decrease in their phenolic acid metabolites, while a corresponding increase was observed in jejunum tissue | Mice with intact GM reduced body weight gain and improved glucose metabolism | [190] | ||
| Anthocyanins | C57BL/6 J mice | Anthocyanins could effectively reduce the expression levels of IL-6 and TNFα genes, markedly increasing SOD and GPx activity | Anthocyanins reduced body weight could also reduce the size of adipocytes, leptin secretion, serum glucose, triglycerides, total cholesterol, LDL-cholesterol, and liver triglycerides | [191] | ||
| Phenolic acid | Curcumin | Mice | A direct effect of bioactive metabolites reaching the adipose tissue rather than from changes in GM composition | Nutritional doses of Curcuma longa is able to decrease proinflammatory cytokine expression in subcutaneous adipose tissue | An effect independent of adiposity, immune-cell recruitment, angiogenesis, or modulation of GM controlling inflammation | [192] |
| Curcumin | LDLR−/− mice | Curcumin improves intestinal barrier function and prevents the development of metabolic diseases | Significantly attenuated the Western diet-induced increase in plasma LPS levels | Significantly reduced WD-induced glucose intolerance and atherosclerosis | [193] | |
| Curcumin | Human IEC lines Caco-2 and HT-29 | Curcumin modulates chronic inflammatory diseases by reducing intestinal barrier dysfunction despite poor bioavailability | Curcumin significantly attenuated LPS-induced secretion of master cytokine IL-1β from IEC and macrophages. Also reduced IL-1β-induced activation of p38 MAPK in IEC and subsequent increase in expression of myosin light-chain kinase | Curcumin attenuates WD-induced development of type 2 diabetes mellitus and atherosclerosis | [194] | |
| Stilbenes | Resveratrol | Kunming mice | HF microbiomes were clearly different from those in CT and HF-RES mice. After treatment, Lactobacillus and Bifidobacterium were significantly increased, whereas Enterococcus faecalis was significantly decreased, resulting in a higher abundance of Bacteroidetes and a lower abundance of Firmicutes | Treatment inhibited increases in body and fat weight in HF mice. Decreased blood glucose to control levels, decreased blood insulin and serum total cholesterol compared with HF mice. Severe steatosis seen in HF mice was well prevented in treated mice. Treatment significantly suppressed expression of PPAR-γ, Acc1, and Fas, suggesting inhibition of triglyceride storage in adipocytes | [195] | |
| Resveratrol | Glp1r−/− mice | Treatment modified GM | Decreased the inflammatory status of mice | Glucoregulatory action of RSV in HFD-fed diabetic wild-type mice, in part through modulation of the enteroendocrine axis in vivo | [196] | |
| Resveratrol | Wistar rats | Trans-resveratrol supplementation alone or in combination with quercetin scarcely modified the GM profile but acted at the intestinal level, altering mRNA expression of tight-junction proteins and inflammation-associated genes | Altering mRNA expression of tight-junction proteins and inflammation-associated genes | Administration of resveratrol and quercetin together prevented body weight gain and reduced serum insulin levels. Effectively reduced serum insulin levels and insulin resistance | [188] | |
| Resveratrol | Adipocytes | Generally, resveratrol opposed the effect induced by LPS, functioning as an ameliorating factor in disease state | LPS altering glycosylation processes of the cell. Resveratrol ameliorates dysfunctioning adipose tissue induced by inflammatory stimulation | [197] | ||
| Resveratrol | Humans | Steroid metabolism of the affected GM should be studied in detail | Subtle but robust effects on several metabolic pathways | [198] | ||
| Piceatannol | C57BL/6 mice | Pic altered the composition of the GM by increasing Firmicutes and Lactobacillus and decreasing Bacteroidetes | Pic significantly reduced mouse body weight in a dose-dependent manner. Significantly decreased the weight of liver, spleen, perigonadal, and retroperitoneal fat compared with the HFD group. Pic significantly reduced adipocyte cell size of perigonadal fat and decreased weight of liver | [199] | ||
| Piceatannol | Zucker obese rats | It did not modify the profusion of the most abundant phyla in GM, though slight changes were observed in the abundance of several Lactobacillus, Clostridium, and Bacteroides species belonging to Firmicutes and Bacteroidetes | Shows a tendency to reduce plasma LPS by 30% | Pic did not reduce either hyperphagia or fat accumulation. There is a tendency toward the decrease of circulating on-esterified fatty acids, LDL-cholesterol, and lactate. While Pic tended to improve lipid handling, it did not mitigate hyperinsulinemia and cardiac hypertrophy | [155] | |
| Organosulfur compounds | GEO (garlic essential oil) DADS (DiAllyl DiSulfide) |
C57BL/6 mice | Significantly decreased the release of proinflammatory cytokines in liver, accompanied by elevated antioxidant capacity via inhibition of cytochrome P450 2E1 expression | GEO and DADS dose-dependently exerted antiobesity and antihyperlipidemic effects by reducing HFD-induced body weight gain, adipose tissue weight, and serum biochemical parameters | [200] |
Gut bacterial diversity is mainly affected by the diet, which may also affect its functional relationships with the host [14–17]. During their gastrointestinal passage, the components of the diet are metabolized by intestinal bacteria [18]. Diets rich in carbohydrates and simple sugars lead to Firmicutes and Proteobacteria proliferation, while those rich in saturated fat and animal protein favor Bacteroidetes and Actinobacteria [19]. Microbial diversity of the intestine decreases in diets with higher fat content [16]. Several physiological aspects of the gut environment can be influenced by the diet, then, including absorption of micronutrients, vitamins, and nutraceutics, and changes in pH of the gut environment, which in turn alters the balance of the GM [20]. Therefore, GM influences the biological activity of food compounds but is also a target for nutritional intervention to improve health [18].
On this basis, phytochemicals, like nutraceuticals that can influence GM, are being studied as coadjuvants to treat obesity and inflammatory diseases. In this review, we will present, organize, and discuss the most recent evidence that points to a relationship of the phytochemical effect on GM that affects obesity and/or inflammation, focusing on the effect of phytochemicals as potential prophylactics and candidates for the treatment of these diseases.
2. Phytochemicals Can Influence Obesity and Inflammatory Diseases through Affecting GM
Phytochemicals can be defined as “bioactive nonnutrient plant compounds present in fruits, vegetables, grains, and other plants, whose ingestion has been linked to reductions in the risk of major chronic diseases” [21]. Held to be phytochemicals, prebiotics are nondigestible food components (mainly carbohydrate polymers, such as fructooligosaccharides and mannooligosaccharides) that benefit the human body because they modulate GM through selective stimulation of some bacterial species proliferation in the colon, named “probiotics” [22]. These include endosymbionts such as lactic acid bacteria, bifidobacteria, yeast, and bacilli, which participate in the metabolism of their hosts [13]. Regarded as functional foods, both prebiotics and probiotics have been considered potential constituents of therapeutic interventions that modify GM in an attempt to modulate in turn some inflammatory diseases (comprehensively reviewed in [23]). On the other hand, the remaining phytochemical compounds may be classified on the basis of some common structural features into groups as follows: polyphenols and derivatives, carotenoids, and thiolsulfides, among others (see Table 1 in [13]). Of the latter, the polyphenols represent the largest group.
Polyphenols are secondary metabolites of plants and represent vastly diverse phytochemicals with complex chemical structures. They are commonly present in plant foods, such as cacao, coffee, dry legumes (seeds), fruits (like apples and berries), nuts, olives, some vegetables (such as lettuce and cabbage), tea, and wine. The daily intake of dietary phenols is estimated to be above 1 g, which is 10 times higher than the vitamin C intake from diet [24]. The interaction between polyphenols and GM has been well established [25]. Polyphenols are frequently conjugated as glycosides, which derive in aglycones when metabolized by GM. Generally, the intestinal metabolism of polyphenols includes hydrolysis of glycosides and esters, reduction of nonaromatic alkenes, and cleavage of the skeletons [26, 27]. Studies have reported that only a low number of polyphenols can be absorbed in the small intestine. The remaining (90–95%) nonabsorbed polyphenols reach the colon in high concentrations (up into the mM range), where they are degradated by microbial enzymes before their absorption [28]. Compared to their parent compounds, the permanence in plasma for metabolites is extended and they are finally eliminated in urine [29, 30]. GM, then, can regulate the health effects of polyphenols, and reciprocally, polyphenols can modulate GM and even interfere with its own bioavailability [31].
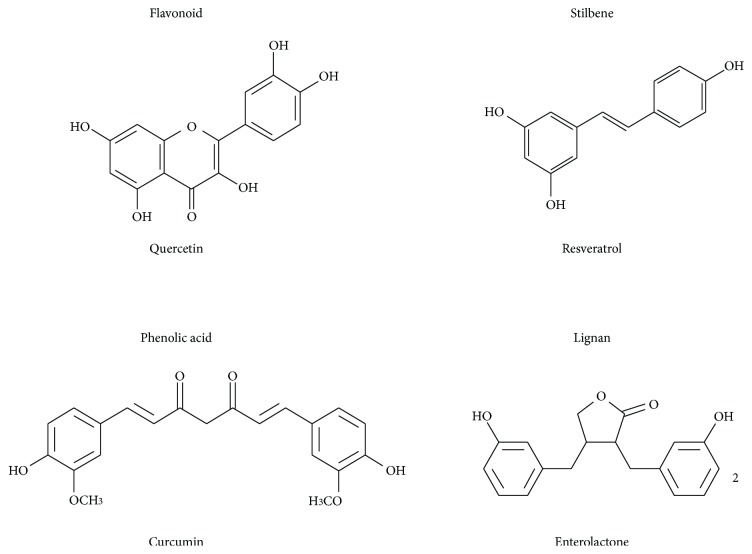
Approximately 8000 structures of polyphenols have been identified [32], which can be classified into four main groups (Figure 1) as follows: (a) flavonoids (with eight subgroups), (b) phenolic acids (curcumin), (c) stilbenoids (resveratrol), and (d) lignanes. Polyphenols have been extensively studied over the past decade because of their strong antioxidant and anti-inflammatory properties and their possible role in the prevention and cotreatment of several chronic diseases, such as hypertension, diabetes, neurodegenerative diseases, and cancer [33–36]. In addition, polyphenols have recently attracted interest in the media and in the research community because of their potential role in reducing obesity, an increasingly serious health issue in different population age ranges [37, 38]. Polyphenols such as catechins, anthocyanins, curcumin, and resveratrol have been suggested as exerting beneficial effects on lipid and energy metabolism [39–41] and potentially on weight status. Multiple mechanisms of action have been proposed mostly as a result of animal and cell studies, such as inhibition of the differentiation of adipocytes [40], increased fatty acid oxidation [42], decreased fatty acid synthesis, increased thermogenesis, the facilitation of energy metabolism and weight management [43], and the inhibition of digestive enzymes [44].
Figure 1.
Chemical structure of representative molecules for the four main polyphenol groups.
Phenolic compounds from tea [45], wine [29], olives [46] and berries [47, 48] have demonstrated antimicrobial properties. Depending on their chemical structure, tea phenolics inhibit the growth of several bacterial species, such as Bacteroides spp., Clostridium spp., Escherichia coli, and Salmonella typhimurium [29]. Furthermore, tea catechins are able to change the mucin content of the ileum, affecting the bacterial adhesion and therefore their colonization [48]. Another study revealed that (+) catechin favored the growth of the Clostridium coccoides-Eubacteriumrectale group and E. coli but inhibited that of Clostridium histolyticum. In addition, the growth of beneficial bacteria, such as Bifidobacterium spp. and Lactobacillus spp., was nonaffected or even slightly favored [45, 49]. Both flavonoids and phenolic compounds reduce the adherence of Lactobacillus rhamnosus to intestinal epithelial cells [50]. The anthocyanins, a type of flavonoid, inhibit the growth of several pathogenic bacteria, including Bacillus cereus, Helicobacter pylori, Salmonella spp., and Staphylococcus spp. [47, 48]. Consequently, phytochemicals that affect the balance of the GM may influence obesity and inflammatory diseases.
Therefore, through the modulation of GM, polyphenols have the potential to generate health benefits. Although there is accumulative evidence concerning the polyphenolic effect on GM, the effects of the interaction between polyphenols and specific GM functions remain mostly uncharacterized; thus, much research remains to be conducted. We will focus on specific polyphenols that have been reported as able to affect GM and, in addition, influence obesity and/or inflammation.
3. Experimental Nutritional Interventions with Phytochemicals That Modify Gut Microbiota Exert an Effect on Obesity and/or Inflammatory Parameters
According to the United States National Agricultural Library, a “nutritional intervention” is “A clinical trial of diets or dietary supplements customized to one or more specific risk groups, such as cancer patients, pregnant women, Down syndrome children, populations with nutrient deficiencies, etc.” [51]. In a broader sense, we review herein the use of phytochemicals in experimental models (mainly polyphenols), which are able to modify GM and exert an effect on obesity and/or inflammatory parameters, in order to analyze and discuss their potential use for the prophylaxis and treatment of obesity and inflammatory diseases by the maintenance and control of GM.
To compile the information from scientific literature on the polyphenols that can be related with GM, we considered the following terms for search in PubMed: “gut microbiota” OR “intestinal microbiota” OR “gut flora” OR “intestinal flora” OR “gut microflora” OR “intestinal microflora,” and we added the specific compound (as listed in Figure 2). From this search, we can conclude that there is at least one report that correlates every polyphenol listed with GM. In addition, of the 40 listed compounds, there are 15 that yield at least 10 works that support the relationship between polyphenols and GM. However, there is still much work to be done in this area in terms of exploring in greater detail the specific actions of each compound on GM. Later, we added to these searches the following terms: “anti-inflammatory OR antiinflamatory” on one subsequent search, or “obesity” for another search. In both cases, the numbers of articles were scarce with a total of 116 and 71, respectively, although this number does not represent a real situation, because there are several articles that are repeated, and those that include more than one compound. From these articles, we extracted information that led to the indication of a relationship among the effects of different phytochemicals on the GM that affects obesity and/or the immune response (Table 1).
Figure 2.
Classification of the eight foremost flavonoid subgroups.
3.1. Flavonoids
The first and largest subgroup of polyphenols is integrated by flavonoids, with >6000 compounds identified and isolated from different plant sources [52], a large family of chemical compounds that constitutes plant and flower pigments and that shares the common function of being free radical scavengers. Due to the thousands of structurally different compounds, it becomes quite difficult to analyze all of them. Therefore, we performed a wide search of different specific compounds that have been reported in the literature and compiled them into eight subgroups, including the most representative compounds within each group (Figure 2). Essentially, all of these are widely recognized by their antioxidant [32, 53, 54] and anti-inflammatory [34, 55, 56] properties. Indeed, they inhibit reactive oxygen species (ROS) synthesis and hypoxia-signaling cascades, modulate cyclooxygenase 2 (COX-2), and block epidermal growth factor receptor (EGFR), insulin-like growth factor receptor-1 (IGFR-1), and nuclear factor-kappa B (NF-κB) signaling pathways. In addition, flavonoids are able to modulate the angiogenic process [57], and the majority of these have been recently involved with obesity [58, 59].
3.1.1. Flavones
Numerous studies have been undertaken on the influence of GM on the intestinal absorption and metabolism of particular flavones, such as apigenin, luteolin, and chrysin, both in rodents and in human cells [60–63]. On the other hand, there are multiple studies that associate different flavones with anti-inflammatory effects. This is the case for apigenin [64–67], luteolin [68, 69], and chrysin [34]. Furthermore, recent studies involve apigenin with the amelioration of obesity-related inflammation [70] and regulating lipid and glucose metabolism [71], luteolin with the amelioration of obesity-associated insulin resistance, hepatic steatosis and fat-diet-induced cognitive deficits [72–75], and chrysin, which inhibits peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer binding protein A (C/EBPα), major adipogenic transcription factors in preadipocytes [75] and which also modulate enhanced lipid metabolism [76]. However, to the best of our knowledge, there is still no study that considers together these following three aspects: GM, inflammation, and obesity as positively affected by these flavones. Consequently, this constitutes a whole new avenue for studying these interactions.
3.1.2. Flavanones
Like the previous subgroup, flavanones also influence and interact with GM [28, 77, 78]. The main compounds included here also exhibit anti-inflammatory properties, such as hesperetin [79, 80], naringenin [81], morin [82–84], and eriodictyol [85–87]. Additionally, they influence lipid metabolism as a potential preventive strategy for obesity. For instance, hesperetin exhibits lipid-lowering efficacy [88, 89]; naringenin regulates lipid and glucose metabolism [71] and also prevents hepatic steatosis and glucose intolerance [90] by suppressing macrophage infiltration into the adipose tissue [91]. In addition, both compounds improve membrane lipid composition [92]. Furthermore, morin exhibits antihyperlipidemic potential by reducing lipid accumulation [31, 93]. Finally, eriodictyol ameliorates lipid disorders and suppresses lipogenesis [94]. Taken together, all of this evidence strongly indicates that these compounds can be usefully applied to prevent or treat obesity and its associated inflammation, but it is relevant to take GM into account in order to incorporate it into the organism's metabolism. Again, there are to our knowledge no studies that correlate all three of these aspects.
3.1.3. Flavonones
In this case, nomenclature represents a problem in the literature search, because the term “flavonones” is usually substituted by “flavanones,” which in fact represent a different subgroup. Due to this, compounds included in this subgroup were individually searched in databases. Three compounds were considered: hesperidin, naringin, and baicalein. In fact, the former two can be confused with similarly named compounds from the flavanone subgroup (see above) but constitute different compounds. As for all the polyphenols, the latter is metabolized by the GM [93, 95] and exhibits strong anti-inflammatory properties [79, 96, 97]. Additionally, these compounds also influence lipid metabolism as follows: hesperidin improves lipid metabolism against alcohol injury by reducing endoplasmic reticulum stress and DNA damage [98] and exhibits an antiobesity effect [99]; naringin also influences the lipid profile and ameliorates obesity [100], and finally, baicalein regulates early adipogenesis by inhibiting lipid accumulation and m-TOR signaling [101]. Again, there is a need for studies that take into account the following elements together, that is, GM metabolism of the polyphenols and their specific effect on lipid metabolism, obesity, and inflammation.
3.1.4. Flavanols
This subgroup mainly comprises catechins, which are more abundant in the skin of fruits than in fruit pulp. Catechins found in cranberries may contribute to cancer prevention [102]. Catechins are abundant in green tea, to which has been attributed several beneficial impacts on health. Traditionally, green tea has been used to improve resistance to disease and to eliminate alcohol and toxins by clearing the urine and improve blood flow [103, 104]. Lately, emerging areas of interest have been the effects of green tea for the prevention of cancer and cardiovascular diseases, as well as their effects on angiogenesis, inflammation, and oxidation [105, 106].
This subgroup of flavonoids is one of the few that has been studied to date under the lens of their relationship with GM and their anti-inflammatory actions [107], as well as their role in lipid metabolism and obesity [105, 108]. Among the compounds included in this group, we find the following: catechin, epicatechin, epigallocatechin, epigallocatechin 3-gallate, and gallocatechin. Practically, all of these have already begun to be studied in the light of their relationship between GM and inflammation, as well as that related with lipid metabolism and obesity (see Table 1 for specific examples). However, much work remains to ascertain the mechanisms by which these compounds are able to benefit health.
3.1.5. Flavonols
Compounds in this subgroup have also been studied as related with GM and inflammation or obesity, mainly quercetin and kaempferol, while another three, rutin, myricetin, and isohamnetin, have not to our knowledge been studied within this context. Quercetin protects against high-fat diet-induced fatty liver disease by modulating GM imbalance and attenuating inflammation [109]. Kaempferol also exhibits protective properties, both anti-inflammatory and antioxidant, in adipocytes in response to proinflammatory stimuli [110]. These two works, by Porras et al., and Le Sage et al., respectively, constitute some clear examples of the experimental approximations that need to be done to increase our knowledge on the relationships already mentioned among phytochemicals, GM, inflammation, and obesity. Therefore, this subgroup constitutes that of the leading compounds in the study of the relationship among these three elements (Figure 3).
Figure 3.
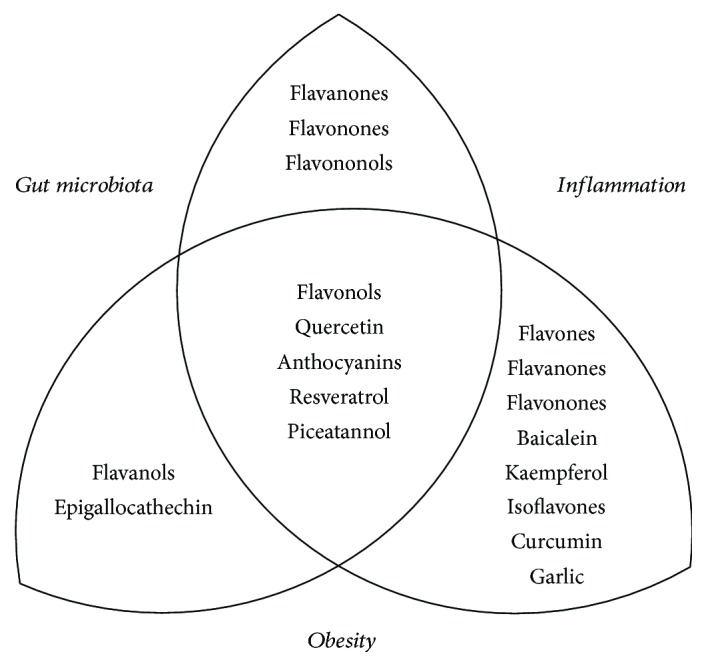
Phytochemicals that affect gut microbiota with anti-inflammatory and/or antiobesity properties.
3.1.6. Flavononols
This is another subgroup with nomenclature problems for the literature search, because the term “flavononols” is usually substituted by “flavonols,” which is a different group (see above). For this reason, compounds included in this group were individually searched. This subgroup includes genistein, taxifolin, engeletin, and astilbin. Again, all of these are metabolized by GM and also exhibit potent anti-inflammatory properties [111–114], as well as being able to influence energy metabolism (both lipid and carbohydrate) [115–117]. Despite this, to our knowledge there is a lack of research regarding the possible effects of this subgroup of flavonoids on obesity and/or inflammation through their effect on GM.
3.1.7. Isoflavones
This subgroup has been partially studied with relation to GM and inflammation or obesity. It is made up of phytoestrogens, which are mainly present in soybeans. Isoflavones are metabolized by GM [30, 118, 119]. They also show an anti-inflammatory effect [120], as well as having had a hypocholesterolemic effect attributed to them [121]. The following are found included in this group: daidzein, genistein, glycitein, formononetin, and daidzin. Daidzein is metabolized by GM mainly into equol, which contributes to the beneficial effects of soybeans [122]; thus, it is relevant that dietary fat intake diminishes GM's ability to synthesize equol [123]. In addition, daidzein and genistein reduced lipid peroxidation in vivo and increased the resistance of low-density lipoproteins (LDL) to oxidation [124] and both exhibit an anti-inflammatory activity [125]. Glycitein affects gene expression in adipose tissue [126] and demonstrates antiobese and antidiabetic effects [127]. Additionally, together with daidzein and genistein, glycitein exhibits an anti-inflammatory and neuroprotective effect on microglial cells [128]. Finally, formononetin and daidzin have also received attention because of their anti-inflammatory properties [129–131]. Once again, this group would be interesting for further studies regarding their metabolism by GM in relation with inflammation and lipid metabolism for obesity.
3.1.8. Anthocyanins
Anthocyanins are a class of flavonoids that are ubiquitously found in fruits and vegetables and they possess many pharmacological properties, for example, lipid-lowering, antioxidant, antiallergic, anti-inflammatory, antimicrobial, anticarcinogenic, and antidiabetic actions [132–135]. Strawberries constitute a source of anthocyanins and have been recently broadly evaluated for their effect on human health, due to their rich phytochemical content, effectiveness in rodent models, and almost no toxicity observed in pilot studies in humans [136, 137]. In rodent models, for example, strawberries have shown anticancer activity in several tissues [138]. This subgroup includes a long list of compounds, such as cyanidin, delphinidin, epigenidin, leucocyanidin, leucodelphinidin, pelargonidin, prodelphinidin, and propelargonidin. Although there are fewer than 70 papers that correlate at least one of these compounds with anti-inflammatory activity or obesity (or lipid metabolism), there are only a dozen papers, to our knowledge, which correlate any of these compounds with their metabolism by GM, and none of them associate this information among these aspects. Therefore, this constitutes a nearly complete virgin area still to be explored.
3.2. Phenolic Acids
3.2.1. Curcumin
A second subgroup of polyphenols is constituted by phenolic acids, such as curcumin (diferuloylmethane), which is abundantly present in the rhizomes of the Curcuma longa, used both in traditional medicine and in cooking. Curcumin has been used for the coadjuvant treatment of a large diversity of diseases, including hepatic disorders, respiratory conditions, and inflammation and also obesity, diabetes, rheumatism, and even certain tumors. One relevant aspect to notice is that even at very high doses, no studies in animals or humans have revealed significant curcumin toxicity [139]. Curcumin possesses a great protective impact on acute alcoholic liver injury in mice and can improve the antioxidant activity of mice after acute administration of alcohol. It can increase the activity of antioxidant enzymes in liver tissues [140]. Curcumin is also metabolized by GM; the biotransformation of turmeric curcuminoids by human GM is reminiscent of equol production from the soybean isoflavone daidzein [141]. Curcumin modulates GM during colitis and colon cancer [142] and improves intestinal barrier function [141]. In addition, it is largely considered a potent anti-inflammatory and neuroprotective agent [143, 144], as well as a possible factor for the treatment of obesity [145–147]. The research on curcumin is extensive; notwithstanding, there are still very few papers that deal with the relationship of curcumin metabolism by GM, its action over intestinal permeability, and effect on obesity and/or inflammation (Table 1).
3.3. Stilbenes
3.3.1. Resveratrol
The third subgroup of polyphenols comprises stilbenoids, such as resveratrol (3,5,4'-trihydroxystilbene) and piceatannol (3,3',4,5'-trans-trihydroxystilbene). Resveratrol is a natural, nonflavonoid polyphenolic compound that can be found in grape wines, grape skins (red wine), pines, peanuts, mulberries, cranberries, and legumes, among other plant species, which synthesize it in response to stress or against pathogen invasion [148, 149]. Resveratrol is studied as a potent antioxidant with neuroprotective activity. Several in vitro and in vivo studies show various properties for resveratrol as a potent antioxidant and antiaging molecule, which also exhibits anti-inflammatory, cardioprotective, and anticancer effects, able to promote vascular endothelial function and enhance lipid metabolism [147, 150]. Principally, it is the anti-inflammatory effect of resveratrol which has been widely reported [151], as well as its antiobesity effect [152]. Regarding the GM effect, resveratrol favored the proliferation of Bifidobacterium and Lactobacillus and counteracts the virulence factors of Proteus mirabilis [29]. In fact, resveratrol exhibits pleiotropic actions, modulates transcription factor NF-κB, and inhibits the cytochrome P450 isoenzyme CYP1 A1, as well as suppressing the expression and activity of cyclooxygenase enzymes, modulating p53, cyclins, and various phosphodiesterases, suppressing proinflammatory molecules, and inhibiting the expression of hypoxia-inducible transcription factor 1 (HIF-1α) and vascular endothelial growth factor (VEGF), among other actions [153]. Some studies analyze the effect of resveratrol on GM combined with their anti-inflammatory and antiobesity actions (Table 1). It constitutes a good example of the potential that the profound study of phytochemicals and their impact on health represents.
3.3.2. Piceatannol
Piceatannol is a hydroxylated analogue of resveratrol found in various plants (mainly grapes and white tea). It is less studied than resveratrol but also exhibits a wide biological activity [154]. It mainly exhibits potent anticancer properties and also antioxidant and anti-inflammatory activities, which make it a potentially useful nutraceutical and possibly an attractive biomolecule for pharmacological use [59]. Recently, Hijona et al. [155] studied its beneficial effects on obesity. Although these are limited, it constitutes a promissory phytochemical molecule.
3.4. Organosulfur Compounds
3.4.1. Garlic
In addition to polyphenols, another group of phytochemicals of relevance for health is the organosulfur compounds. For instance, garlic (Allium sativum) is a rich source of organosulfur compounds and exhibits a plethora of beneficial effects against microbial infections as well as cardioprotective, anticarcinogenic, and anti-inflammatory activity [156].
Nearly 80% of garlic's cysteine sulfoxide is constituted by alliin (allylcysteine sulfoxide). When raw or crushed garlic is chopped, the “allinase” enzyme is released which catalyzes sulfonic acid formation from cysteine sulfoxides and when the two react with each other, they produce an unstable compound: thiosulfinate or allicin. The in vitro breakdown of allicin produces numerous fat-soluble components: diallyl sulfide; DiAllylDiSulfide (DADS), and DiAllylTriSulfide (DATS). Likewise, vinyldithiins, S-allylcysteine, ajoene, S-1-prpenylcysteine, and S-allylmercaptocysteine are important constituents of garlic powder, oil, and extracts [157, 158].
Naturally occurring products have attracted the attention of researchers as sources of novel drugs and drug leads for the treatment of obesity [159–161]. Allium species have been used in herbolary or traditional medicine for the treatment of metabolic diseases, and Allium-derived extracts have recently become of interest for their antiobesity effects [162].
The chemical constituents of garlic are enzymes (asalliinase) and organosulfur compounds (such as alliin and its derived agent, allicin). The effect of garlic on different medical conditions (such as hypertension, hyperlipidemia, diabetes mellitus, rheumatic disease, the common cold, arteriosclerosis, and cancer) has been widely investigated. Garlic is known as a hypolipidemic agent because of its role in increasing the hydrolysis of triacylglycerols due to increased lipase activity [163]. Moreover, garlic reduces the biosynthesis of triacylglycerols through its blocking of nicotinamide adenine dinucleotide phosphate. On the other hand, garlic contains abundant antioxidants and can induce antioxidant enzymes [164]. Thus, garlic is a potential hepatoprotective agent against liver disorders [165]. Experimental studies have shown that garlic and its organosulfur compounds might reduce alcohol-related liver enzymes, glutathione reductase, alkaline phosphatase, lactate dehydrogenase, and alcohol dehydrogenase, as well as enhance liver antioxidant enzymes, and alleviate hepatic-fat accumulation [165–172]. However, there has been no clinical trial on patients with liver disorders [164].
4. Concluding Remarks and Perspectives
Several issues need to be solved before natural products can be effectively translated into the clinic. With regard to the best source of bioactive molecules or compounds, the following aspects should be considered: (a) if they are better acquired directly from food in the diet or from pharmacological sources (purified or through synthetic analogues) and (b) if they should be used alone or as a cotreatment in combination with approved drugs. Therefore, there is a need to develop specific clinical trials. Disadvantages of commercial nutraceutic preparations include the high variability in formulations (preparation methods and chemical composition), as well as the dosage quantification and the different means of administration. Research devoted to the optimization of phytochemical formulation and dosage has become of critical importance. Given the low bioavailability of phytochemicals, the development of more useful synthetic derivatives has become a great concern [173].
Once nutrients and nutraceuticals have been incorporated into the body, the gut environment is essential in maintaining homeostasis; in this sense, like GM, the surface of the intestinal mucous membrane plays a fundamental role in the preservation of homeostasis. Consequently, the correct functioning of its permeability is of great importance [174]. Several pathologies, as well as susceptibility to metabolic diseases, have been linked to alterations in the permeability of the intestinal barrier. Humans possess two interacting genomes: their own and that of their host microbiome, the majority of which resides in the gut, in the layer of mucin glycoproteins (mucus) produced by the cells called goblet cells [168]. The microbiome provides products such as vitamins and nutrients to host cells, thereby establishing a beneficial ecosystem for host physiology and preventing the arrival of pathogens [175]. Thus, a symbiotic relationship is established between both genomes, through the expression of pattern recognition receptors (PRRs) for the sense of the presence of intestinal microbiota, through the microbe-associated molecular patterns (MAMPs). This communication between the two genomes results in the accuracy of the mucosal barrier function, by regulating the production of its components: mucus, antimicrobial peptides, IgA and IL-22, facilitating homeostasis, and immune tolerance [175–177]. Therefore, GM and the human host influence each other by exchanging their metabolic active molecules [178], working together, as a hologenome, to maintain mutual health [179].
Another current challenge is convincing a skeptical health sector of the use of such compounds as medicines, or at least in conjunction with pharmaceutical medicines, which could serve both practitioners and patients better [180]. For instance, research on traditional Chinese medicine has substantially increased recently through the search for its molecular, cellular, and pharmacological bases, with the identification of active substances and the investigation of mechanisms of action [181]. Although the available cumulative data strongly suggest the positive effects of a large variety of phytochemicals in terms of health, it remains insufficient in order to directly extract solid conclusions, due mainly to the lack of confirmation, in human trials, of the results obtained by the animal model studies. Consequently, more research must be focused on the analysis of different phenolic compounds metabolized by GM and their influence on human health [182]. Results are crucial for the precise understanding of the influence of GM on the metabolism of micronutrients and phytochemicals within the human organism, and their metabolism undergone upon ingestion, in order to correctly attribute beneficial health properties to specific polyphenols with a more complete knowledge of their bioavailability, metabolism, and effects on carbohydrate and lipid metabolism, and therefore their use in treating obesity and inflammatory diseases.
Acknowledgments
The work was partially supported by Universidad de Guadalajara Grant PRO-SNI 2017 to Daniel Ortuño-Sahagún and SEP-UDG-CA-454 to Barbara Vizmanos and Lucrecia Carrera-Quintanar and CONACyT-México Grant CB-2015-256736 to Rocío I. López Roa. Fellowship support was provided by CONACyT-México Grant 622462 to Marina A. Sánchez-Sánchez.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors' Contributions
Lucrecia Carrera-Quintanar and Rocío I. López Roa contributed equally to this work.
References
- 1.Gallagher E. J., Leroith D., Karnieli E. The metabolic syndrome—from insulin resistance to obesity and diabetes. Medical Clinics of North America. 2011;95(5):855–873. doi: 10.1016/j.mcna.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero-Garcia J. J., Carrera-Quintanar L., Lopez-Roa R. I., Marquez-Aguirre A. L., Rojas-Mayorquin A. E., Ortuno-Sahagun D. Multiple sclerosis and obesity: possible roles of adipokines. Mediators of Inflammation. 2016;2016:24. doi: 10.1155/2016/4036232.4036232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahiya D. K., Renuka P. M., Shandilya U. K., et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Frontiers in Microbiology. 2017;8:p. 563. doi: 10.3389/fmicb.2017.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Chierico F., Vernocchi P., Bonizzi L., et al. Early-life gut microbiota under physiological and pathological conditions: the central role of combined meta-omics-based approaches. Journal of Proteomics. 2012;75(15):4580–4587. doi: 10.1016/j.jprot.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Clemente J. C., Ursell L. K., Parfrey L. W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith C. C., Snowberg L. K., Gregory Caporaso J., Knight R., Bolnick D. I. Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. The ISME Journal. 2015;9(11):2515–2526. doi: 10.1038/ismej.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung C., Rivera L., Furness J. B., Angus P. W. The role of the gut microbiota in NAFLD. Nature Reviews Gastroenterology & Hepatology. 2016;13(7):412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Chanona E., Trinchieri G. The role of microbiota in cancer therapy. Current Opinion in Immunology. 2016;39:75–81. doi: 10.1016/j.coi.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson C. T., Sharma V., Elmen L., Peterson S. N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clinical & Experimental Immunology. 2015;179(3):363–377. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R. Toll-like receptors and innate immunity. Nature Reviews Immunology. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 11.Fava F., Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? The World Journal of Gastroenterology. 2011;17(5):557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberfroid M. B. Prebiotics and probiotics: are they functional foods? The American Journal of Clinical Nutrition. 2000;71(6) Supplement:1682S–1687S. doi: 10.1093/ajcn/71.6.1682S. [DOI] [PubMed] [Google Scholar]
- 13.Ortuno Sahagun D., Marquez-Aguirre A. L., Quintero-Fabian S., Lopez-Roa R. I., Rojas-Mayorquin A. E. Modulation of PPAR-γ by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Research. 2012;2012:17. doi: 10.1155/2012/318613.318613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kok R. G., de Waal A., Schut F., Welling G. W., Weenk G., Hellingwerf K. J. Specific detection and analysis of a probiotic bifidobacterium strain in infant feces. Applied and Environmental Microbiology. 1996;62(10):3668–3672. doi: 10.1128/aem.62.10.3668-3672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley R. E., Lozupone C. A., Hamady M., Knight R., Gordon J. I. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Reviews Microbiology. 2008;6(10):776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G. D., Chen J., Hoffmann C., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyra K. A., Saha D. C., Reimer R. A. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. The Journal of Nutrition. 2012;142(2):213–220. doi: 10.3945/jn.111.147132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laparra J. M., Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacological Research. 2010;61(3):219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Eid H. M., Wright M. L., Anil Kumar N. V., et al. Significance of microbiota in obesity and metabolic diseases and the modulatory potential by medicinal plant and food ingredients. Frontiers in Pharmacology. 2017;8:p. 387. doi: 10.3389/fphar.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott K. P., Gratz S. W., Sheridan P. O., Flint H. J., Duncan S. H. The influence of diet on the gut microbiota. Pharmacological Research. 2013;69(1):52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Liu R. H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition. 2004;134(12):3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 22.Schrezenmeir J., de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. The American Journal of Clinical Nutrition. 2001;73(2):361s–364s. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 23.Vieira A. T., Fukumori C., Ferreira C. M. New insights into therapeutic strategies for gut microbiota modulation in inflammatory diseases. Clinical & Translational Immunology. 2016;5(6, article e87) doi: 10.1038/cti.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalbert A., Johnson I. T., Saltmarsh M. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 2005;81(1):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 25.Tomas-Barberan F. A., Selma M. V., Espin J. C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Current Opinion in Clinical Nutrition and Metabolic Care. 2016;19(6):471–476. doi: 10.1097/MCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 26.Kim M., Lee J., Han J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. Journal of the Science of Food and Agriculture. 2015;95(9):1925–1931. doi: 10.1002/jsfa.6900. [DOI] [PubMed] [Google Scholar]
- 27.Lewandowska U., Szewczyk K., Hrabec E., Janecka A., Gorlach S. Overview of metabolism and bioavailability enhancement of polyphenols. Journal of Agricultural and Food Chemistry. 2013;61(50):12183–12199. doi: 10.1021/jf404439b. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J. F., Maier C. S. The chemistry of gut microbial metabolism of polyphenols. Phytochemistry Reviews. 2016;15(3):425–444. doi: 10.1007/s11101-016-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larrosa M., Luceri C., Vivoli E., et al. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Molecular Nutrition & Food Research. 2009;53(8):1044–1054. doi: 10.1002/mnfr.200800446. [DOI] [PubMed] [Google Scholar]
- 30.Bowey E., Adlercreutz H., Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food and Chemical Toxicology. 2003;41(5):631–636. doi: 10.1016/S0278-6915(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 31.Duda-Chodak A. The inhibitory effect of polyphenols on human gut microbiota. Journal of Physiology and Pharmacology. 2012;63(5):497–503. [PubMed] [Google Scholar]
- 32.Pandey K. B., Rizvi S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2009;2(5):278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Remon A., Casas R., Tressserra-Rimbau A., et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. British Journal of Clinical Pharmacology. 2017;83(1):114–128. doi: 10.1111/bcp.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeinali M., Rezaee S. A., Hosseinzadeh H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomedicine & Pharmacotherapy. 2017;92:998–1009. doi: 10.1016/j.biopha.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Conte R., Marturano V., Peluso G., Calarco A., Cerruti P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. International Journal of Molecular Sciences. 2017;18(4):p. 709. doi: 10.3390/ijms18040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu D. P., Li Y., Meng X., et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. International Journal of Molecular Sciences. 2017;18(1):p. 96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y., Keogh J. B., Clifton P. M. Polyphenols and glycemic control. Nutrients. 2016;8(1):17. doi: 10.3390/nu8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amiot M. J., Riva C., Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obesity Reviews. 2016;17(7):573–586. doi: 10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- 39.Meydani M., Hasan S. T. Dietary polyphenols and obesity. Nutrients. 2010;2(7):737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min S. Y., Yang H., Seo S. G., et al. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. International Journal of Obesity. 2013;37(4):584–592. doi: 10.1038/ijo.2012.85. [DOI] [PubMed] [Google Scholar]
- 41.Kunnumakkara A. B., Bordoloi D., Padmavathi G., et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimoda H., Tanaka J., Kikuchi M., et al. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. Journal of Agricultural and Food Chemistry. 2009;57(5):1786–1792. doi: 10.1021/jf803441c. [DOI] [PubMed] [Google Scholar]
- 43.Stohs S. J., Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytotherapy Research. 2016;30(5):732–740. doi: 10.1002/ptr.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Y., Hurst W. J., Stuart D. A., Lambert J. D. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. Journal of Agricultural and Food Chemistry. 2011;59(10):5305–5311. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H. C., Jenner A. M., Low C. S., Lee Y. K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Research in Microbiology. 2006;157(9):876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Medina E., García A., Romero C., De Castro A., Brenes M. Study of the anti-lactic acid bacteria compounds in table olives. International Journal of Food Science & Technology. 2009;44(7):1286–1291. doi: 10.1111/j.1365-2621.2009.01950.x. [DOI] [Google Scholar]
- 47.Nohynek L. J., Alakomi H. L., Kähkönen M. P., et al. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutrition and Cancer. 2006;54(1):18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 48.Puupponen-Pimia R., Nohynek L., Hartmann-Schmidlin S., et al. Berry phenolics selectively inhibit the growth of intestinal pathogens. Journal of Applied Microbiology. 2005;98(4):991–1000. doi: 10.1111/j.1365-2672.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 49.Tzounis X., Vulevic J., Kuhnle G. G., et al. Flavanol monomer-induced changes to the human faecal microflora. The British Journal of Nutrition. 2008;99(4):782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 50.Parkar S. G., Stevenson D. E., Skinner M. A. The potential influence of fruit polyphenols on colonic microflora and human gut health. International Journal of Food Microbiology. 2008;124(3):295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 51.N. A. L. f. United States Department of Agriculture. Defenition: nutritional intervention. 2017, https://definedterm.com/nutritional_intervention.
- 52.Kumar S., Pandey A. K. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;2013:16. doi: 10.1155/2013/162750.162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Science. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 54.Bubols G. B., Vianna Dda R., Medina-Remon A., et al. The antioxidant activity of coumarins and flavonoids. Mini Reviews in Medicinal Chemistry. 2013;13(3):318–334. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 55.Marzocchella L., Fantini M., Benvenuto M., et al. Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Patents on Inflammation & Allergy Drug Discovery. 2011;5(3):200–220. doi: 10.2174/187221311797264937. [DOI] [PubMed] [Google Scholar]
- 56.Antunes-Ricardo M., Gutierrez-Uribe J., Serna-Saldivar S. O. Anti-inflammatory glycosylated flavonoids as therapeutic agents for treatment of diabetes-impaired wounds. Current Topics in Medicinal Chemistry. 2015;15(23):2456–2463. doi: 10.2174/1568026615666150619141702. [DOI] [PubMed] [Google Scholar]
- 57.van Meeteren M. E., Hendriks J. J., Dijkstra C. D., van Tol E. A. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochemical Pharmacology. 2004;67(5):967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Hurt R. T., Wilson T. Geriatric obesity: evaluating the evidence for the use of flavonoids to promote weight loss. Journal of Nutrition in Gerontology and Geriatrics. 2012;31(3):269–289. doi: 10.1080/21551197.2012.698222. [DOI] [PubMed] [Google Scholar]
- 59.Kawser Hossain M., Abdal Dayem A., Han J., et al. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. International Journal of Molecular Sciences. 2016;17(4):p. 569. doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simons A. L., Renouf M., Hendrich S., Murphy P. A. Human gut microbial degradation of flavonoids: structure–function relationships. Journal of Agricultural and Food Chemistry. 2005;53(10):4258–4263. doi: 10.1021/jf0500177. [DOI] [PubMed] [Google Scholar]
- 61.Hanske L., Loh G., Sczesny S., Blaut M., Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. The Journal of Nutrition. 2009;139(6):1095–1102. doi: 10.3945/jn.108.102814. [DOI] [PubMed] [Google Scholar]
- 62.Braune A., Blaut M. Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environmental Microbiology. 2011;13(2):482–494. doi: 10.1111/j.1462-2920.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 63.Angelino D., Berhow M., Ninfali P., Jeffery E. H. Caecal absorption of vitexin-2-O-xyloside and its aglycone apigenin, in the rat. Food & Function. 2013;4(9):1339–1345. doi: 10.1039/c3fo60047e. [DOI] [PubMed] [Google Scholar]
- 64.Lee J. H., Zhou H. Y., Cho S. Y., Kim Y. S., Lee Y. S., Jeong C. S. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Archives of Pharmacal Research. 2007;30(10):1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- 65.Karamese M., Erol H. S., Albayrak M., Findik Guvendi G., Aydin E., Aksak Karamese S. Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: an immunological, biochemical, and histopathological study. Immunopharmacology and Immunotoxicology. 2016;38(3):228–237. doi: 10.3109/08923973.2016.1173058. [DOI] [PubMed] [Google Scholar]
- 66.Lee J. A., Ha S. K., Cho E., Choi I. Resveratrol as a bioenhancer to improve anti-inflammatory activities of apigenin. Nutrients. 2015;7(11):9650–9661. doi: 10.3390/nu7115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mascaraque C., Gonzalez R., Suarez M. D., Zarzuelo A., Sanchez de Medina F., Martinez-Augustin O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. The British Journal of Nutrition. 2015;113(04):618–626. doi: 10.1017/S0007114514004292. [DOI] [PubMed] [Google Scholar]
- 68.Seelinger G., Merfort I., Schempp C. M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica. 2008;74(14):1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 69.Nabavi S. F., Braidy N., Gortzi O., et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Research Bulletin. 2015;119(Part A):1–11. doi: 10.1016/j.brainresbull.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Feng X., Weng D., Zhou F., et al. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. eBioMedicine. 2016;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren B., Qin W., Wu F., et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. European Journal of Pharmacology. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Yang Y., Wu Z., Weng P. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. Journal of Agricultural and Food Chemistry. 2016;64(12):2582–2590. doi: 10.1021/acs.jafc.6b00586. [DOI] [PubMed] [Google Scholar]
- 73.Kwon E. Y., Jung U. J., Park T., Yun J. W., Choi M. S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015;64(5):1658–1669. doi: 10.2337/db14-0631. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Fu X., Lan N., et al. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behavioural Brain Research. 2014;267:178–188. doi: 10.1016/j.bbr.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 75.Xu N., Zhang L., Dong J., et al. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Molecular Nutrition & Food Research. 2014;58(6):1258–1268. doi: 10.1002/mnfr.201300830. [DOI] [PubMed] [Google Scholar]
- 76.Choi J. H., Yun J. W. Chrysin induces brown fat–like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition. 2016;32(9):1002–1010. doi: 10.1016/j.nut.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Lin W., Wang W., Yang H., Wang D., Ling W. Influence of intestinal microbiota on the catabolism of flavonoids in mice. Journal of Food Science. 2016;81(12):H3026–H3034. doi: 10.1111/1750-3841.13544. [DOI] [PubMed] [Google Scholar]
- 78.Braune A., Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7(3):216–234. doi: 10.1080/19490976.2016.1158395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Q. Q., Shi J. B., Chen C., Huang C., Tang W. J., Li J. Hesperetin derivatives: synthesis and anti-inflammatory activity. Bioorganic & Medicinal Chemistry Letters. 2016;26(5):1460–1465. doi: 10.1016/j.bmcl.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 80.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytotherapy Research. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 81.Manchope M. F., Casagrande R., Verri W. A., Jr. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 2017;8(3):3766–3767. doi: 10.18632/oncotarget.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee K. M., Lee Y., Chun H. J., et al. Neuroprotective and anti-inflammatory effects of morin in a murine model of Parkinson’s disease. Journal of Neuroscience Research. 2016;94(10):865–878. doi: 10.1002/jnr.23764. [DOI] [PubMed] [Google Scholar]
- 83.Franova S., Kazimierova I., Pappova L., Joskova M., Plank L., Sutovska M. Bronchodilatory, antitussive and anti-inflammatory effect of morin in the setting of experimentally induced allergic asthma. The Journal of Pharmacy and Pharmacology. 2016;68(8):1064–1072. doi: 10.1111/jphp.12576. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Y., Cao Z. Q., Wang H. Y., et al. The anti-inflammatory effects of Morin hydrate in atherosclerosis is associated with autophagy induction through cAMP signaling. Molecular Nutrition & Food Research. 2017;61(9, article 1600966) doi: 10.1002/mnfr.201600966. [DOI] [PubMed] [Google Scholar]
- 85.Mokdad-Bzeouich I., Mustapha N., Sassi A., et al. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular anti-oxidant activity. Cell Stress and Chaperones. 2016;21(5):773–781. doi: 10.1007/s12192-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee J. K. Anti-inflammatory effects of eriodictyol in lipopolysaccharidestimulated raw 264.7 murine macrophages. Archives of Pharmacal Research. 2011;34(4):671–679. doi: 10.1007/s12272-011-0418-3. [DOI] [PubMed] [Google Scholar]
- 87.Zhu G. F., Guo H. J., Huang Y., Wu C. T., Zhang X. F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Experimental and Therapeutic Medicine. 2015;10(6):2259–2266. doi: 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim H. K., Jeong T. S., Lee M. K., Park Y. B., Choi M. S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clinica Chimica Acta. 2003;327(1-2):129–137. doi: 10.1016/S0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 89.Choi G. S., Lee S., Jeong T. S., et al. Evaluation of hesperetin 7-O-lauryl ether as lipid-lowering agent in high-cholesterol-fed rats. Bioorganic & Medicinal Chemistry. 2004;12(13):3599–3605. doi: 10.1016/j.bmc.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 90.Assini J. M., Mulvihill E. E., Burke A. C., et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology. 2015;156(6):2087–2102. doi: 10.1210/en.2014-2003. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida H., Watanabe H., Ishida A., et al. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochemical and Biophysical Research Communications. 2014;454(1):95–101. doi: 10.1016/j.bbrc.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 92.Miler M., Živanović J., Ajdžanović V., et al. Citrus flavanones naringenin and hesperetin improve antioxidant status and membrane lipid compositions in the liver of old-aged Wistar rats. Experimental Gerontology. 2016;84:49–60. doi: 10.1016/j.exger.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 93.Prahalathan P., Saravanakumar M., Raja B. The flavonoid morin restores blood pressure and lipid metabolism in DOCA-salt hypertensive rats. Redox Report. 2012;17(4):167–175. doi: 10.1179/1351000212Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X., Zhang D. M., Gu T. T., et al. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochemical Pharmacology. 2013;86(12):1791–1804. doi: 10.1016/j.bcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 95.Kim D. H., Jung E. A., Sohng I. S., Han J. A., Kim T. H., Han M. J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Archives of Pharmacal Research. 1998;21(1):17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 96.Bharti S., Rani N., Krishnamurthy B., Arya D. S. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Medica. 2014;80(6):437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- 97.Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. European Journal of Medicinal Chemistry. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Z., Zhong W., Lin H., et al. Hesperidin protects against acute alcoholic injury through improving lipid metabolism and cell damage in zebrafish larvae. Evidence-based Complementary and Alternative Medicine. 2017;2017:9. doi: 10.1155/2017/7282653.7282653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohara T., Muroyama K., Yamamoto Y., Murosaki S. Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutrition Journal. 2016;15:p. 6. doi: 10.1186/s12937-016-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alam M. A., Kauter K., Brown L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients. 2013;5(3):637–650. doi: 10.3390/nu5030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seo M. J., Choi H. S., Jeon H. J., Woo M. S., Lee B. Y. Baicalein inhibits lipid accumulation by regulating early adipogenesis and m-TOR signaling. Food and Chemical Toxicology. 2014;67:57–64. doi: 10.1016/j.fct.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Duthie S. J., Jenkinson A. M., Crozier A., et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. European Journal of Nutrition. 2006;45(2):113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 103.Rameshrad M., Razavi B. M., Hosseinzadeh H. Protective effects of green tea and its main constituents against natural and chemical toxins: a comprehensive review. Food and Chemical Toxicology. 2017;100:115–137. doi: 10.1016/j.fct.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 104.Chen L., Mo H., Zhao L., et al. Therapeutic properties of green tea against environmental insults. The Journal of Nutritional Biochemistry. 2017;40:1–13. doi: 10.1016/j.jnutbio.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Janssens P. L., Hursel R., Westerterp-Plantenga M. S. Nutraceuticals for body-weight management: the role of green tea catechins. Physiology & Behavior. 2016;162:83–87. doi: 10.1016/j.physbeh.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 106.Thielecke F., Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome – a review. Phytochemistry. 2009;70(1):11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 107.Fan F. Y., Sang L. X., Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22(3):p. 484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hursel R., Westerterp-Plantenga M. S. Catechin- and caffeine-rich teas for control of body weight in humans. The American Journal of Clinical Nutrition. 2013;98(6):1682S–1693S. doi: 10.3945/ajcn.113.058396. [DOI] [PubMed] [Google Scholar]
- 109.Porras D., Nistal E., Martinez-Florez S., et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radical Biology and Medicine. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 110.Le Sage F., Meilhac O., Gonthier M. P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacological Research. 2017;119:303–312. doi: 10.1016/j.phrs.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 111.Jeong J. W., Lee H. H., Han M. H., Kim G. Y., Kim W. J., Choi Y. H. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chemico-Biological Interactions. 2014;212:30–39. doi: 10.1016/j.cbi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 112.Gupta M. B., Bhalla T. N., Gupta G. P., Mitra C. R., Bhargava K. P. Anti-inflammatory activity of taxifolin. Japanese Journal of Pharmacology. 1971;21(3):377–382. doi: 10.1254/jjp.21.377. [DOI] [PubMed] [Google Scholar]
- 113.Huang H., Cheng Z., Shi H., Xin W., Wang T. T., Yu L. L. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. Journal of Agricultural and Food Chemistry. 2011;59(9):4562–4569. doi: 10.1021/jf2002969. [DOI] [PubMed] [Google Scholar]
- 114.Lu C. L., Zhu Y. F., Hu M. M., et al. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules. 2015;20(1):625–644. doi: 10.3390/molecules20010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao J., Echelberger R., Liu M., et al. Soy but not bisphenol A (BPA) or the phytoestrogen genistin alters developmental weight gain and food intake in pregnant rats and their offspring. Reproductive Toxicology. 2015;58:282–294. doi: 10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pisonero-Vaquero S., Garcia-Mediavilla M. V., Jorquera F., et al. Modulation of PI3K-LXRα-dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin. Laboratory Investigation; a Journal of Technical Methods and Pathology. 2014;94(3):262–274. doi: 10.1038/labinvest.2013.156. [DOI] [PubMed] [Google Scholar]
- 117.Haraguchi H., Mochida Y., Sakai S., et al. Protection against oxidative damage by dihydroflavonols in Engelhardtia chrysolepis. Bioscience, Biotechnology, and Biochemistry. 1996;60(6):945–948. doi: 10.1271/bbb.60.945. [DOI] [PubMed] [Google Scholar]
- 118.Turner N. J., Thomson B. M., Shaw I. C. Bioactive isoflavones in functional foods: the importance of gut microflora on bioavailability. Nutrition Reviews. 2003;61(6):204–213. doi: 10.1301/nr.2003.jun.204-213. [DOI] [PubMed] [Google Scholar]
- 119.Yuan J. P., Wang J. H., Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora – implications for health. Molecular Nutrition & Food Research. 2007;51(7):765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 120.Park J. S., Woo M. S., Kim D. H., et al. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. The Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):1237–1245. doi: 10.1124/jpet.106.114322. [DOI] [PubMed] [Google Scholar]
- 121.Ramdath D. D., Padhi E. M., Sarfaraz S., Renwick S., Duncan A. M. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):p. 324. doi: 10.3390/nu9040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reverri E. J., Slupsky C. M., Mishchuk D. O., Steinberg F. M. Metabolomics reveals differences between three daidzein metabolizing phenotypes in adults with cardiometabolic risk factors. Molecular Nutrition & Food Research. 2017;61(1, article 1600132) doi: 10.1002/mnfr.201600132. [DOI] [PubMed] [Google Scholar]
- 123.Rowland I. R., Wiseman H., Sanders T. A., Adlercreutz H., Bowey E. A. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutrition and Cancer. 2000;36(1):27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 124.Wiseman H., O'Reilly J. D., Adlercreutz H., et al. Isoflavone phytoestrogens consumed in soy decrease F2-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. The American Journal of Clinical Nutrition. 2000;72(2):395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 125.Hämäläinen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators of Inflammation. 2007;2007:10. doi: 10.1155/2007/45673.45673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Velpen V., Geelen A., Hollman P. C., Schouten E. G., van 't Veer P., Afman L. A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: a double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. The American Journal of Clinical Nutrition. 2014;100(5):1269–1277. doi: 10.3945/ajcn.114.088484. [DOI] [PubMed] [Google Scholar]
- 127.Zang Y., Igarashi K., Yu C. Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet. Bioscience, Biotechnology, and Biochemistry. 2015;79(1):117–123. doi: 10.1080/09168451.2014.955453. [DOI] [PubMed] [Google Scholar]
- 128.Marotta F., Mao G. S., Liu T., et al. Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Annals of the New York Academy of Sciences. 2006;1089(1):276–281. doi: 10.1196/annals.1386.033. [DOI] [PubMed] [Google Scholar]
- 129.Lima Cavendish R., de Souza Santos J., Belo Neto R., et al. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. Journal of Ethnopharmacology. 2015;173:127–133. doi: 10.1016/j.jep.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 130.Ma Z., Ji W., Fu Q., Ma S. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation. 2013;36(6):1560–1566. doi: 10.1007/s10753-013-9700-5. [DOI] [PubMed] [Google Scholar]
- 131.Jin S. E., Son Y. K., Min B. S., Jung H. A., Choi J. S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Archives of Pharmacal Research. 2012;35(5):823–837. doi: 10.1007/s12272-012-0508-x. [DOI] [PubMed] [Google Scholar]
- 132.He J., Giusti M. M. Anthocyanins: natural colorants with health-promoting properties. Annual Review of Food Science and Technology. 2010;1(1):163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 133.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutrition Reviews. 2015;73(6):348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 134.Guo H., Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Reviews in Endocrine and Metabolic Disorders. 2015;16(1):1–13. doi: 10.1007/s11154-014-9302-z. [DOI] [PubMed] [Google Scholar]
- 135.Asgary S., Rafieian-Kopaei M., Shamsi F., Najafi S., Sahebkar A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. Journal of Complementary and Integrative Medicine. 2014;11(2):63–69. doi: 10.1515/jcim-2013-0022. [DOI] [PubMed] [Google Scholar]
- 136.Basu A., Nguyen A., Betts N. M., Lyons T. J. Strawberry as a functional food: an evidence-based review. Critical Reviews in Food Science and Nutrition. 2014;54(6):790–806. doi: 10.1080/10408398.2011.608174. [DOI] [PubMed] [Google Scholar]
- 137.Giampieri F., Tulipani S., Alvarez-Suarez J. M., Quiles J. L., Mezzetti B., Battino M. The strawberry: composition, nutritional quality, and impact on human health. Nutrition. 2012;28(1):9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Shi N., Clinton S. K., Liu Z., et al. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients. 2015;7(3):1696–1715. doi: 10.3390/nu7031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Talero E., Avila-Roman J., Motilva V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Current Pharmaceutical Design. 2012;18(26):3939–3965. doi: 10.2174/138161212802083725. [DOI] [PubMed] [Google Scholar]
- 140.Zeng Y., Liu J., Huang Z., Pan X., Zhang L. Effect of curcumin on antioxidant function in the mice with acute alcoholic liver injury. Wei Sheng Yan Jiu. 2014;43(2):282–285. [PubMed] [Google Scholar]
- 141.Burapan S., Kim M., Han J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. Journal of Agricultural and Food Chemistry. 2017;65(16):3305–3310. doi: 10.1021/acs.jafc.7b00943. [DOI] [PubMed] [Google Scholar]
- 142.McFadden R. M., Larmonier C. B., Shehab K. W., et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflammatory Bowel Diseases. 2015;21(11):2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Munch G. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Archives of Toxicology. 2017;91(4):1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- 144.Tizabi Y., Hurley L. L., Qualls Z., Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014;19(12):20864–20879. doi: 10.3390/molecules191220864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shehzad A., Ha T., Subhan F., Lee Y. S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. European Journal of Nutrition. 2011;50(3):151–161. doi: 10.1007/s00394-011-0188-1. [DOI] [PubMed] [Google Scholar]
- 146.Aggarwal B. B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annual Review of Nutrition. 2010;30(1):173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bradford P. G. Curcumin and obesity. BioFactors. 2013;39(1):78–87. doi: 10.1002/biof.1074. [DOI] [PubMed] [Google Scholar]
- 148.Catalgol B., Batirel S., Taga Y., Ozer N. K. Resveratrol: French paradox revisited. Frontiers in Pharmacology. 2012;3:p. 141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vastano B. C., Chen Y., Zhu N., Ho C. T., Zhou Z., Rosen R. T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. Journal of Agricultural and Food Chemistry. 2000;48(2):253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 150.Gambini J., Inglés M., Olaso G., et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Medicine and Cellular Longevity. 2015;2015:13. doi: 10.1155/2015/837042.837042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malhotra A., Bath S., Elbarbry F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxidative Medicine and Cellular Longevity. 2015;2015:15. doi: 10.1155/2015/803971.803971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aguirre L., Fernandez-Quintela A., Arias N., Portillo M. P. Resveratrol: anti-obesity mechanisms of action. Molecules. 2014;19(11):18632–18655. doi: 10.3390/molecules191118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Diaz-Gerevini G. T., Repossi G., Dain A., Tarres M. C., Das U. N., Eynard A. R. Beneficial action of resveratrol: how and why? Nutrition. 2016;32(2):174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 154.Piotrowska H., Kucinska M., Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutation Research/Reviews in Mutation Research. 2012;750(1):60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 155.Hijona E., Aguirre L., Pérez-Matute P., et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: gut microbiota, metabolic, endocrine, and cardiac aspects. Journal of Physiology and Biochemistry. 2016;72(3):567–582. doi: 10.1007/s13105-015-0464-2. [DOI] [PubMed] [Google Scholar]
- 156.Arreola R., Quintero-Fabián S., López-Roa R. I., et al. Immunomodulation and anti-inflammatory effects of garlic compounds. Journal of Immunology Research. 2015;2015:13. doi: 10.1155/2015/401630.401630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Amagase H., Petesch B. L., Matsuura H., Kasuga S., Itakura Y. Intake of garlic and its bioactive components. The Journal of Nutrition. 2001;131(3):955S–962S. doi: 10.1093/jn/131.3.955s. [DOI] [PubMed] [Google Scholar]
- 158.Butt M. S., Naz A., Sultan M. T., Qayyum M. M. Anti-oncogenic perspectives of spices/herbs: a comprehensive review. EXCLI Journal. 2013;12:1043–1065. [PMC free article] [PubMed] [Google Scholar]
- 159.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 160.Sergent T., Vanderstraeten J., Winand J., Beguin P., Schneider Y.-J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chemistry. 2012;135(1):68–73. doi: 10.1016/j.foodchem.2012.04.074. [DOI] [Google Scholar]
- 161.Yun J. W. Possible anti-obesity therapeutics from nature – a review. Phytochemistry. 2010;71(14-15):1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 162.Yang M. H., Kim N. H., Heo J. D., et al. Comparative evaluation of sulfur compounds contents and antiobesity properties of Allium hookeri prepared by different drying methods. Evidence-Based Complementary and Alternative Medicine. 2017;2017:10. doi: 10.1155/2017/2436927.2436927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aviello G., Abenavoli L., Borrelli F., et al. Garlic: empiricism or science? Natural Product Communications. 2009;4(12):1785–1796. [PubMed] [Google Scholar]
- 164.Ghorbani Z., Hajizadeh M., Hekmatdoost A. Dietary supplementation in patients with alcoholic liver disease: a review on current evidence. Hepatobiliary & Pancreatic Diseases International. 2016;15(4):348–360. doi: 10.1016/S1499-3872(16)60096-6. [DOI] [PubMed] [Google Scholar]
- 165.Raghu R., Liu C. T., Tsai M. H., et al. Transcriptome analysis of garlic-induced hepatoprotection against alcoholic fatty liver. Journal of Agricultural and Food Chemistry. 2012;60(44):11104–11119. doi: 10.1021/jf303800p. [DOI] [PubMed] [Google Scholar]
- 166.Zeng T., Guo F. F., Zhang C. L., et al. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chemico-Biological Interactions. 2008;176(2-3):234–242. doi: 10.1016/j.cbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 167.Zeng T., Xie K. Q. Could garlic partially-counteract excess alcohol consumption? A postulated role of garlic oil in prevention of ethanol-induced hepatotoxicity. Medical Hypotheses. 2008;71(6):984–985. doi: 10.1016/j.mehy.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 168.Zeng T., Zhang C. L., Song F. Y., Zhao X. L., Xie K. Q. Garlic oil alleviated ethanol-induced fat accumulation via modulation of SREBP-1, PPAR-α, and CYP2E1. Food and Chemical Toxicology. 2012;50(3-4):485–491. doi: 10.1016/j.fct.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 169.Adoga G. I. Effect of garlic oil extract on glutathione reductase levels in rats fed on high sucrose and alcohol diets: a possible mechanism of the activity of the oil. Bioscience Reports. 1986;6(10):909–912. doi: 10.1007/BF01116245. [DOI] [PubMed] [Google Scholar]
- 170.Adoga G. I. The mechanism of the hypolipidemic effect of garlic oil extract in rats fed on high sucrose and alcohol diets. Biochemical and Biophysical Research Communications. 1987;142(3):1046–1052. doi: 10.1016/0006-291X(87)91520-8. [DOI] [PubMed] [Google Scholar]
- 171.Adoga G. I., Osuji J. Effect of garlic oil extract on serum, liver and kidney enzymes of rats fed on high sucrose and alcohol diets. Biochemistry International. 1986;13(4):615–624. [PubMed] [Google Scholar]
- 172.Kim M. H., Kim M. J., Lee J. H., et al. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. Journal of Medicinal Food. 2011;14(7-8):732–738. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 173.Morbidelli L. Polyphenol-based nutraceuticals for the control of angiogenesis: analysis of the critical issues for human use. Pharmacological Research. 2016;111:384–393. doi: 10.1016/j.phrs.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 174.Spiljar M., Merkler D., Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Frontiers in Immunology. 2017;8:p. 1353. doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brown E. M., Sadarangani M., Finlay B. B. The role of the immune system in governing host-microbe interactions in the intestine. Nature Immunology. 2013;14(7):660–667. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]
- 176.Carvalho F. A., Aitken J. D., Vijay-Kumar M., Gewirtz A. T. Toll-like receptor–gut microbiota interactions: perturb at your own risk! Annual Review of Physiology. 2012;74(1):177–198. doi: 10.1146/annurev-physiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 177.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 178.Hooper L. V., Midtvedt T., Gordon J. I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition. 2002;22(1):283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 179.Zhao L., Shen J. Whole-body systems approaches for gut microbiota-targeted, preventive healthcare. Journal of Biotechnology. 2010;149(3):183–190. doi: 10.1016/j.jbiotec.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 180.Chen F., Wen Q., Jiang J., et al. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? Journal of Ethnopharmacology. 2016;179:253–264. doi: 10.1016/j.jep.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 181.Tang J. L. Research priorities in traditional Chinese medicine. BMJ. 2006;333(7564):391–394. doi: 10.1136/bmj.333.7564.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mosele J. I., Macia A., Motilva M. J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: a review. Molecules. 2015;20(9):17429–17468. doi: 10.3390/molecules200917429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Päivärinta E., Niku M., Maukonen J., et al. Changes in intestinal immunity, gut microbiota, and expression of energy metabolism–related genes explain adenoma growth in bilberry and cloudberry-fed ApcMin mice. Nutrition Research. 2016;36(11):1285–1297. doi: 10.1016/j.nutres.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 184.Pu P., Wang X. A., Salim M., et al. Baicalein, a natural product, selectively activating AMPKα2 and ameliorates metabolic disorder in diet-induced mice. Molecular and Cellular Endocrinology. 2012;362(1-2):128–138. doi: 10.1016/j.mce.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 185.Remely M., Ferk F., Sterneder S., et al. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxidative Medicine and Cellular Longevity. 2017;2017:17. doi: 10.1155/2017/3079148.3079148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cheng M., Zhang X., Miao Y., Cao J., Wu Z., Weng P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3′′Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Research International. 2017;92:9–16. doi: 10.1016/j.foodres.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 187.Unno T., Sakuma M., Mitsuhashi S. Effect of dietary supplementation of (-)-epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. Journal of Nutritional Science and Vitaminology. 2014;60(3):213–219. doi: 10.3177/jnsv.60.213. [DOI] [PubMed] [Google Scholar]
- 188.Etxeberria U., Arias N., Boqué N., et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. The Journal of Nutritional Biochemistry. 2015;26(6):651–660. doi: 10.1016/j.jnutbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 189.Roldán-Marín E., Krath B. N., Poulsen M., et al. Effects of an onion by-product on bioactivity and safety markers in healthy rats. The British Journal of Nutrition. 2009;102(11):1574–1582. doi: 10.1017/S0007114509990870. [DOI] [PubMed] [Google Scholar]
- 190.Esposito D., Damsud T., Wilson M., et al. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. Journal of Agricultural and Food Chemistry. 2015;63(27):6172–6180. doi: 10.1021/acs.jafc.5b00963. [DOI] [PubMed] [Google Scholar]
- 191.Wu T., Tang Q., Yu Z., et al. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. International Journal of Food Sciences and Nutrition. 2014;65(3):351–359. doi: 10.3109/09637486.2013.854749. [DOI] [PubMed] [Google Scholar]
- 192.Neyrinck A. M., Alligier M., Memvanga P. B., et al. Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS One. 2013;8(11, article e81252) doi: 10.1371/journal.pone.0081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ghosh S. S., Bie J., Wang J., Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice – role of intestinal permeability and macrophage activation. PLoS One. 2014;9(9, article e108577) doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J., Ghosh S. S., Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. American Journal of Physiology Cell Physiology. 2017;312(4):C438–C445. doi: 10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food & Function. 2014;5(6):1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 196.Dao T. M., Waget A., Klopp P., et al. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS One. 2011;6(6, article e20700) doi: 10.1371/journal.pone.0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nøhr M. K., Kroager T. P., Sanggaard K. W., et al. SILAC-MS based characterization of LPS and resveratrol induced changes in adipocyte proteomics – resveratrol as ameliorating factor on LPS induced changes. PLoS One. 2016;11(7, article e0159747) doi: 10.1371/journal.pone.0159747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Korsholm A. S., Kjaer T. N., Ornstrup M. J., Pedersen S. B. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: a randomized, placebo-controlled clinical trial on the effects of resveratrol after four months’ treatment. International Journal of Molecular Sciences. 2017;18(3):p. 554. doi: 10.3390/ijms18030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tung Y. C., Lin Y. H., Chen H. J., et al. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules. 2016;21(11):p. 1419. doi: 10.3390/molecules21111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lai Y. S., Chen W. C., Ho C. T., et al. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. Journal of Agricultural and Food Chemistry. 2014;62(25):5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]