Abstract
Introduction. In the discovery of more potent and selective anticancer drugs, the research continually expands and explores new bioactive metabolites coming from different natural sources. Gallnuts are a group of very special natural products formed through parasitic interaction between plants and insects. Though it has been traditionally used as a source of drugs for the treatment of cancerous diseases in traditional and folk medicinal systems through centuries, the anticancer properties of gallnuts are barely systematically reviewed. Objective. To evidence the traditional uses and phytochemicals and pharmacological mechanisms in anticancer aspects of gallnuts, a literature review was performed. Materials and Methods. The systematic review approach consisted of searching web-based scientific databases including PubMed, Web of Science, and Science Direct. The keywords for searching include gallnut, Galla Chinensis, Rhus chinensis, Rhus potaninii, Rhus punjabensis, nutgall, gall oak, Quercus infectoria, Quercus lusitanica, and galla turcica. Two reviewers extracted papers independently to remove the papers unrelated to the anticancer properties of gallnuts. Patents, abstracts, case reports, and abstracts in symposium and congress were excluded. Results and Conclusion. As a result, 14 articles were eligible to be evaluated. It is primarily evident that gallnuts contain a number of bioactive metabolites, which account for anticancer activities. The phytochemical and pharmacological studies reviewed strongly underpin a fundamental understanding of anticancer properties for gallnuts (Galla Chinensis and Galla Turcica) and support their ongoing clinical uses in China. The further bioactive compounds screening and evaluation, pharmacological investigation, and clinical trials are expected to progress gallnut-based development to finally transform the wild medicinal gallnuts to the valuable authorized anticancer drugs.
1. Introduction
Cancer is a generic term for a vast diversity of diseases that can affect any organ system throughout the body. Though great efforts have been invested over recent decades, the leading morbidity and mortality rates of human cancer have not dramatically changed [1]. The development of more effective anticancer agents thus remains an ongoing challenge. Natural products are the valuable treasury offering approximately 75% of drugs currently used for cancer treatment [2]. With the aim of discovering more potent, more selective, and less toxic compounds than today's drugs, the research of anticancer agents continually expands and explores new natural products coming from different sources, among which the insect gallnuts could be quoted as an almost wild resource rarely systematically studied.
Gallnuts are a group of very special natural products characterized as the plant-insect symbiont. They are formed as the pathological excrescences on the young branches or twigs of plants as a result of the insect attack and deposition of the eggs [3]. Historically, gallnuts have been used by both Western and Eastern cultures as a traditional medicine for various body disorders, as an astringent in painful hemorrhoids, an antiphlogistic for inflammatory conditions, a treatment for diarrhea and dysentery, and a remedy for toothache and dental caries [4, 5]. Galla Chinensis and Galla Turcica are the two most significant gallnuts as a source of medicine widely used in different countries [5].
Galla Chinensis is formed when the Chinese sumac aphid Baker (mainly Melaphis chinensis Bell) parasitizes the leaves or petioles of the plants of family Anacardiaceae (mainly Rhus chinensis Mill, Rhus potaninii Maxim, and Rhus punjabensis var. sinica (Diels) Rehd. Et Wils) [6]. Galla Chinensis is native to and mainly distributed in areas of southern provinces of China, Sumatra, and Malaysia, but it is also produced in small amounts in India, Japan, and Korea [7]. As shown in Figure 1, these gallnuts are typically 4-5 × 1.5 cm in diameter, horned, and reddish-brown in colour, covered with velvety down. The galls are usually harvested between September and October. The medicine Galla Chinensis is the dry and clean gallnuts after removal of the larvae [5, 6].
Figure 1.
Galla Chinensis (a) and Galla Turcica (b).
Galla Turcica is formed on the young branches or twigs of Quercus infectoria Olivier parasitized by the gall wasps, Cynips gallae-tinctoriae Olivier [8]. Quercus infectoria Olivier is a small tree or shrub, native to and widely distributed in the Mediterranean coast countries, mainly Greece and Turkey, and also Iran and Syria [5, 8]. Galla Turcica is typically 1–2.5 cm in diameter, being almost spherical in shape, with the tuberculated surface on the upper part. Its colour is gray, white-brown, olive green, or dark bluish-green (Figure 1). The galls of Quercus infectoria Olivier are usually harvested between August and September [5].
2. Records in TCM Encyclopedia
As the important traditional medicine dates back centuries, the medicinal uses of gallnuts (Galla Chinensis and Galla Turcica) have been widely recorded in more than sixteen classical Traditional Chinese Medicine (TCM) pharmacopeias compiled in different dynasties of China (Table 1). Though those books were written in numerous obscure TCM terminologies, many of recorded uses of gallnuts have been proved for treating clinical features associated with cancer. According to the theories of TCM, cancer is caused by the invasion of exogenous pathogenic factors (accumulated toxins and heat) and the imbalance of endogenous physical conditions (air and blood stasis) [36]. The main therapeutic effects of gallnuts recorded in TCM pharmacopeias are to clear the heat and toxins and rebalance the pathological conditions of human body.
Table 1.
Anticancer uses in Chinese classical pharmaceutical books.
| Gallnuts | Book | Medical uses | Dynasty | Year of publication | References |
|---|---|---|---|---|---|
| Galla Turcica | Tang Materia Medica | Diarrhea | Tang | 659 AD | [9] |
|
| |||||
| Galla Chinensis | Chinese Materia Medica Gleanings | Intestinal dysfunction, diarrhea | Tang | 741 AD | [10] |
|
| |||||
| Galla Turcica | Kai Bao Materia Medica | Ulcer, chancre, night sweats | Song | 974 AD | [11] |
|
| |||||
| Galla Turcica | Drug Properties | Uroclepsia | Tang | - | [12] |
|
| |||||
| Galla Turcica | Oversea Materia Medica | Diarrhea, toxins | Tang | - | [13] |
|
| |||||
| Galla Chinensis | Ri Hua Zi Materia Medica | Toxins | Song | - | [14] |
|
| |||||
| Galla Chinensis | Kai Bao Ben Cao | Ulcer, haemorrhoids, chancre | Song | 974 AD | [11] |
|
| |||||
| Galla Chinensis | Commentaries on the Illustrations | Body fluid deficiency | Song | 1061 AD | [15] |
|
| |||||
| Galla Chinensis | Amplification on Materia Medica | Aphtha | Song | 1116 AD | [16] |
|
| |||||
| Galla Chinensis | Amplification on Materia Medica Addendum | Obstinate phlegm, heat | Yuan | - | [17] |
|
| |||||
| Galla Chinensis | Materia Medica Meng Quan | Analgesic | Ming | 1565 | [18] |
|
| |||||
| Galla Chinensis | Compendium of materia medica | Heat, toxins, phlegm, cough, wasting-thirst, night sweats, emesia, hemorrhage, analgesic, diarrhea, ulcer, chancre | Ming | 1578 | [19] |
|
| |||||
| Galla Turcica | Fresh Chinese Materia Medica | Diarrhea, uroclepsia | Qing | 1757 | [20] |
|
| |||||
| Galla Chinensis | Truth Chinese Materia Medica | Heat, phlegm, cough, diarrhea, haemorrhoids | Qing | 1769 | [21] |
|
| |||||
| Galla Turcica | Truth Chinese Materia Medica | Heat, ulcer, diarrhea | Qing | 1769 | [21] |
An important medical compendium, ⟪Tang Materia Medica⟫ in 659 AD, is the earliest official medical monograph compiled by the state authority in the world [37]. To the best of our knowledge, it is also the first book to record gallnuts medicine (Galla Turcica), as well as their activities in treating diarrhea through eliminating the toxins and rebuilding the normal gastrointestinal environment [9]. The medical uses of the other gallnut, Galla Chinensis, were also firstly recorded to treat the intestinal dysfunction and diarrhea in ⟪Chinese Materia Medica Gleanings⟫ at 741 AD [10]. The microenvironment rebalance of digestive system could thus be considered as one of the major therapeutic effects of gallnuts, which was claimed to be a significant progress for cancer treatment in the TCM theories. This therapeutic effect was further recorded by ⟪Oversea Materia Medica⟫, ⟪Compendium of materia medica⟫, ⟪Fresh Chinese Materia Medica⟫, and ⟪Truth Chinese Materia Medica⟫ in the following several centuries [13, 19–21].
The other therapeutic effect of gallnuts related to cancer treatment is the pathogen (toxins and heat in TCM theories) scavenging. According to ⟪Compendium of materia medica⟫, one of the most authoritative encyclopedias of TCM, Galla Chinensis could be used in the remedy of phlegm, cough, emesia, analgesic, ulcer, and chancre through clearing the exogenous pathogenic heat and toxins [19]. ⟪Oversea Materia Medica⟫, ⟪Ri Hua Zi Materia Medica⟫, ⟪Amplification on Materia Medica Addendum⟫, and ⟪Truth Chinese Materia Medica⟫ also recorded the antipathogenic activity of gallnuts (Galla Chinensis and Galla Turcica) in different periods [13, 14, 17, 21]. Therefore, the accumulated toxins and heat, which were believed as the major carcinogenic factors in TCM theories, could be effectively eliminated under gallnuts treatment.
Taken together, though there's no straight conclusion, the abstracted information from the ancient TCM encyclopedia has highlighted the potentials of gallnuts as the effective anticancer candidates, thus providing an valuable natural resource for further scientific study. A systematic review was performed with the aims of collecting and analyzing current knowledge of anticancer aspects for gallnuts, which may underpin the fundamental understanding and inspire the gallnuts-sourced drug development.
3. Articles Selection
The authors searched numbers of electronic databases, including PubMed, Web of Science, and Science Direct up to the date on 31 August 2017. The keywords for searching include gallnut, Galla Chinensis, Rhus chinensis, Rhus potaninii, Rhus punjabensis, nutgall, gall oak, Quercus infectoria, Quercus lusitanica, and galla turcica. These keywords were searched individually and in combination with cancer and tumour. Searching was limited to articles in either English or Chinese. Two reviewers extracted papers independently. The duplication articles were firstly deleted. The papers unrelated to the anticancer properties of gallnuts were then excluded. Patents, abstracts, case reports, and abstracts in symposium and congress were excluded as they did not contain sufficient information for evaluation and comparison with other studies. The review articles did not contain the original data. The articles were reviewed to determined compatibility with the inclusion criteria above. Based on the above criteria, 14 articles were eligible to be evaluated (Figure 2). Characteristics of the studies have been summarized in Table 2.
Figure 2.
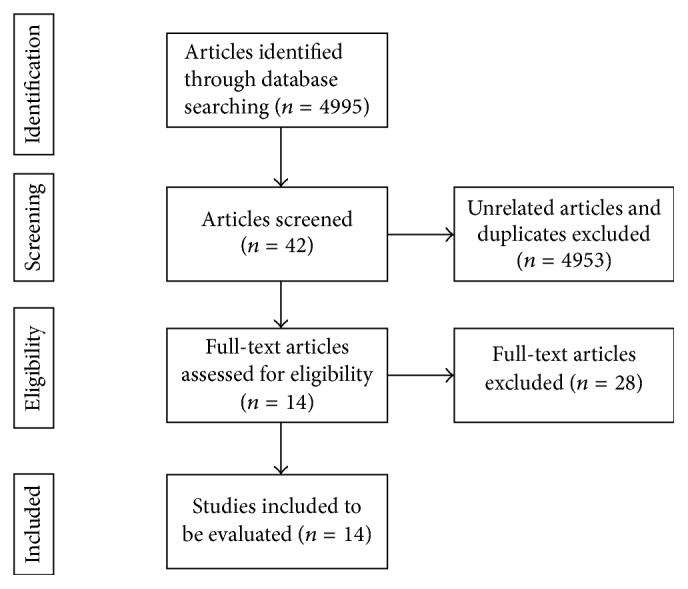
Trend of screening and choosing articles.
Table 2.
Information summary of selected literature research for gallnuts.
| Gallnuts | Active ingredients | Action related to anticancer | References |
|---|---|---|---|
| Galla Turcica | Purpurogallin | Inhibit the tyrosine-specific protein kinase of the human oncogene product epidermal growth factor receptor | [22] |
|
| |||
| Galla Turcica | 50% ethanol extract | Protect against oxidative stress-induced liver injury | [23] |
|
| |||
| Galla Chinensis | Gallic acid | Increase the activities of phase II xenobiotic-metabolizing enzyme and decrease the activities of phase I xenobiotic-metabolizing enzyme, and thus decrease tumor incidence | [24] |
|
| |||
| Galla Chinensis | Gallic acid | Increase p53 and NF-κB, decrease I-κB protein, and thus induce apoptosis and inhibit cell viability in U937 cells | [25] |
|
| |||
| Galla Chinensis | Gallic acid | Upregulate p27(Kip1) level via disruption of p27(Kip1)/skp2 complex and the consequent degradation of p27(Kip1) and lead to G2/M phase arrest of MCF-7 cells | [26] |
|
| |||
| Galla Chinensis | 1,2,3,4,6-Penta-O-galloyl-β-D-glucose | Regulate the expression of genes involved in cancer pyruvate metabolism, glycolysis/gluconeogenesis, and tyrosine metabolism | [27] |
|
| |||
| Galla Chinensis | Gallic acid | Downregulate expression of COX-2 and stathmin and inhibit the proliferation of 5-8F cells | [28] |
|
| |||
| Galla Chinensis | 1,2,3,4,6-Penta-O-galloyl-β-D-glucose | Enhance cytotoxicity and PARP cleavage in cisplatin-treated Caki-2 cells | [29] |
|
| |||
| Galla Chinensis | Gallic acid | Suppress the matrix metalloproteinase-2/-9, protein kinase B and C signaling, and thus inhibit migration and invasion of U-2 OS cells | [30] |
|
| |||
| Galla Chinensis | Ether fraction | Inhibit the proliferation of U251 | [31] |
|
| |||
| Galla Turcica | Aqueous extract | Show similar acute, chronic toxicity, and mortality to that of control | [32] |
|
| |||
| Galla Chinensis | Ethyl acetate extract | Inhibit the activities of epidermal growth factor receptor | [33] |
|
| |||
| Galla Chinensis | 1,2,3,4,6-Penta-O-galloyl-β-D-glucose | Inhibit human lactic acid dehydrogenase-A and attenuate proliferation of MDA-MB-231 cells | [34] |
|
| |||
| Galla Chinensis | Water extract | Do not produce significant acute and chronic toxicity until overdose (3 times higher than that of the recommended dose), no side effects to rats in central nervous system, cardiovascular system, and respiratory system | [35] |
4. Bioactive Metabolites from Gallnuts
The primary and secondary metabolic processes of natural products can produce huge numbers of chemical constituents [38]. Specifically, the genesis of gallnuts also consists of an additional interaction between the plant host and invasion parasites. The complex processes of gallnuts formation thus should generate abundance of bioactive metabolites, which includes compounds originating from plants, insects, and mutual stimulation.
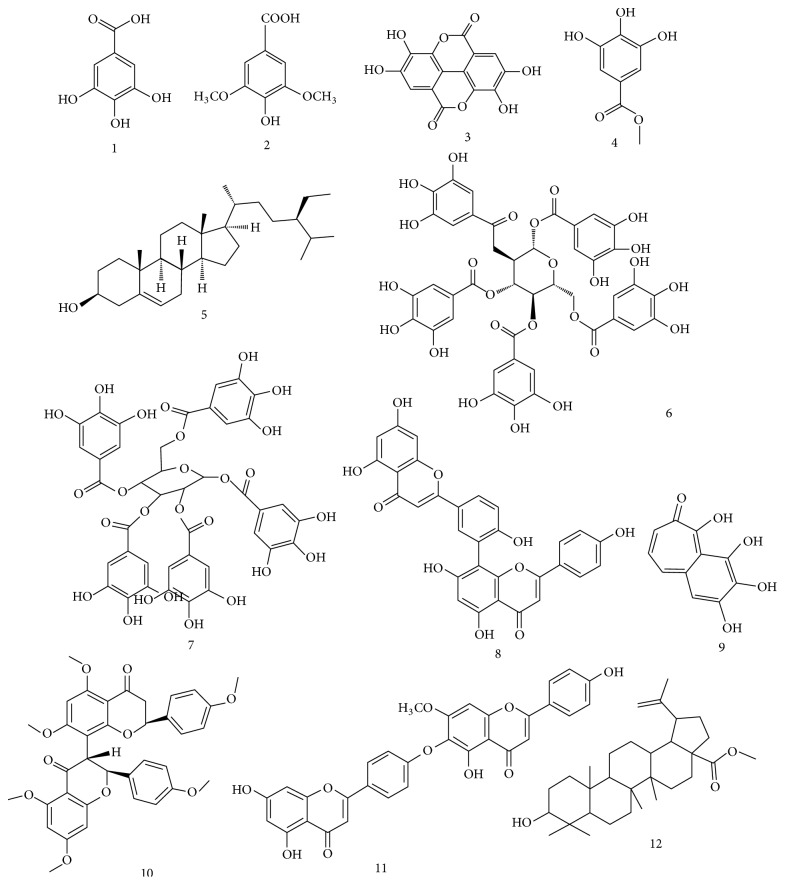
Both Galla Chinensis and Galla Turcica contain a large amount (50–70%) of tannin (gallotannin), mainly of penta-undeca-galloylglucoses which have depside galloyl groups randomly distributed at the C-2, C-3, and C-4 positions on a penta-O-galloyl-β-D-glucose core [31]. According to the literature reported (Figure 3), twelve chemical components have been identified from gallnuts to the date, including gallic acid (1), syringic acid (2), ellagic acid (3), methyl gallate (4), β-sitosterol (5), 1,2,3,4,6-penta-O-galloyl-β-D-glucose (6), 1,2,3,4,6-tetra-O-galloyl-β-D-glucose (7), amentoflavone (8), purpurogallin (9), hexamethyl ether (10), isocryptomerin (11), and methyl betulate (12) [22, 32, 39–42]. Among them, gallic acid (1), ellagic acid (3), methyl gallate (4), β-sitosterol (5), 1,2,3,4,6-penta-O-galloyl-β-D-glucose (6), amentoflavone (8), and purpurogallin (9) have been reported to present anticancer activities in either in vitro or in vivo studies [22, 23, 34, 43–48]. However, the current researches of identification and isolation of gallnuts compounds were very few and mainly focused on the gallotannin only. The understanding of phytochemical knowledge for gallnuts thus clearly needs further complement.
Figure 3.
Chemical compounds identified from gallnuts.
The complex development of the plant-insect symbiont highlights the great potentials to produce a much higher variety of bioactive metabolites within gallnuts. In the future studies, the phytochemical researches are highly suggested to investigate the nontannin groups of gallnuts, such as flavones and terpenoids. Two flavones, amentoflavone and isocryptomerin, identified from Galla Turcica and the traditional anticancer uses of gallnuts indicated the possibility of abundance existence of flavones and other cytotoxic components.
5. Pharmacological Insights of Anticancer Aspect
Unlike many researches on the natural products, the anticancer effects of gallnuts were rarely studied using crude extracts. To the best of our knowledge, only Tong and his colleagues reported that the ether fraction of Galla Chinensis presented an in vitro inhibition ratio to the human glioblastoma cell U251 ranging from 42.34% to 72.54% in a dose-dependent manner, with IC50 being 12.76 μg/ml. This activity was comparable to that of fluorouracil (from 40.6 to 75.5%, IC50 = 9.01 μg/ml) [31]. Moreover, another anticancer-related study indicated that the ethyl acetate extracts of Galla Chinensis exhibited the significant inhibitory activities to the epidermal growth factor receptor, which was a validated target for different human malignancies, with IC50 values at 4.339 μg/ml [33]. Both studies above somehow demonstrated that Galla Chinensis, as a medicinal agent individually, presented an effective anticancer activity in the laboratory tests.
As discussed previously, numbers of compounds identified from gallnuts have been reported to behave as anticancer agents in literature and thus could be considered as the material foundation of anticancer activities of gallnuts. Among them, gallic acid is the most reported compound shown to inhibit carcinogenesis in animal models and in vitro cancerous cell lines [24, 49, 50]. Ellagic acid is another polyphenolic compound that has been well researched for the anticancer properties. The growth inhibitory effects of ellagic acid have been determined on ranges of in vitro and in vivo experimental models of cancer [51–55]. Methyl gallate, isolated from three plants Parthenocissus tricuspidata, Spondias pinnata, and Acacia hydaspica, has also been reported to be cytotoxic to cancer cells in vitro [43, 56, 57]. Deiab et al. reported that 1,2,3,4,6-tetra-O-galloyl-β-D-glucose demonstrated significant antiproliferative effects on human MDA-MB-231 breast cancer cells with IC50 value as low as 1.2 μM [34]. The bioflavonoid compound, amentoflavone, has been previously recognized as a cancer chemopreventive agent. It was found to stimulate the cell cycle arrest and apoptosis on many types of cancer cell lines in vitro [58, 59]. Taken together, the chemical profile somehow has conferred the anticancer property to gallnuts. Five compounds, including gallic acid, ellagic acid, methyl gallate, 1,2,3,4,6-tetra-O-galloyl-β-D-glucose, and amentoflavone, of gallnuts have been broadly reported to exhibit inhibitory effects against carcinogenesis through mechanisms of action on apoptosis, cell cycle, angiogenesis, or metastasis.
Gallic acid exerts anticancer activities through action on cell cycle, apoptosis, angiogenesis, invasion, and metastasis [48, 60, 61]. The molecular mechanisms of gallic acid identified in various types of human or rodent cancerous cells include modulation of apoptosis-related proteins, activation of ATM kinase, ribonucleotide reductase inhibition, cyclooxygenase inhibition, GSH depletion, UDP-glucose dehydrogenase inhibition, vascular endothelial growth factor (VEGF) inhibition, ADAMs inhibition, and NF-кB inhibition [25, 26, 30, 47, 62–67].
The ellagic acid is able to trigger the apoptosis and inhibit the proliferation, angiogenesis, migration, and invasion of cancer cells via regulation of several subcellular signaling pathways such as mitochondria-dependent signaling pathway, cell cycle signaling cascade, the protein kinase C signaling pathway, TGF-β/Smad3 pathway, and Wnt/β-catenin signaling pathway [46, 52, 53, 68–71].
As the results of methyl gallate treatment, the alteration of apoptotic molecules, and blockage of AKT, NF-кB, ERK1/2, PI3K, and JAK/STAT signaling pathways led to the apoptosis of cancer cells [43, 57]. In addition, methyl gallate also could exert antitumour activities through reversing immune suppression by inhibiting tumour infiltration of CD4+CD25+ regulatory T cells [72].
This impressive effect of 1,2,3,4,6-tetra-O-galloyl-β-D-glucose could be achieved through targeting on the overexpression of lactic acid dehydrogenase-A and metabolism genes of MDA-MB-231 cancer cells [34, 58]. The molecular mechanisms underlying the activities of amentoflavone mainly involve the action on human peroxisome proliferator-activated receptor gamma and mitochondria-emanated intrinsic pathways [73, 74]. Moreover, amentoflavone also could suppress the expression of NF-кB and VEGF and thus obstruct the tumour angiogenesis and metastasis [44, 75].
Arguably, these findings above partly provide the scientific evidence to support its traditional records of anticancer uses based on the TCM theory. However, the pharmacological research for anticancer properties of gallnuts is just at the beginning. The anticancer effects of individual gallnuts medicine need to be evaluated as a whole in a broad of in vitro and in vivo cancerous models. Their underlying mechanisms are expected to be elucidated in detail as well. More important, anticancer activity-guided isolation should be employed to systemically screen chemicals within gallnuts, aiming to obtain powerful anticancer agents or lead compounds.
6. Safety Verification
For the safety and healthy consideration, the use of gallnuts either orally or topically is not recommended over a long period at high doses [5]. However, both Galla Chinensis and Galla Turcica did not present significant, either acute or chronic, toxicity in the safety evaluative tests [32, 35].
In the toxicity studies using specific pathogen-free (SPF) Sprague-Dawley (SD) rats, the acute toxic test showed that the oral single dose of 5760 mg/kg of Galla Chinensis solution did not cause acute toxicity and the LD50 for rats was thus determined in excess of 5000 mg/kg. The subchronic toxicity study indicated that the no-observed effect of Galla Chinensis solution was lesser than 1500 mg/kg bw day, which is three times lower than that of the recommended dose of clinical uses. Moreover, Galla Chinensis solution did not produce any side effects on rats in central nervous system, cardiovascular system, and respiratory system [35].
In the acute toxicity test, the enema administration of Galla Turcica aqueous extracts at 10 g/kg did not cause any death or even significant adverse effects noted in the imprinting control region (ICR) mice. In the chronic use of Galla Turcica (up to 2 g/kg/day for 180 days), there are also no noticed changes in mice body weight and nutrient consumption as well as minor histological changes of organs such as the brain, heart, kidneys, lung stomach, liver, spleen, and uterus [32].
Though no significant toxicity in animal tests was reported, the gallnuts contain large amounts (over 50%) of tannins, which may cause negative health effects. Overdose intake of gall tannins active components could lead to the irritation of the gastric mucosa, nausea, vomiting, and even the fatal liver damage. Moreover, tannins were known as the metal ion chelators and digestive enzymes inhibitors. Therefore, they can cause anemia and dyspepsia in cases of long-term usage [5].
Overall, the gallnuts including Galla Chinensis and Galla Turcica could be considered safe in lower doses over a short period. However, the higher doses and longer term administration are not recommended.
7. Concluding Remarks
As the very special insect-plant symbiont in natural products, the gallnuts (Galla Chinensis and Galla Turcica) have been used as traditional herbal medicine for centuries. According to the TCM theory, gallnuts are claimed as the effective anticancer agents recorded in many ancient TCM encyclopedias and used in the clinical formulae in China. Based on the data accumulated in this review, it is primarily evident that gallnuts contain a number of bioactive phytochemical components, which at least partly account for the anticancer uses, and their medicinal uses are considered safe in vivo. The gallnuts thus could be proposed as a novel resource to develop the effective new chemotherapy drugs in future.
However, the number of current studies carried out on chemical constituents and pharmacological activities regarding the anticancer properties of gallnuts is surprisingly low. The following gaps are urgently noteworthy. First, as the complex symbiont formed through interaction between different living races, the current bioactive compounds identified from gallnuts should be just a corner of the whole chemical profile. Further study is required to systematically screen the chemical constitutes of gallnuts using bioassay-guided isolation to obtain more powerful anticancer components or lead compounds. Second, the pharmacological aspects of anticancer activities are barely investigated on either Galla Chinensis or Galla Turcica. Though anticancer actions of several compounds of gallnuts could be confirmed using information extracted from the research on other natural products, the pharmacological studies are still insufficient to determine their effects and to validate the ethno-anticancer uses. Both gallnuts themselves and pure bioactive compounds directly isolated from gallnuts thus should be comprehensively investigated into the molecule mechanisms underlying cancer effects to better understand the traditional medicine theory on gallnuts. Thirdly, anticancer activities of the extracts and compounds were mainly conducted to test in either in vitro or in vivo models, but not in clinical trials. Therefore, there are few reported data focused on clinical efficiency and side effects, and the results obtained may not be accurate and applicable in humans. Comprehensive well-controlled and double-blind clinical trials are therefore urgently required to reevaluate the efficacy and safety of anticancer uses of gallnuts.
8. Summary
Overall, the phytochemical and pharmacological studies reviewed herein are evident that gallnuts contain a number of anticancer phytochemical components and their medicinal uses are considered safe in vivo. It thus strongly underpins a fundamental understanding of anticancer activities of gallnuts medicine and supports their ongoing clinical uses in China. The further phytochemical evaluation, pharmacological investigation, and clinical trials are expected to progress gallnuts-based development to finally transform the wild traditional medicinal resource gallnuts to the valuable authorized anticancer drugs.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Project no. U1504830).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Powers S., Pollack R. E. Inducing stable reversion to achieve cancer control. Nature Reviews Cancer. 2016;16(4):266–270. doi: 10.1038/nrc.2016.12. [DOI] [PubMed] [Google Scholar]
- 2.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giron D., Huguet E., Stone G. N., Body M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. Journal of Insect Physiology. 2016;84:70–89. doi: 10.1016/j.jinsphys.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Larew H. G. Oak galls preserved by the eruption of mount vesuvius in A.D. 79, and their probable use. Economic Botany. 1987;41(1):33–40. doi: 10.1007/BF02859343. [DOI] [Google Scholar]
- 5.Sariozlu N. Y., Kivanc M. Gallnuts (Quercus infectoria Oliv. and Rhus chinensis Mill.) and Their Usage in Health. Nuts and Seeds in Health and Disease Prevention. 2011:505–511. doi: 10.1016/B978-0-12-375688-6.10060-X. [DOI] [Google Scholar]
- 6.State Pharmacopoeia Committee of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. 2015. Beijing, China: Chemical Industry Press; 2015. [Google Scholar]
- 7.Djakpo O., Yao W. Rhus chinensis and Galla Chinensis - Folklore to modern evidence: Review. Phytotherapy Research. 2010;24(12):1739–1747. doi: 10.1002/ptr.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hapidin H., Rozelan D., Abdullah H., Hanaffi W. N. W., Soelaiman I. N. Quercus infectoria gall extract enhanced the proliferation and activity of human fetal osteoblast cell line (hFOB 1.19) Malaysian Journal of Medical Sciences. 2015;22(1):12–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Su J. Tang Materia Medica. Hefei, China: Anhui Science and Technology Press; 1981. [Google Scholar]
- 10.Chen Z. Q. Chinese Materia Medica Gleanings. Hefei, China: Gleanings Anhui Science and Technology Press; 2004. [Google Scholar]
- 11.Lu D. X., Li F. Kai Bao Materia Medica. Hefei, China: Anhui Science and Technology Press; 1998. [Google Scholar]
- 12.Zhen Q. Drug Properties. Hefei, China: Anhui Science and Technology Press; 2006. [Google Scholar]
- 13.Li X. Oversea Materia Medica. Shijiazhuang, China: Hubei Science and Technology Press; 2016. [Google Scholar]
- 14.Shang Z. J. Ri Hua Zi Materia Medica. Hefei, China: Anhui Science and Technology Press; 2007. [Google Scholar]
- 15.Su S. Commentaries on the Illustrations. Hefei, China: Anhui Science and Technology Press; 1994. [Google Scholar]
- 16.Kou Z. S. Amplification on Materia Medica. Beijing, China: Commercial Press; 1957. [Google Scholar]
- 17.Zhu D. X. Amplification on Materia Medica Addendum, Dan Xi Medical Set. Beijing, China: People's Medical Publishing House; 2014. [Google Scholar]
- 18.Cheng J. M. Materia Medica Meng Quan. Beijing, China: Chinese Medicine Publishing House of China; 2013. [Google Scholar]
- 19.Li Z. Compendium of Materia Medica. Beijing, China: People's Medical Publishing Hous; 2012. [Google Scholar]
- 20.Wu Y. L. Fresh Chinese Materia Medica. Beijing, China: Science and Technology Health Publishing House; 1958. [Google Scholar]
- 21.Huang G. X. Truth Chinese Materia Medica. Shanghai, China: Shanghai Science and Technology Press; 1959. [Google Scholar]
- 22.Abou-Karam M., Shier W. T. Inhibition of oncogene product enzyme activity as an approach to cancer chemoprevention. Tyrosine-specific protein kinase inhibition by purpurogallin from Quercus sp. nutgall. Phytotherapy Research. 1999;13(4):337–340. doi: 10.1002/(SICI)1099-1573(199906)13:4<337::AID-PTR451>3.0.CO;2-J. doi: 10.1002/(SICI)1099-1573(199906)13:4<337::AID-PTR451>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Pithayanukul P., Nithitanakool S., Bavovada R. Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules. 2009;14(12):4987–5000. doi: 10.3390/molecules14124987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giftson Senapathy J., Jayanthi S., Viswanathan P., Umadevi P., Nalini N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats - A chemopreventive approach. Food and Chemical Toxicology. 2011;49(4):887–892. doi: 10.1016/j.fct.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Kim N.-S., Jeong S.-I., Hwang B.-S., et al. Gallic acid inhibits cell viability and induces apoptosis in human monocytic cell line U937. Journal of Medicinal Food. 2011;14(3):240–246. doi: 10.1089/jmf.2010.1160. [DOI] [PubMed] [Google Scholar]
- 26.Hsu J.-D., Kao S.-H., Ou T.-T., Chen Y.-J., Li Y.-J., Wang C.-J. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27Kip1 attributed to disruption of p27 Kip1/Skp2 complex. Journal of Agricultural and Food Chemistry. 2011;59(5):1996–2003. doi: 10.1021/jf103656v. [DOI] [PubMed] [Google Scholar]
- 27.Yu W. S., Jeong S.-J., Kim J.-H., et al. The genome-wide expression profile of 1,2,3,4,6-penta-O-galloyl-β-D- glucose-treated MDA-MB-231 breast cancer cells: molecular target on cancer metabolism. Molecules and Cells. 2011;32(2):123–132. doi: 10.1007/s10059-011-2254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Q., Fan C. W., Xiao S. J., et al. Effect of gallnut extract on nasopharyngeal carcinoma 5-8F cells and its mechanism. Journal of Central South University (Medical Sciences) 2012;37(9):871–875. doi: 10.3969/j.issn.1672-7347.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Ryu H.-G., Jeong S.-J., Kwon H.-Y., et al. Penta-O-galloyl-β-D-glucose attenuates cisplatin-induced nephrotoxicity via reactive oxygen species reduction in renal epithelial cells and enhances antitumor activity in Caki-2 renal cancer cells. Toxicology in Vitro. 2012;26(2):206–214. doi: 10.1016/j.tiv.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Liao C.-L., Lai K.-C., Huang A.-C., et al. Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food and Chemical Toxicology. 2012;50(5):1734–1740. doi: 10.1016/j.fct.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Tong S., Fu M., Cao X., et al. Lipid raft stationary phase chromatography for screening anti-tumor components from Galla chinensis. Chromatographia. 2014;77(5-6):419–429. doi: 10.1007/s10337-014-2623-y. [DOI] [Google Scholar]
- 32.Iminjan M., Amat N., Li X.-H., Upur H., Ahmat D., He B. Investigation into the toxicity of traditional uyghur medicine quercus infectoria galls water extract. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0090756.e90756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Lin S., Wang D., Huang L. Anti-epidermal growth factor receptor tyrosine kinase activities of traditional Chinese medicine for cancer treatment. European Journal of Integrative Medicine. 2014;6(5):565–570. doi: 10.1016/j.eujim.2014.05.006. [DOI] [Google Scholar]
- 34.Deiab S., Mazzio E., Eyunni S., et al. 1,2,3,4,6-Penta-O-galloylglucose within Galla Chinensis inhibits human LDH-A and attenuates cell proliferation in MDA-MB-231 breast cancer cells. Evidence-Based Complementary and Alternative Medicine. 2015;2015 doi: 10.1155/2015/276946.276946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang F., Peng L., Yin Z., et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of Galla chinensis solution. Journal of Ethnopharmacology. 2015;162:181–190. doi: 10.1016/j.jep.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao W. L. W., Liu L. The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Medica. 2010;76(11):1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y. H., Liu Y. Z. Pharmacognosy. Beijing, China: The Peking University Publishing House; 2011. [Google Scholar]
- 38.David B., Wolfender J.-L., Dias D. A. The pharmaceutical industry and natural products: historical status and new trends. Phytochemistry Reviews. 2015;14(2):299–315. doi: 10.1007/s11101-014-9367-z. [DOI] [Google Scholar]
- 39.Dar M. S., Ikram M., Fakouhi T. Pharmacology of Quercus infectoria. Journal of Pharmaceutical Sciences. 1976;65(12):1791–1794. doi: 10.1002/jps.2600651224. [DOI] [PubMed] [Google Scholar]
- 40.Ikram M., Nowshad F. Constituents of Quercus infectoria. Planta Medica. 1977;31(3):286–287. doi: 10.1055/s-0028-1097531. [DOI] [PubMed] [Google Scholar]
- 41.Kaur G., Hamid H., Ali A., Alam M. S., Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. Journal of Ethnopharmacology. 2004;90(2-3):285–292. doi: 10.1016/j.jep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Jang S.-E., Hyam S. R., Jeong J.-J., Han M. J., Kim D.-H. Penta-O-galloyl-β-D-glucose ameliorates inflammation by inhibiting MyD88/NF-κB and MyD88/MAPK signalling pathways. British Journal of Pharmacology. 2013;170(5):1078–1091. doi: 10.1111/bph.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhuri D., Ghate N. B., Singh S. S., Mandal N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacognosy Magazine. 2015;11(42):269–276. doi: 10.4103/0973-1296.153078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J.-H., Chen W.-L., Liu Y.-C. Amentoflavone Induces Anti-angiogenic and Anti-metastatic Effects Through Suppression of NF-κB Activation in MCF-7 cells. Anticancer Reseach. 2015;35(12):6685–6693. [PubMed] [Google Scholar]
- 45.Han N.-R., Kim H.-M., Jeong H.-J. The potential anti-proliferative effect of β-sitosterol on human mast cell line-1 cells. Canadian Journal of Physiology and Pharmacology. 2015;93(11):979–983. doi: 10.1139/cjpp-2015-0166. [DOI] [PubMed] [Google Scholar]
- 46.Salimi A., Roudkenar M. H., Sadeghi L., et al. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biology. 2015;6:461–471. doi: 10.1016/j.redox.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y.-C., Lin M.-L., Su H.-L., Chen S.-S. ER-Dependent Ca++-mediated Cytosolic ROS as an Effector for Induction of Mitochondrial Apoptotic and ATM-JNK Signal Pathways in Gallic Acid-treated Human Oral Cancer Cells. Anticancer Reseach. 2016;36(2):697–705. [PubMed] [Google Scholar]
- 48.Sun G., Zhang S., Xie Y., Zhang Z., Zhao W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncology Letters. 2016;11(1):150–158. doi: 10.3892/ol.2015.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K., Zhu X., Zhang K., Zhu L., Zhou F. Investigation of Gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. Journal of Biochemical and Molecular Toxicology. 2014;28(9):387–393. doi: 10.1002/jbt.21575. [DOI] [PubMed] [Google Scholar]
- 50.Weng S.-W., Hsu S.-C., Liu H.-C., et al. Gallic acid induces DNA damage and inhibits DNA repair-associated protein expression in human oral cancer SCC-4 Cells. Anticancer Reseach. 2015;35(4):2077–2084. [PubMed] [Google Scholar]
- 51.Han D. H., Lee M. J., Kim J. H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Reseach. 2006;26(5 A):3601–3606. [PubMed] [Google Scholar]
- 52.Vanella L., Di Giacomo C., Acquaviva R., et al. Apoptotic markers in a prostate cancer cell line: Effect of ellagic acid. Oncology Reports. 2013;30(6):2804–2810. doi: 10.3892/or.2013.2757. [DOI] [PubMed] [Google Scholar]
- 53.Vanella L., Barbagallo I., Acquaviva R., et al. Ellagic acid: Cytodifferentiating and antiproliferative effects in human prostatic cancer cell lines. Current Pharmaceutical Design. 2013;19(15):2728–2736. doi: 10.2174/1381612811319150008. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M., Tang S., Marsh J. L., Shankar S., Srivastava R. K. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Letters. 2013;337(2):210–217. doi: 10.1016/j.canlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Umesalma S., Nagendraprabhu P., Sudhandiran G. Ellagic acid inhibits proliferation and induced apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells. Molecular and Cellular Biochemistry. 2015;399(1-2):303–313. doi: 10.1007/s11010-014-2257-2. [DOI] [PubMed] [Google Scholar]
- 56.Woo M. H., Zhao B. T., Hung T. M., Jeong S. Y., Ma E. S., Min B. S. DNA topoisomerases i and II inhibitory activity and cytotoxicity of compounds from the stems of Parthenocissus tricuspidata. Bulletin of the Korean Chemical Society. 2013;34(9):2675–2679. doi: 10.5012/bkcs.2013.34.9.2675. [DOI] [Google Scholar]
- 57.Afsar T., Trembley J. H., Salomon C. E., Razak S., Khan M. R., Ahmed K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Scientific Reports. 2016;6 doi: 10.1038/srep23077.23077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siveen K. S., Kuttan G. Effect of amentoflavone, a phenolic component from Biophytum sensitivum, on cell cycling and apoptosis of B16F-10 melanoma cells. Journal of Environmental Pathology, Toxicology and Oncology. 2011;30(4):301–309. doi: 10.1615/JEnvironPatholToxicolOncol.v30.i4.30. [DOI] [PubMed] [Google Scholar]
- 59.Pei J. S., Liu C. C., Hsu Y. N., et al. Amntoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In Vivo. 2012;26(6):963–970. [PubMed] [Google Scholar]
- 60.Kuo C.-L., Lai K.-C., Ma Y.-S., Weng S.-W., Lin J.-P., Chung J.-G. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-κB, Ras and matrix metalloproteinase-2 and -9. Oncology Reports. 2014;32(1):355–361. doi: 10.3892/or.2014.3209. [DOI] [PubMed] [Google Scholar]
- 61.Tan S., Guan X., Grün C., Zhou Z., Schepers U., Nick P. Gallic acid induces mitotic catastrophe and inhibits centrosomal clustering in HeLa cells. Toxicology in Vitro. 2015;30(1):506–513. doi: 10.1016/j.tiv.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Madlener S., Illmer C., Horvath Z., et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Letters. 2007;245(1-2):156–162. doi: 10.1016/j.canlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Lu Y., Jiang F., Jiang H., et al. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. European Journal of Pharmacology. 2010;641(2-3):102–107. doi: 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You B. R., Park W. H. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicology in Vitro. 2010;24(5):1356–1362. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Chandramohan Reddy T., Bharat Reddy D., Aparna A., et al. Anti-leukemic effects of gallic acid on human leukemia K562 cells: Downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicology in Vitro. 2012;26(3):396–405. doi: 10.1016/j.tiv.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 66.Ho H. H., Chang C.-S., Ho W.-C., Liao S.-Y., Lin W.-L., Wang C.-J. Gallic acid inhibits gastric cancer cells metastasis and invasive growth via increased expression of RhoB, downregulation of AKT/small GTPase signals and inhibition of NF-kappaB activity. Toxicology and Applied Pharmacology. 2013;266(1):76–85. doi: 10.1016/j.taap.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 67.He Z., Chen A. Y., Rojanasakul Y., Rankin G. O., Chen Y. C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling pathway in ovarian cancer cells. Oncology Reports. 2016;35(1):291–297. doi: 10.3892/or.2015.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang T., Chen H.-S., Wang L.-F., et al. Ellagic acid exerts anti-proliferation effects via modulation of Tgf-B/Smad3 signaling in MCF-7 breast cancer cells. Asian Pacific Journal of Cancer Prevention. 2014;15(1):273–276. doi: 10.7314/APJCP.2014.15.1.273. [DOI] [PubMed] [Google Scholar]
- 69.Chen H.-S., Bai M.-H., Zhang T., Li G.-D., Liu M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. International Journal of Oncology. 2015;46(4):1730–1738. doi: 10.3892/ijo.2015.2870. [DOI] [PubMed] [Google Scholar]
- 70.Fang Y., Zhou H., Xia J.-F., et al. Ellagic acid regulates wnt/β-catenin signaling pathway and CDK8 in HCT 116 and HT 29 colon cancer cells. Bangladesh Journal of Pharmacology. 2015;10(1):47–56. doi: 10.3329/bjp.v10i1.21068. [DOI] [Google Scholar]
- 71.Mishra S., Vinayak M. Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complementary and Alternative Medicine. 2015;15(1, article no. 281) doi: 10.1186/s12906-015-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heekyung L., Hyojung L., Youngjoo K., et al. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. The Journal of Immunology. 2010;185(11):6698–6705. doi: 10.4049/jimmunol.1001373. [DOI] [PubMed] [Google Scholar]
- 73.Guruvayoorappan C., Kuttan G. Amentoflavone stimulates apoptosis in B16F-10 melanoma cells by regulating bcl-2, p53 as well as caspase-3 genes and regulates the nitric oxide as well as proinflammatory cytokine production in B16F-10 melanoma cells, tumor associated macrophages and peritoneal macrophages. Journal of Experimental Therapeutics and Oncology. 2008;7(3):207–218. [PubMed] [Google Scholar]
- 74.Lee E., Shin S., Lee J.-Y., et al. Cytotoxic activities of amentoflavone against human breast and cervical cancers are mediated by increasing of pten expression levels due to peroxisome proliferator-activated receptor γ activation. Bulletin of the Korean Chemical Society. 2012;33(7):2219–2223. doi: 10.5012/bkcs.2012.33.7.2219. [DOI] [Google Scholar]
- 75.Guruvayoorappan C., Kuttan G. Inhibition of tumor specific angiogenesis by amentoflavone. Biochemistry (Moscow) 2008;73(2):209–218. doi: 10.1007/s10541-008-2013-x. doi: 10.1007/s10541-008-2013-x. [DOI] [PubMed] [Google Scholar]