Abstract
Objective and Design
Work in multiple organs has suggested that toll-like receptor 4 (TLR4) may play a role in insulin resistance. Additional studies have shown a negative role for TLR4 on retinal health. We have previously reported that β-adrenergic receptors can regulate both TLR4 signal transduction, as well as insulin signaling in the retina and in retinal endothelial cells (REC). Thus, we hypothesized that TLR4 would regulate retinal insulin signaling.
Materials and Methods
We used endothelial cell specific TLR4 knockout mice, as well as TLR4 overexpressing mice for these studies.
Methods
Western blotting and ELISA analyses were done for investigations of insulin receptor, insulin receptor substrate 1 (IRS-1) serine 307, and Akt phosphorylation, as well as cleaved caspase 3 levels in the mouse retina.
Results
We found that loss of TLR4 led to increased insulin receptor and Akt phosphorylation, as well as decreased IRS-1Ser307 levels. In support of these results, TLR4 overexpression decreased insulin signaling and the cleavage of caspase 3.
Conclusions
Therefore, these results suggest that TLR4 plays a key role in insulin signaling in the retina. Reduction of TLR4 levels may be protective to the retina.
Keywords: insulin, TLR4, retinal endothelial cells, retina
1.0 Introduction
A number of pathologies have been suggested to result from a low-grade chronic inflammation, including diabetic retinopathy, specifically type 2. Retinal damage in type 2 diabetes results from insulin resistance [1]. There is ample evidence that inhibition of various inflammatory pathways can protect the diabetic retina [2–5]. A key question then arises, is there a common link between insulin resistance and inflammation? One potential link may be toll-like receptor 4 (TLR4) [6]. We have previously reported that β-adrenergic receptors can regulate TLR4 in the diabetic retina, as well as retinal endothelial cells (REC) an Müller cells [7]. Additionally, we have reported that a β-adrenergic receptor agonist can reduce functional, neuronal, and vascular phenotypes associated with retinopathy with rodents [8], as well as restore normal insulin signal transduction in cells grown in high glucose [9, 10].
TLR4 is reported to be both protective and harmful to some systems such as cardiomyocytes, [11] and can mediate a multitude of inflammatory actions in the aorta [12], arterioles [13], adipocytes and macrophages [6]. The key in the response to TLR4 appears to be cell- and disease-specific. In hepatocytes, loss of TLR4 led to improved glucose tolerance, insulin sensitivity and reduced hepatic stenosis, despite a high fat diet in mice [14]. In the same study, the authors found that loss of TLR4 in myeloid cells had little effect on insulin sensitivity [14]. In human aortic endothelial cells, TLR4 caused ER stress [13], when TLR4 was activated by saturated fatty acids. If TLR4 was eliminated, the arterioles were protected against a high fat diet [13]. Similarly, loss of TLR4 was protective to the thoracic aorta, through decreased NFkB, ICAM1, and IL-6, with increased levels of Akt and eNOS [12]. Work has also suggested that one pathway by which TLR4 is detrimental is through activation of resistin, an adipokine. TLR4 knockout mice have reduced resistin, which results in cardiovascular damage [15]. Recent work also points to a role for TLR4 in neuroinflammation in the CNS [16].
While much has been done to explore TLR4 in the cardiovascular and adipocyte systems, this study focused on the role of TLR4 in the retina. Work in TLR4 knockout mice on a high fat diet showed that loss of TLR4 decreased insulin resistance, obesity and improved glucose tolerance. This was associated with decreased phosphorylation of NFkB, IL-6, and other inflammatory pathways [17]. Furthermore, work in the streptozotocin-induced diabetic rat showed that diabetes increased TLR4 signaling, leading to increased levels of key inflammatory mediators [18]. Taken together, there is literature to demonstrate localization and activation of TLR4 signaling in the retina, leading to retinopathy-associated complications.
To further the work in the previous literature, we used endothelial-cell specific conditional knockout mice for TLR4 and TLR4 overexpressing mice with normal glucose levels to investigate whether TLR4 regulates retinal insulin receptor signaling.
2.0 Materials and Methods
2.1 Mice
All animal procedures meet the Association for Research in Vision and Ophthalmology requirements and were approved by the Institutional Animal Care and Use Committee of Wayne State University and conform to NIH guidelines.
2.2 Endothelial cell specific TLR4 knockout mice
The TLR4 floxed mice (B6(Cg)-Tlr4tm1.1Karp/J mice) and B6 FVB-Tg (cdh5-cre)7Mlia/J Cre mice were purchased from Jackson Laboratories. After 2 generations, TLR4 floxed mice were bred with cdh5-Cre mice to generate conditional knockout mice in which TLR4 is eliminated in vascular endothelial cells. Around 3 months of age, TLR4 floxed and TLR4 Cre-Lox mice were used for experiments.
2.3 TLR4 overexpressing mice
TLR4 overexpressing mice were a kind gift from Dr. Danielle Malo, McGill University [19]. A pathogen-free line of 6xTLR4 (Tg390) carrying 6 copies of the TLR4 gene was established in C57BL/10ScNJ background mice. Wildtype controls used were C57BL/10SnJ mice. Mice were genotyped at approximately 2–3 weeks of age. At 3 months, TLR4 overexpression mice and corresponding wild type mice were used for experiments.
2.4 Genotyping
Genomic DNA was extracted from ear punch samples from 2–3 week-old mice. Ear punches were digested with one step tail DNA extraction buffer (100mM Tris, 5mM EDTA, 200mM NaCL, 1% Triton) plus proteinase K (10mg/ml) at 55°C overnight, followed by heat-inactivation at 85°C for 45 min.
Sequences of primer pairs used to screen the TLR4 cdh5 cre-lox conditional knock out mice were as follows: TLR4 floxed: 5′->3′ mutant forward: TGA CCA CCC ATA TTG CCT ATA C; reverse: TGA TGG TGT GAG CAG GAG AG; Cdh5-cre forward: AGG CAG CTC ACA AAG GAA CAA T; reverse: TCG TTG CAT CGA CCG GTA A; Cdh5-cre internal positive control forward: CTA GGC CAC AGA ATT GAA AGA TCT; reverse: GTA GGT GGA AAT TCT AGC ATC ATC C. The standard PCR reaction was done using KAPA2G HotStart Genotyping PCR Mix (KK5621, KAPA Biosystems). The PCR reaction was performed with following temperatures and times: denature: 95°C 3 min, 35 cycles at 95°C, 15 sec, 60°C 15 sec, 72°C sec/kb, with final extension at 72°C for 1 min.
For TLR4 overexpression mice: forward primer: 5′->3′ AGA AGA GCT GCA GCA CCT GGA TTT; reverse primer 5′→3′ GAT GAA ATT GGA ATG AAG ACC TCT CA. The PCR reaction was performed with following temperatures and times: 94°C 3 min, 35 cycles at 94°C, 30 sec, 50°C 30 sec 72°C 30sec with the final extension at 72°C for 10 min.
2.5 Western blotting
Whole retinal lysates from mice were collected into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein were separated onto a pre-cast tris-glycine gel (Invitrogen, Carlsbad, CA), and blotted onto nitrocellulose membrane. After blocking in TBST (10mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, the membranes were treated with TLR4 (Abcam, Cambridge, MA), Akt, phosphorylated Akt (Ser473), insulin receptor, phosphorylated insulin receptor (Tyr 1150/1151), insulin receptor substrate 1, phosphorylated insulin receptor substrate 1 ((Ser 307), Cell Signaling Technology, Danvers, MA)) and beta actin (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies followed by incubation with secondary antibodies labeled with horseradish peroxidase. Antigen-antibody complexes were detected by chemilluminescence reagent kit (Thermo Scientific, Pittsburgh, PA) and data was acquired using an Azure C500 (Azure Biosystems, Dublin, CA). Western blot data were assessed using Image Studio Lite software.
2.6 ELISA
A cleaved caspase 3 ELISA was done according to manufacturer’s instructions (Cell Signaling Technology, Danvers, MA) with equal protein loaded to allow for comparison using the optical density measurement (O.D).
2.7 Statistics
One-way ANOVA with Student Newman Keul’s post-hoc test was used. P<0.05 was considered statistically significant. A representative Western blot is shown where appropriate.
3.0 Results
Genotyping and protein analyses confirm TLR4 conditional knockout mice and TLR4 overexpressing mice
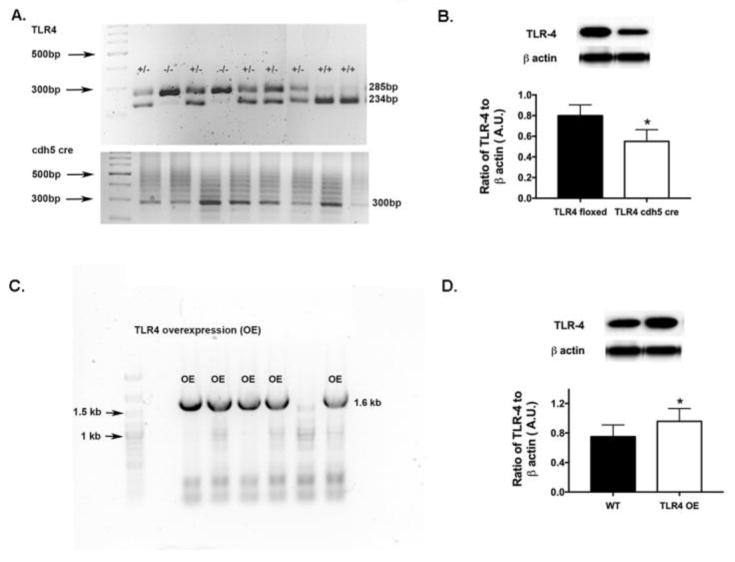
Figure 1A shows the genotyping results for the TLR4 floxed and TLR4/cdh5 Cre-Lox mice to confirm knockdown of TLR4. Figure 1B shows that TLR4 protein levels are reduced in the TLR4 Cre-Lox mice. Figure 1C shows overexpression of TLR4 in the TLR4 OE mice versus the C57BL/10SnJ wildtype controls. Overexpression of TLR4 protein levels were noted in the TLR4 OE mice when compared to littermates (Figure 1D).
Figure 1.
TLR4 is reduced in the TLR4 endothelial cell knockout mice. Figure 1A provides the genotyping data for the TLR4 floxed versus Cre-Lox mice. Figure 1C shows genotyping results for TLR4 overexpressing mice vs. WT. Figures 1B, D provide western blot results showing decreased TLR4 in the conditional knockout mice (B) and increased TLR4 in the TLR4 overexpressing mice (D). N=5 for each group. Data are mean ± SEM. *P<0.05 vs. TLR4 floxed or wildtype littermates.
Overexpression of TLR4 reduces insulin receptor phosphorylation, leading to increased IRS-1Ser307 phosphorylation
We have previously reported that TNFα decreased insulin receptor activation, which occurred concurrently with increased IRS1Ser307 phosphorylation [9]. Since we showed that TLR4 increased TNFα levels in the mice, we wanted to determine if TLR4 could drive insulin resistance in the mouse retina. Figure 2A shows that loss of TLR4 in the vascular cells of the retina leads to increased insulin receptor phosphorylation. TLR4 overexpression significantly decreased insulin receptor phosphorylation (Figure 2B). In contrast to insulin receptor, loss of TLR4 decreased IRS-1Ser307 phosphorylation (Figure 2C), which would suggest reduced insulin resistance. Overexpression of TLR4 increased IRS-1Ser307 phosphorylation (Figure 2D).
Figure 2.
TLR4 modulates insulin and IRS-1 phosphorylation. Panel A shows that loss of TLR4 increased insulin receptor phosphorylation, while panel C shows a significant decrease in IRS-1Ser307 phosphorylation. IRS-1Ser307 is inhibitory to insulin signaling. Panel B shows that overexpression of TLR4 significantly reduced insulin receptor activation, which was associated with increased IRS-1Ser307 phosphorylation (D). N=5 for each group. Data are mean ± SEM. *P<0.05 vs. TLR4 floxed or wildtype littermates.
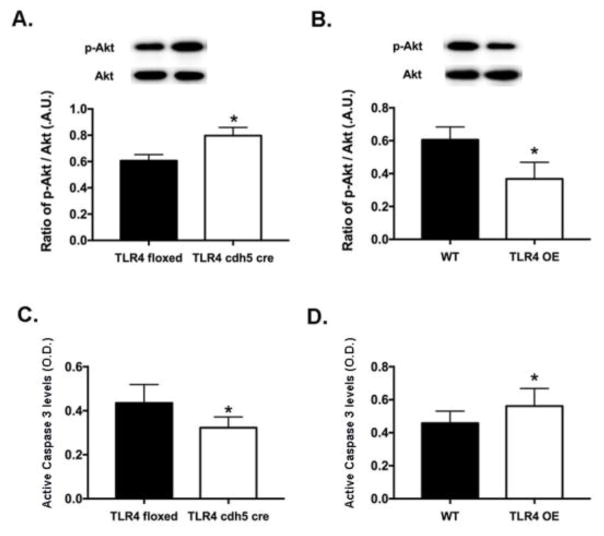
TLR4 regulates Akt phosphorylation to control apoptosis in the retina
Increased IRS-1Ser307 often leads to decreased Akt phosphorylation and increased apoptosis [20]. Figure 3A shows that loss of TLR4 increased Akt phosphorylation, while TLR4 overexpression reduced Akt phosphorylation (Figure 3B). Since Akt can decrease the cleavage of caspase 3 in REC [9], Figure 3C shows that loss of TLR4 decreased cleaved caspase 3 levels. Overexpression of TLR4 increased cleavage of caspase 3. These data suggest that TLR4 is involved in the insulin signal transduction pathway in the mouse retina.
Figure 3.
Akt and caspase 3 are regulated by TLR4. TLR4 knockout in endothelial cells led to increased Akt phosphorylation (A), leading to decreased cleavage of caspase 3 (C). In support of this, increased TLR4 expression increased cleavage of caspase 3 in retinal lysates (D), which occurred with decreased Akt phosphorylation (B). N=5 for each group. Data are mean ± SEM. *P<0.05 vs. TLR4 floxed or wildtype littermates.
4.0 Discussion
Insulin insensitivity is a key component of type 2 diabetes. More recently, the role of a low-grade inflammation has become of increasing interest, as it may relate to insulin responsiveness [6]. Furthermore, data suggest that insulin responsiveness is organ specific [14]. Work in hepatocytes suggested that loss of TLR4 was strongly associated with improved glucose tolerance and insulin sensitivity, despite maintenance of a high fat diet [14]. Similarly, TLR4 regulated insulin receptor signaling in muscle following systemic lipid infusion [6]. Work in the aorta has shown that palmitate blocked insulin signal transduction, which was blocked in TLR4 knockout mice [12]. Using interferon regulatory factor 3 (IRF3) knockout mice, work showed that these mice are protected against obesity through decreased TLR4 signaling [21]. In contrast to work on the aorta and insulin responsive organs, a study did show that TLR4 is protective to cardiomyocytes, producing decreased apoptosis through iNOS and MyD88 [11]. But, work in these same cells showed that TLR4/MyD88 can induce insulin resistance if retinal binding protein 4 (RBP4) levels are high [22].
While there is much literature in known insulin sensitive organs, such as adipocytes and liver, less work has focused on the retina. Using systemic TLR4 knockout mice, work has shown that loss of TLR4 leads to increased neuronal thickness of the retina in response to LPS [23]. TLR4 knockout mice on a high fat diet had less retinal degeneration and DNA damage than their wild type littermates on the same diet [17], likely through reduced macrophage and microglial activation. While there is work to show that systemic loss of TLR4 in mice led to retinal protection, less was known about TLR4 regulation of insulin signaling. In this study, we used endothelial-cell specific knockout mice and TLR4 overexpressing mice (Figure 1) to show that TLR4 has a significant response on insulin signal transduction and retinal cell apoptosis. Loss of TLR4 in endothelial cells led to improved insulin receptor phosphorylation and Akt phosphorylation, with reduced IRS-1Ser307 and TNFα levels. In contrast, overexpression of TLR4 increased cleaved caspase 3 levels in retinal lysates, likely due to the increased TNFα and IRS-1Ser307 phosphorylation. Thus, our data strongly supports work in other organs that TLR4 can reduce insulin signaling. These data agree with other studies on IGFBP-3 and β-adrenergic receptors, which showed altered insulin signaling, despite normal glucose levels [24, 25]. Further studies on insulin signal transduction and TLR4 will be done once these mice are made diabetic.
5.0 Conclusions
In conclusion, the data support that TLR4 is involved in the insulin signaling pathway in the retina. In endothelial cell specific TLR4 knockout mice, insulin receptor and Akt phosphorylation were significantly increased, while IRS-1Ser307 phosphorylation was decreased. In support of these findings, TLR4 overexpression led to decreased insulin receptor signaling and increased cleavage of caspase 3. These data suggest that TLR4 regulates insulin resistance proteins in the retina.
Acknowledgments
This work was supported by R01EY022330 (JJS), P30EY04068 (PI: Hazlett) and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute).
References
- 1.Velloso LA, Folli F, Saad MJ. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr Rev. 2015;36:245–71. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- 2.Joussen AM, Doehmen S, Le ML, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Molecular vision. 2009;15:1418–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 4.Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011;30:343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abcouwer SF, Lin CM, Shanmugam S, et al. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. Journal of neuroinflammation. 2013;10:149. doi: 10.1186/1742-2094-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger EA, Carion TW, Jiang Y, et al. beta-adrenergic receptor agonist, Compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Guy K, Pagadala J, et al. Compound 49b Prevents Diabetes-Induced Apoptosis through Increased IGFBP-3 Levels. Investigative ophthalmology & visual science. 2012;53:3004–13. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24:1086–92. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker RJ, Anderson NM, Jiang Y, Bahouth S, Steinle JJ. Role of beta-adrenergic receptors regulation of TNF-alpha and insulin signaling in retinal Muller cells. Investigative ophthalmology & visual science. 2011;52:9527–33. doi: 10.1167/iovs.11-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Zhao H, Graveline AR, et al. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291:H1900–9. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circulation research. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 13.Kim JA, Jang HJ, Hwang DH. Toll-like receptor 4-induced endoplasmic reticulum stress contributes to impairment of vasodilator action of insulin. Am J Physiol Endocrinol Metab. 2015;309:E767–76. doi: 10.1152/ajpendo.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia L, Vianna CR, Fukuda M, et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Lu L, Hu Y, et al. Resistin Induces Hypertension and Insulin Resistance in Mice via a TLR4-Dependent Pathway. Sci Rep. 2016;6:22193. doi: 10.1038/srep22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang NQ, Jin H, Zhou SY, Shi JS, Jin F. TLR4 is a link between diabetes and Alzheimer’s disease. Behav Brain Res. 2017;316:234–244. doi: 10.1016/j.bbr.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Wang PW, Yang IH, et al. High-fat diet induces toll-like receptor 4-dependent macrophage/microglial cell activation and retinal impairment. Investigative ophthalmology & visual science. 2015;56:3041–50. doi: 10.1167/iovs.15-16504. [DOI] [PubMed] [Google Scholar]
- 18.Wang YL, Wang K, Yu SJ, et al. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:389–98. doi: 10.1007/s00417-014-2832-y. [DOI] [PubMed] [Google Scholar]
- 19.Vogel SN, Johnson D, Perera PY, et al. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–70. [PubMed] [Google Scholar]
- 20.Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–9. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumari M, Wang X, Lantier L, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest. 2016;126:2839–54. doi: 10.1172/JCI86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W, Wang H, Zhang L, et al. Retinol-Binding Protein 4 Induces Cardiomyocyte Hypertrophy by Activating TLR4/MyD88 Pathway. Endocrinology. 2016;157:2282–93. doi: 10.1210/en.2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halder SK, Matsunaga H, Ishii KJ, et al. Retinal cell type-specific prevention of ischemia-induced damages by LPS-TLR4 signaling through microglia. J Neurochem. 2013;126:243–60. doi: 10.1111/jnc.12262. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Zhang Q, Liu L, et al. beta2-adrenergic receptor knockout mice exhibit A diabetic retinopathy phenotype. PloS one. 2013;8:e70555. doi: 10.1371/journal.pone.0070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Jiang Y, Miller MJ, et al. IGFBP-3 and TNF-alpha Regulate Retinal Endothelial Cell Apoptosis. Investigative ophthalmology & visual science. 2013;54:5376–84. doi: 10.1167/iovs.13-12497. [DOI] [PMC free article] [PubMed] [Google Scholar]