Abstract
Background
There is growing recognition that chronic cocaine users have alterations in sensorimotor control that are positively related to low frontal-striatal connectivity within the motor system. These frontal-striatal motor circuits however, are modulated by circuits governing attention, which are also disrupted in cocaine users. This study’s aim was to determine if sensorimotor control deficits are positively related to the difficulty of a motor task or exist independent of the increasing cognitive demand.
Methods
Functional MRI data was collected from 40 individuals (20 non-treatment seeking chronic cocaine users, 20 age and gender matched non-drug using controls) as they mimicked an unpredictable finger-tapping sequence at various speeds. Dependent measures included task accuracy, percent BOLD signal change in sensorimotor regions of interest (ROIs), and functional connectivity (temporal correlations) between ROIs.
Results
In both groups, as speed increased, the BOLD signal change increased in the primary motor cortex, supplementary motor area (SMA), cerebellum, and anterior cingulate cortex. Compared to controls, cocaine user SMA-Caudate and ACC-Putamen connectivity was lower at all speeds in the contralateral hemisphere. Furthermore, as speed increased there was a decrease in connectivity between additional ROI pairs among users.
Conclusions
These data support previous observations of sensorimotor performance deficits and dorsal frontal-striatal connectivity impairments among cocaine users. While previous studies demonstrate these deficits when performing a finger-tapping task at a single speed, we show that these same impairments exist at multiple levels of task difficulty. These data suggest that previously observed frontal-striatal connectivity in cocaine users during sensorimotor task performance are stable and not directly related to cognitive demands of the task.
Keywords: Addiction, Connectivity, Neuroimaging, Sensorimotor, Cognitive, Neural networks
1. Introduction
Chronic cocaine use has been associated with motivational (Risinger et al., 2005; Sinha et al., 2005), cognitive (Hester and Garavan, 2004), and sensorimotor impairments (Bauer, 1996); (Pascual-Leone and Dhuna, 1990; Preller et al., 2013). Although disruptions in the frontal-striatal circuits that govern executive control and limbic arousal in cocaine users is a very active field of research, there is a limited understanding of the functional implications to sensorimotor circuits (Hanlon et al., 2009, 2010, 2011).
In healthy individuals ongoing motor control requires temporally coordinated activity among a network of cortical and subcortical regions including the primary motor cortex (MC), supplementary motor area (SMA), anterior cingulate cortex (ACC), caudate nucleus (Caud), putamen (Put), thalamus (Thal), and cerebellum (Cb) (Pollok et al., 2006; van Donkelaar et al., 2000). As task complexity increases, however, additional cortical regions such as the pre-supplementary motor area (pre-SMA) are recruited in response to increased cognitive demand, response inhibition and reaction time. A common experimental paradigm in motor control literature requires individuals to mimic an ongoing, unpredictable pattern of finger tapping movements. During this externally-guided finger tapping task, right-handed individuals will engage a network of left-sided frontal-striatal nodes (Foulkes and Miall, 2000; Inoue et al., 1998; Miall et al., 2000). Furthermore, there is a significant temporal correlation between the cortical and subcortical nodes of this network during task performance (Pollok et al., 2005). Functional connectivity within this network, therefore, is presumed to be critical in the successful performance of motor control. Meanwhile, studies have investigated functional integrity of frontal-striatal nodes during the performance of cognitive tasks among healthy controls. Reductions in resting state functional connectivity, for example, are reduced between caudate and anterior cingulate among individuals who perform poorly on a working memory n-back task (Gordon et al., 2015).
While healthy controls have highly correlated BOLD signals amongst all cortical and subcortical connections of this network, cocaine users have a selective impairment in connectivity between the frontal-striatal nodes of this network. Specifically, MC-SMA connectivity is intact in cocaine users, but they have lower connectivity between the SMA and its striatal targets (Caud and Put). Furthermore, lower SMA-striatal connectivity was positively correlated with performance deficits (Hanlon et al., 2011). These data suggest that, in addition to the well-established irregularities in functional connectivity in the limbic and executive control networks (Bolla et al., 2003; Hester and Garavan, 2004; Lundqvist, 2010; Moeller et al., 2010), chronic cocaine users also have functional connectivity irregularities in the motor system which is behaviorally relevant (Li et al., 2000).
One interpretation of these data however, is that the high error rates and slower reaction times in cocaine users reflect a deficit in cognitive processing rather than a deficit in motor performance. Previous studies indicate that cognitive tasks with increasing complexity impair motor task performance and cognitive abilities. Cognitive systems such as working memory play an important role in the successful execution of complex upper extremity movements (Boyd et al., 2009; Fitts, 1954; Spiegel et al., 2014). An increase in SMA and MC regional cerebral blood flow, using PET for example, has been shown to be dependent on task complexity (Grafton et al., 1992; Shibasaki et al., 1993). The aim of this study was to understand the effects of varying cognitive load on frontal-striatal connections involved in execution of a sensorimotor task among chronic cocaine users. We hypothesized that there would be a strong interaction between the attentional demands of the task and sensorimotor accuracy and neural engagement. Specifically, we tested the hypotheses that an increase in cognitive load would cause cocaine users to have: 1.) higher error rates, and 2.) reduced functional coupling within frontal-striatal connections.
2. Methods and materials
2.1. Participants
Twenty non-treatment seeking, right-handed, cocaine dependent individuals and twenty age and gender matched non-drug using controls were recruited from the Charleston, SC metropolitan area through advertisements and word-of-mouth. All participants underwent a urine drug screen and an evaluation of co-morbid disorders via the DSM-IV criteria for current or past substance dependence other than cocaine, nicotine, marijuana, or caffeine. The MINI (Mini-International Neuropsychiatric Interview) was administered to all participants to determine the presence of participants meeting DSM-IV criteria for SUD, PTSD, and major depression. Recent use was documented by a 5-panel urine drug screen sensitive to cocaine (last 48 h), benzodiazepines (last 3–7 days), opiates (last 37 days), THC (last 5 days) (Quickvue, 5-panel urine screen, Quidel, San Diego, CA). Exclusionary criteria included left hand dominance, an AUDIT score of >15, >1 pack of tobacco cigarettes per day; use of illicit psychoactive drugs other than cocaine and marijuana; a lifetime history of head injury with loss of consciousness; more than 100 lifetime uses of any drug of abuse other than cocaine; being or planning to become pregnant; having an unstable medical illness, or color blindness.
The control and user groups were age matched (± standard deviation, Controls: 34.85 ± 7.5 years old, Users: 35.55 ± 8.4 years old), and gender matched (50% female, 50% male). All cocaine users met DSM-IV criteria for cocaine dependence and had positive urine drug screens for cocaine. Average time since last use was 1.5 days (verbal report). Among users, positive urine drug screens for THC (13), benzodiazepines (2), and opiates (1) were detected. One control participant tested positive for benzodiazepines. More users (17) were cigarette smokers than controls (2). Two users met criteria alcohol dependence. The average age of first cocaine use was 20.05.35 ± 3.5 years old. One of the cocaine users had a history of major depression disorder and all had relatively low levels of depressive symptoms. Beck Depression Inventory scores were significantly higher in the users (Users: 5.1 ± 4.8, Controls: 0.25 ± 0.44, p < 0.001). Harmful alcohol drinking behavior was also significantly higher among users (Alcohol Use Disorders Inventory Test (AUDIT), Users: 8.7 ± 7.0, Controls: 3.6 ± 1.9; p < 0.001). State anxiety scores were significantly greater in users (Controls: 21.8 ± 3.3, Users: 28.6 ± 8.9, p < 0.01) and trait anxiety scores were significantly higher in users (Controls: 22.8 ± 4.0, Users: 32.5 ± 9.6, p < 0.001). A medical history of heart attack was reported for one of the users. None of the controls had a history of cocaine use, nor did they meet criteria for abuse or dependence on any other drugs.
2.2. Tasks and procedures
As in previous studies, during this dynamic, externally guided finger-tapping task participants were asked to mimic an unpredictable sequence of finger tapping movements with their right hand. The finger tapping movements were presented to them on a video monitor as they lay in the MRI scanner. Six blocks of finger tapping (VIDEO 1) and rest (30 s) were interspersed with preparation blocks (9 s) for each run. All participants performed 2 runs of the task. Blocks of the finger-tapping task became increasingly difficult throughout the run. Six distinct speed levels were repeated twice during each run. The two first finger tapping blocks were “slow” (.56–0.67 Hz), the 3rd and 4th blocks were “medium” (1.0–1.3 Hz) and the 5th and 6th blocks were “fast” (1.67–2.0 Hz). After each finger tap was displayed visually, recording of the finger tapping response began. Subjects had 1000 ms to respond during the 0.56–0.67 Hz task, 1000 ms to respond during the 1.0 Hz task, 750 ms during the 1.3 Hz task, 600 ms during the 1.67 Hz task, and 500 ms during the 2.0 Hz task. Responses outside of these recording windows were considered to be an omitted response. Responses to the finger-tapping task were recorded with an MR compatible button box. Participants were familiarized with the task prior to the finger-tapping task in the scanner in order to reduce the effects of learning while performing the task in the scanner.
Functional data were acquired on a 3-T Siemens TIM Trio scanner (Siemens Medical, Erlangen, Germany). For functional imaging, BOLD images were acquired using gradient echo-planar imaging (EPI) protocol (repetition time (TR) = 2200 ms, echo time (TE) = 30 ms, flip angle 90, 3.0 mm × 3.0 mm × 3.0 mm in-plane resolution, and 64 × 64 matrix). High-resolution T1-weighted anatomical images were acquired for each participant (3.0 T Siemens Trio, 3D SPGR, TR = 1750 ms, TE = 4 ms, voxel dimensions 1.0 × 1.0 × 1.0 mm, and 160 slices).
2.3. Functional MRI preprocessing
Spatial preprocessing was performed with standard parametric mapping techniques (SPM12, London, UK). Data were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute (MNI) brain template), and smoothed using a Gaussian kernel of 8 mm for the group analysis to reduce the variance due to anatomical variability. Region of interest (ROI) extractions were performed through SPM12 MarsBaR 0.44 toolbox in SPM.
2.4. Statistical analysis
The primary dependent measures for this experiment were: 1.) task accuracy, 2.) percent BOLD signal change in a network or established motor regions of interest, and 3.) functional connectivity between these ROIs, as defined by the average correlation coefficient of BOLD signal timecourse between anatomically connected ROIs (Hanlon et al., 2010). Statistical analysis between groups was performed using standard statistical software (IBM SPSS Statistics 23).
2.4.1. Task accuracy
Accuracy on the visual-motor finger-tapping task was quantified by dividing the sum of correct button presses within a given speed block by the total number of possible correct button responses within that block. Mean accuracy was compared between groups across the three finger-tapping speeds (group × speed). Additionally, a one way repeated measures ANOVA was used to assess changes in response accuracy during the across speeds within each group. To test the relationship of alcohol use (AUDIT score) and motor response accuracy, a Pearson’s correlation was used to determine a linear correlation within the user group. Task accuracy in users, which tested positive for THC prior to scanning, were compared to THC negative users by a Student t-test. All post-hoc t-tests in this study were performed with Bonferroni correction for multiple comparisons.
2.4.2. Percent BOLD signal change
BOLD time series were extracted from regions or interest that has been previously shown to be involved in the finger-tapping task (Hanlon et al., 2010). Anatomical ROIs extracted from MNI based atlas (WFU Pick Atlas, Wake Forest University) included left and right primary motor cortex (M1), supplementary motor area (SMA), anterior cingulate cortex (ACC), caudate (Caud), putamen (Put), thalamus (Thal), and the cerebellum (Cereb). A 8 mm spherical pre-supplementary motor area (pre-SMA) ROI was created based on previously reported MNI coordinates (x = −4, y = 36, z = 56). ROI time courses for individual subjects were extracted via MarsBaR. For each individual subject timecourses for each of these regions were extracted over an entire task run. The average BOLD signal across rest blocks was compared to movement blocks to determine percent signal change. A 3-way ANOVA was used to test the interaction of Group × Time × Speed. ROI% percent signal change was compared between groups using 2-way repeated measures ANOVA (Group × Speed) for each ROI.
2.4.3. Functional coupling
Using the timecourses extracted from each ROI correlation coefficients were calculated for all anatomically connected ROIs in the contralateral and ipsilateral motor network for each individual (Pre-SMA-Caudate, Pre-SMA-Putamen, SMA-Caud, SMA-Put, ACC-Caud, ACC-Put, Caud-Thal, Put-Thal, Thal-M1, Thal-Cereb, Cereb-SMA, Cereb-M1). The correlation coefficients were used as measures for functional coupling and their significance was determined by critical values of the Pearson Correlation coefficient r. Correlation coefficients of each couplet were averaged across subjects in each group for each of the slow, medium and fast conditions. The differences in couplet connectivity between groups were assessed using a 2-way between groups repeated measured ANOVA (Group × Speed) for each ROI couplet.
3. Results
3.1. Motor task performance
A two-way repeated measures ANOVA (group × speed) showed a significant main effect of group (F (1,38) = 4.432, P < 0.05) revealing performance accuracy of finger tapping task among controls is significantly higher than users across speed levels. There was no significant interaction of group × speed. A one-way, repeated measures ANOVA among controls (F = 59.02(2,38), P < 0.001) and users (F = 63.307(2,38), P < 0.001) revealed a significant main effects of task speed. Pairwise comparisons between slow, medium and fast task speed indicated a significant difference between slow and fast (P < 0.001), as well as between medium and fast (P < 0.001). There were no significant differences between the slow and medium condition. Average accuracy rates for the users were 75.2% for the slow task, 88.1% for the medium task and 34% for the fast task. The average accuracy rates for the controls were 86.4% for the slow task, 91.3% for the medium task, and 49.8% for the fast task (Fig. 1). 3 unpaired t-tests comparing users to controls for each speed level (slow, medium and fast) revealed that users performed the task with significantly lower accuracy levels (P < 0.05, Bonferroni corrected) during the fast speed.
Fig. 1.
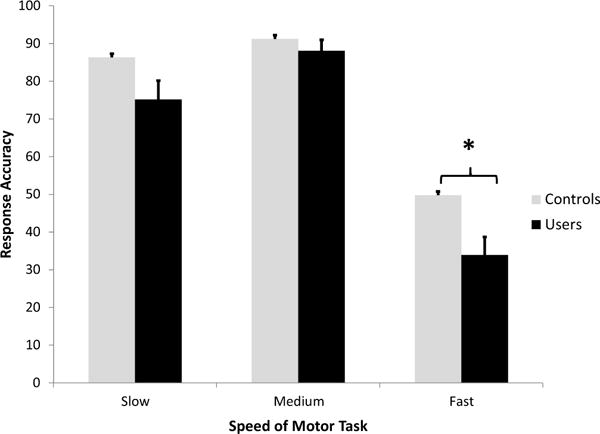
Task performance in cocaine users and controls. The mean (with standard error) accuracy rate (% correct responses) is plotted for the slow, medium and fast speed levels of the visually guided motor task. Response accuracy is shown for controls (light grey bars), and cocaine users (black bars). There was a significant main effect of group (with cocaine users performing consistently worse) and a main effect of speed, with no interaction between group and speed. Significant between group differences for each speed are noted (* = p < 0.05, Bonferroni corrected).
Given the presence of alcohol use in the cocaine using group, the effects of this co-drug use was analyzed in relationship to task performance. AUDIT score across users was normally distributed (Shapiro-Wilk test), and a linear correlation between motor accuracy and AUDIT score was performed. The Pearson correlation (r = −0.301, p > 0.05) of average motor performance accuracy and AUDIT score did not reach significance. Additionally, there was no significant correlation between AUDIT score and motor latency (r=0.048, p > 0.05). No significant differences in mean accuracy scores were seen in users whose urine tested positive for THC compared to those who did not.
3.2. ROI percent signal change: finger-tapping task
Percent signal change for ROIs involved in the finger-tapping task are shown at various task speed levels (Fig. 2). A 3 way ANOVA (group × ROI × speed) revealed a significant interaction of ROI × speed (F(14,546) = 6.122, P < 0.001), main effect of task speed (F (2,76) = 8.052, P < . 01), a significant main effect of ROI (F(7,273) = 14.457, P < 0.0001). Subsequent 2 way ANOVAs for each ROI (group × speed) demonstrated that, consistent with prior literature, there was a main effect of speed on BOLD signal activity in multiple left sided cortical regions (contralateral motor network: left primary motor cortex (F (2,76) = 26.132, P < 0.001), left SMA (F (2,76) = 39.625, P < 0.001), the left cingulate (F (2,76) = 5.213, P < 0.05) and the right cerebellum (F (2,76) = 12.849, P < 0.001). Additionally, the right hemisphere (ipsilateral motor network) showed a main effect of speed (right primary motor cortex (F (2,76) = 4.343, P < 0.05), right SMA (F (2,76) = 18.496, P < 0.001), the right cingulate (F (2,76) = 5.165, P < 0.05) and the left cerebellum (F (2,76) = 9.738, P < 0.001). There were no significant effects related to group.
Fig. 2.
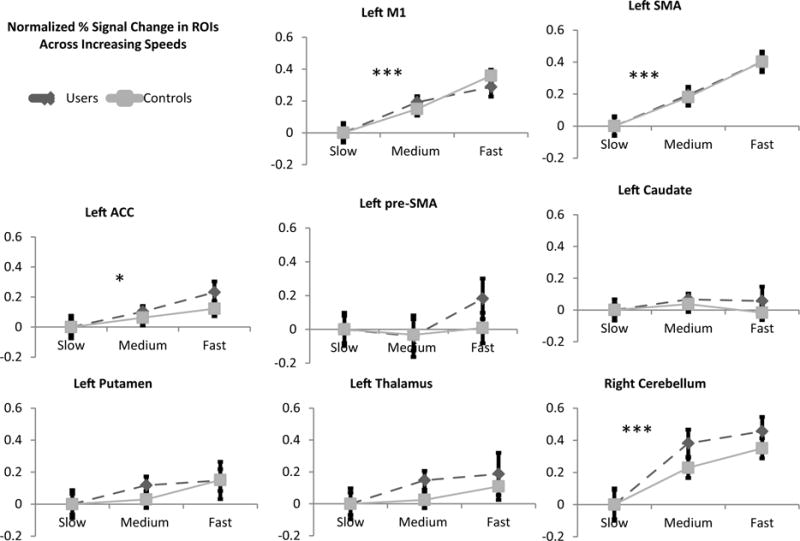
Normalized percent signal change in ROIs across finger tapping speeds (Contralateral Network). BOLD percent signal change within the medium and fast task are shown relative to the slow condition. There was no main effect of group when comparing % signal change for various difficulty levels between users (dashed line) and controls (solid gray line). ROIs with a significant main effect of task speed included left M1 (p < 0.001), left SMA (p < 0.001), left ACC (p < 0.05), and right cerebellum (p < 0.001). Left thalamus, left caudate and left putamen showed no main effect of speed. Significant main effects of speed are noted (* = p < 0.05, ** = p < 0.01, ** = p < 0.001).
3.3. Connectivity: finger-tapping task
Within the controls, there was a significant correlation in all 7 anatomically connected ROI pairs for all 3 speeds (SMA-M1, ACC-Caud, SMA-Put, SMA-Caud, M1-Thal, Thal-Cereb, and SMA-Cereb; Pearson correlation coefficient (α= 0.05), corrected for multiple comparisons) (Fig. 3). Pre-SMA-Caudate and Pre-SMA-Putamen did not show significant correlation coefficients in healthy controls or users (Supp Fig. 21 (contralateral) and 31 (ipsilateral)).
Fig. 3.
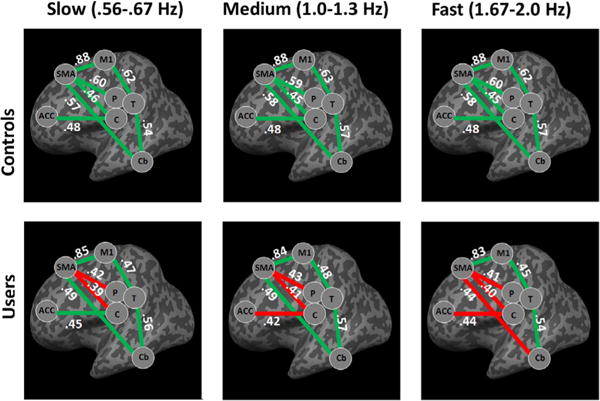
Functional coupling between ROIs involved in finger-tapping task (Contralateral Network). Significant Pearson’s correlation coefficients (p < 0.05) are signified by a green line, while non-significant correlation coefficients are signified by a red line. Values in white are shown for each ROI pair and represent the correlation coefficient r. Controls show significant coupling between all 7 ROI pairs across the various task speeds. Meanwhile, cocaine users show significant functional coupling between ROIs with exception of frontal-striatal pairs during the slow task. During the medium and fast speeds functional decoupling occurs between additional ROI pairs. Abbreviations are used for anatomical ROIs are SMA for supplementary motor area, ACC for anterior cingulate cortex, T for thalamus, P for putamen, C for caudate, Cb for cerebellum, and M1 for primary motor cortex. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Within the cocaine users, 5 pairs were significantly coupled during “slow”, 4 pairs were significantly coupled during “medium”, and 3 pairs were significantly coupled during “fast.” The two frontal striatal ROI pairs (SMA-Caud, SMA-Put) were not significantly coupled at any of the 3 speeds for the cocaine users.
Between groups there was a significant difference in the contralateral SMA-Put (F (2,76) = 6.062, P < 0.05), ACC-Put (F (2,76) = 5.114, P < 0.05), and Thal-M1 (F (2,76) = 6.264, P < 0.05) as well as the ipsilateral Thal-M1 (F (2,76) = 4.341, P < 0.05) and Cereb-M1 (F (2,76) = 5.534, P < 0.05). There was no effect of speed nor an interaction between speed and ROI pair.
4. Discussion
Given the direct effects of cocaine on dopamine in the dorsal striatum and the dense projections the dorsal striatum receives from the prefrontal cortex, frontal-striatal circuitry abnormalities have been an active area of investigation in cocaine research. Recent data has demonstrated that chronic cocaine users have fundamental deficits in basic visually-guided finger tapping which is correlated with lower dorsal frontal-striatal connectivity (Hanlon et al., 2011). Given that dorsal prefrontal and striatal areas are also implicated in cognitive disruptions, it has been difficult to discern whether observed sensorimotor deficits exist independent of the cognitive demands of the task. The aim of this study was to resolve this gap in knowledge by evaluating the effect of task difficulty on motor performance and frontal-striatal connectivity in cocaine users. The results demonstrate that at multiple levels of attentional demand (e.g., task speed) cocaine users perform less accurately and have lower temporal coupling in the dorsal frontal-striatal motor network than controls.
This study was motivated by a series of previous studies that have demonstrated sensorimotor control deficits in cocaine users. In 2010 our group demonstrated that when cocaine users perform a basic visually guided finger tapping task in an MRI scanner, they make more errors of commission and have longer reaction times than age and gender matched controls (Hanlon et al., 2010). Additionally, while the control participants had a significant increase in BOLD signal (measured by percent signal change) in a network of left sided brain regions involved in motor control and error monitoring (primary motor cortex, ACC, SMA, putamen), the cocaine users had an increase in BOLD signal in both the left and the right-sided motor network. These were the same ROIs used in the present investigation (with the addition of the PreSMA). In the initial study in 2010, the BOLD signal activity in the cocaine users was significantly higher in both the left and right motor cortex, the left and right SMA, the left and right ACC and the left and right putamen than the controls. This pattern was also observed in the present study, with controls relying primarily on the left primary motor cortex and putamen during the task, while cocaine users had elevated BOLD signal in the left and the right motor cortex.
While it was tempting to conclude that this elevated bilateral BOLD signal must be either a compensatory strategy (more neural effort) or a lack of appropriate neural organization (less neural efficiency), there was no association between behavioral performance and percent BOLD signal change in that initial study. In a subsequent data analysis effort, we tested the hypothesis that, instead of an association with individual nodes of the network, behavioral performance may be related to the functional connectivity between these structurally connected nodes (Hanlon et al., 2011). In this analysis we demonstrated that within the controls there was a significant correlation in BOLD signal timecourses between each of the anatomically connected pairs. In the cocaine users however, the cortical-striatal coupling was not significant (e.g., SMA-caudate, SMA-putamen). Additionally the strength of the SMA-caudate and SMA-putamen coupling was correlated with motor performance.
In the present study these same pairs were used (with the addition of the Pre-SMA), and the observation of lower SMA-putamen and caudate BOLD signal was observed at all speeds. The only pair which was not significantly correlated in the controls was the PreSMA-Caudate/Putamen. The lack of observed functional correlation with the PreSMA and striatal ROIs may be because, unlike the SMA, the pre-SMA has less dense anatomical connectivity with subcortical brain regions that are involved with ongoing motor control (e.g., the caudate, putamen, cerebellum). All of the other pairs have strong structural connectivity and revealed a significant correlation in BOLD timecourse for the controls. That said, the PreSMA was included in this study given its role in cognitive control and attentional monitoring of movement, so it should not be overlooked in future studies.
One large question that remained after those initial two investigations however, was if the observed differences in percent signal change and in functional connectivity in the cocaine users were related to a fundamental motor related deficit, or if they were an indirect reflection of attentional engagement of task difficulty. Hence, as a next logical step in this line of inquiry the present investigation evaluated task performance, percent BOLD signal change and functional connectivity in cocaine users and controls performing a motor task at three levels of difficulty. Speed was chosen as the domain on which h to vary difficulty because of its established role as a factor that alters motor performance in controls. That said, subsequent investigations which alter cognitive load by something such as working memory demand or drug cue distractors may be equally interesting given their involvement in mesocortical and mesolimbic fronto-stratal circuit function. While many of the results observed in the present study are consistent with our prior observations in different cohorts of cocaine users which performed the task at a fixed speed. Although varying the speed led to a significant decrease in accuracy, there was not a significant relationship between speed and either percent signal change or functional connectivity in the nodes of the ipsilateral or contralateral motor network of cocaine users. These data suggest that previously reported deficits in functional connectivity between the SMA and striatum in cocaine users at a single speed, are present in these individuals even when performing the task at a slower or faster speed.
While cognitive control and attention have been well studied in cocaine users, less is known about motor planning and execution. Behaviorally it is likely that increasing speed of a task produces a greater number of errors due to the reduced time to plan movements as well as the increased information load on the participant. A critical component to successful motor planning and preparation requires a direct modulation of attention to critical somatosensory and visuospatial information (Fagioli et al., 2007; Galazky et al., 2009). Some previous studies have shown that patterns of brain activation are altered when the amount of time available to plan a movement changes (Boyd et al., 2009; Elsinger et al., 2006). One study in particular showed that while SMA, sensorimotor cortex and putamen were recruited during increased task difficulty, a cognitive associated region- the medial frontal gyrus was important for fast movements and high accuracy (Boyd et al., 2009). Evidence from the current study supports the relationship between complex motor tasks and ROIs such as the SMA, and putamen. Disruption of the connectivity between these two regions, as seen in users across levels of difficulty, therefore is likely to be significantly involved in the reduced motor performance. However, non-significant coupling in the medium and fast finger-tapping tasks were associated with additional regions, which are functionally correlated among controls. Specifically, correlation values in the medium task and fast task did not reach significance in ACC to caudate and SMA to cerebellum, respectively. Interestingly, ACC and cerebellum have been implicated in successful motor task performance and cognitive processes. The anterior cingulate is involved in monitoring action performance and cognitive modalities including attention to actions (Isomura et al., 2003). Similarly, the cerebellum is involved in many aspects of visual motor coordination, and executive processing (Bellebaum and Daum, 2007; Miall et al., 2000).
The relationship between attentional demand and motor circuit activity in cocaine users is also germane to one of the most common behavioral deficits found in chronic cocaine users –response inhibition and impulsivity (Lane et al., 2007; Vonmoos et al., 2013). Impulsivity can result in risky behaviors such as drug seeking and poor decision making (Hulka et al., 2015; Wittwer et al., 2016). Despite numerous studies on impulsivity among drug users, the exact mechanisms underlying these impairments have not yet been elucidated (Fillmore and Rush, 2002). Similar resources required to successfuly perform a complex motor task are impaired during implusive behaviors including cognition and motor control. ACC hypoactivity for example has been implicated in cocaine users when inhibiting motor response to visual stimuli (Kaufman et al., 2003).
Although the results of this investigation are consistent with previous studies among cocaine users, there are limitations due to the distinctive behavioral profile of chronic cocaine users. One prevalent characteristic among these participants includes smoking of tobacco and marijuana. Drug screening and self reporting indicate that participants actively and regularly smoked which has been shown to have implications on both motor and cognitive performance (Campos et al., 2016; Prashad and Filbey, 2017). However, users who tested positive for THC use prior to the scanning session did not perform significantly worse than users which tested negative for THC. In order to reduce the influence of nicotine withdrawl on motor performance, smokers were given the option to smoke up to an hour prior to image acquisition (Wang et al., 2007). The present cohort of users also had higher AUDIT scores, indicating the presence of alcohol use. We directly tested the effects of concurrent alcohol use, and showed there was no significant correlation between AUDIT score and performacnce accuracy. These results indicate the effects of alcohol on behavioral motor performance among users was minimal. Additionally, cocaine users had a greater prevalence of depressive symptoms relative to controls. While the effects of depression on cocaine user’s motor performance has not been assesed directly, psychomotor slowing and visual attention deficits are often observed in patients with depression (Goodwin, 1997; Pardo et al., 2006; Weiland-Fiedler et al., 2004). Although there is some evidence that IQ affects motor performance (Sen et al., 1983), IQ score was not measured in this study and cannot be ruled out as an explanation for the poor motor performance across all speeds of the task. Finally, while no individual appeared to be experiencing the acute effects of cocaine during the experimental protocol, all cocaine users had positive urine screens for cocaine at the time of scanning. Although this appears like it might be a confound, Goldstein and colleagues (Woicik et al., 2009) demonstrated that cocaine dependent individuals with positive urine drug screens were less impaired on multiple cognitive performance measures than users with negative urine drug screens.
In summary, this investigation confirms prior reports of sensori-motor processing deficits and dorsal frontal-striatal impairment in cocaine users and demonstrates that, while some of these deficits are independent of the attentional demands of the task, increasing the attentional load results in a more global decay of functional coupling in the brains of cocaine users. Although there has been extensive literature regarding the executive and affective deficits among users, impairments to sensorimotor modalities indicate that widespread changes in neural function characterize cocaine abuse and dependence in addition to striatal dysregulation. Testing of an additional group of participants that are nicotine smokers and/or marijuana smokers, but not cocaine dependent is necessary to determine the relative contribution that these drugs (commonly co-abused with cocaine) have on motor performance and neural circuit abnormalities in cocaine users.
Supplementary Material
Acknowledgments
Thank you to Logan Dowdle for his help with ROI extractions.
Role of funding
This work was supported by the National Institutes of Health [grant numbers K01DA027756, R01DA036617, TL1TR001451, P20GM109040].
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2016.12.008.
Footnotes
Contributors
Daniel H. Lench performed analysis on data, and wrote introduction, methods, results and discussion sections of the manuscript. William DeVries collected data and recruited participants. Colleen A. Hanlon designed experimental procedures, and edited manuscript. All authors reviewed and approved of the final manuscript.
Conflict of interest
Nothing declared.
References
- Bauer LO. Resting hand tremor in abstinent cocaine-dependent, alcohol-dependent, and polydrug-dependent patients. Alcohol Clin Exp Res. 1996;20:1196–1201. doi: 10.1111/j.1530-0277.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. http://dx.doi.org/10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, Siengsukon CF, Wessel BD. Manipulating time-to-plan alters patterns of brain activation during the Fitts’ task. Exp Brain Res. 2009;194:527–539. doi: 10.1007/s00221-009-1726-4. http://dx.doi.org/10.1007/s00221-009-1726-4. [DOI] [PubMed] [Google Scholar]
- Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016 doi: 10.2174/1874473709666160803101633. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. http://dx.doi.org/10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Fagioli S, Hommel B, Schubotz RI. Intentional control of attention: action planning primes action-related stimulus dimensions. Psychol Res. 2007;71:22–29. doi: 10.1007/s00426-005-0033-3. http://dx.doi.org/10.1007/s00426-005-0033-3. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- Foulkes AJ, Miall RC. Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res. 2000;131:101–110. doi: 10.1007/s002219900286. [DOI] [PubMed] [Google Scholar]
- Galazky I, Schütze H, Noesselt T, Hopf J-M, Heinze H-J, Schoenfeld MA. Attention to somatosensory events is directly linked to the preparation for action. J Neurol Sci. 2009;279:93–98. doi: 10.1016/j.jns.2008.12.006. http://dx.doi.org/10.1016/j.jns.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Goodwin GM. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol (Oxford) 1997;11:115–122. doi: 10.1177/026988119701100204. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Devaney JM, Bean S, Vaidya CJ. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb Cortex. 2015;25:336–345. doi: 10.1093/cercor/bht229. http://dx.doi.org/10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992;115:565–587. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. http://dx.doi.org/10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. http://dx.doi.org/10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 2011;115:240–243. doi: 10.1016/j.drugalcdep.2010.11.008. http://dx.doi.org/10.1016/j.drugalcdep.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Vonmoos M, Preller KH, Baumgartner MR, Seifritz E, Gamma A, Quednow BB. Changes in cocaine consumption are associated with fluctuations in self-reported impulsivity and gambling decision-making. Psychol Med. 2015;45:3097–3110. doi: 10.1017/S0033291715001063. http://dx.doi.org/10.1017/S0033291715001063. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Koyama M, Sugiura M, Ito M, Fukuda H. PET study of pointing with visual feedback of moving hands. J Neurophysiol. 1998;79:117–125. doi: 10.1152/jn.1998.79.1.117. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. Neural coding of attention for action and response selection in primate anterior cingulate cortex. J Neurosci. 2003;23:8002–8012. doi: 10.1523/JNEUROSCI.23-22-08002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. http://dx.doi.org/10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. http://dx.doi.org/10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Miall RC, Imamizu H, Miyauchi S. Activation of the cerebellum in co-ordinated eye and hand tracking movements: an fMRI study. Exp Brain Res. 2000;135:22–33. doi: 10.1007/s002210000491. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. http://dx.doi.org/10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Humes SW, Posner IM. Neurocognitive dysfunction in antidepressant-free, non-elderly patients with unipolar depression: alerting and covert orienting of visuospatial attention. J Affect Disord. 2006;92:71–78. doi: 10.1016/j.jad.2005.12.037. http://dx.doi.org/10.1016/j.jad.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dhuna A. Cocaine-associated multifocal tics. Neurology. 1990;40:999–1000. doi: 10.1212/wnl.40.6.999. [DOI] [PubMed] [Google Scholar]
- Pollok B, Südmeyer M, Gross J, Schnitzler A. The oscillatory network of simple repetitive bimanual movements. Brain Res Cogn Brain Res. 2005;25:300–311. doi: 10.1016/j.cogbrainres.2005.06.004. http://dx.doi.org/10.1016/j.cogbrainres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Schnitzler A. How the brain controls repetitive finger movements. J Physiol Paris. 2006;99:8–13. doi: 10.1016/j.jphysparis.2005.06.002. http://dx.doi.org/10.1016/j.jphysparis.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Preller KH, Ingold N, Hulka LM, Vonmoos M, Jenni D, Baumgartner MR, Vollenweider FX, Quednow BB. Increased sensorimotor gating in recreational and dependent cocaine users is modulated by craving and attention-deficit/hyperactivity disorder symptoms. Biol Psychiatry. 2013;73(3):225–234. doi: 10.1016/j.biopsych.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Prashad S, Filbey FM. Cognitive motor deficits in cannabis users. Curr Opin Behav Sci. 2017;13:1–7. doi: 10.1016/j.cobeha.2016.07.001. http://dx.doi.org/10.1016/j.cobeha.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. http://dx.doi.org/10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Sen A, Jensen AR, Sen AK, Arora I. Correlation between reaction time and intelligence in psychometrically similar groups in America and India. Appl Res Ment Retard. 1983;4:139–152. doi: 10.1016/0270-3092(83)90006-1. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. http://dx.doi.org/10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Spiegel MA, Koester D, Schack T. Movement planning and attentional control of visuospatial working memory: evidence from a grasp-to-place task. Psychol Res. 2014;78:494–505. doi: 10.1007/s00426-013-0499-3. http://dx.doi.org/10.1007/s00426-013-0499-3. [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Schulz C, Baumgartner MR, Quednow BB. Differences in self-reported and behavioral measures of impulsivity in recreational and dependent cocaine users. Drug Alcohol Depend. 2013;133:61–70. doi: 10.1016/j.drugalcdep.2013.05.032. http://dx.doi.org/10.1016/j.drugalcdep.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. http://dx.doi.org/10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Wittwer A, Hulka LM, Heinimann HR, Vonmoos M, Quednow BB. Risky decisions in a lottery task are associated with an increase of cocaine use. Front Psychol. 2016;7:640. doi: 10.3389/fpsyg.2016.00640. http://dx.doi.org/10.3389/fpsyg.2016.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang G-J, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. http://dx.doi.org/10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar P, Stein JF, Passingham RE, Miall RC. Temporary inactivation in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 2000;83:2780–2790. doi: 10.1152/jn.2000.83.5.2780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


