Abstract
We reported that β-adrenergic receptors regulate toll-like receptor 4 (TLR4) signaling in the retina of diabetic mice and in retinal endothelial cells (REC) and Müller cells. We hypothesized that TLR4 regulates retinal permeability both in vitro and in vivo in the retinal vasculature. We used REC cultured in normal and high-glucose conditions and TLR4 siRNA treatments for cell culture studies of permeability and protein analyses of tumor necrosis factor a, occludin, and zonula occludens 1 (ZO-1). We used endothelial cell (EC)-specific Cre-Lox TLR4 knockout mice to study retinal permeability and neuronal and vascular changes following exposure to ocular ischemia/reperfusion (I/R) used as a retinal stressor. We found that the loss of TLR4 in the EC led to the reduced permeability following I/R and fewer degenerate capillaries. Retinal permeability was increased in REC grown in high-glucose conditions but was inhibited by TLR4 siRNA treatment. High-glucose culture conditions significantly reduced occludin and ZO-1 levels in REC, and TLR4 siRNA treatment restored levels to baseline. In conclusion, these studies demonstrate that TLR4 in EC strongly regulates retinal permeability and neuronal and vascular changes following exposure to stressors such as I/R.
Keywords: Permeability, Inflammation, Toll-like receptor 4, Retina, Endothelial cell, Ischemia/reperfusion
Introduction
While a plethora of potential factors are likely at play in retinal damage, the role of inflammation has recently taken a larger role. Studies show that a multitude of inflammatory mediators are increased in the retina in response to high glucose/hyperglycemia or other stressors [1–3]. Inhibition of any of these factors shows promise in reducing retinal stress-associated neuronal and vascular damage [4–8]. Additionally, the role of the innate immune system may also contribute to retinal damage associated with diabetes, specifically toll-like receptor 4 (TLR4) [9, 10]. TLR4 is one of a class of pathogen-associated molecular pattern receptors [11]. In humans, 10 TLRs have been identified [12]. TLR4 is a receptor for lipopolysaccharide (LPS), and LPS can lead to a number of inflammatory diseases including sepsis [12]. TLR4 signals downstream through the MyD88 or IRF3 pathways to activate NF-κB and other inflammatory factors, including tumor necrosis factor (TNF)α [13].
A role for TLR4 in barrier permeability is well established in multiple organ systems. Work in Caco-2 cells treated with ethanol or C57/B6 mice receiving ethanol as a liquid diet showed increased TLR4 and intestinal permeability [14]. The increase in TLR4 was associated with a decrease in occludin phosphorylation [14]. Similarly, work with occludin knockout (KO) mice showed a 4-fold reduction in liver permeability after ethanol treatment when compared to wild-type (WT) mice [15]. Other work on intracranial hemorrhage showed that methylprednisolone sodium succinate reduced blood-brain barrier permeability in C57/B6 mice with reduced TLR4 levels and increased zonula occludin 1 (ZO-1) and occludin levels [16]. Work with an intestinal burn model showed that the loss of TLR4 in KO mice led to decreased permeability when compared to WT mice [17]. Similarly, colon permeability following a high-fat diet was reduced in TLR4 KO mice compared to in their WT littermates [18]. The reduction in colon permeability was associated with reduced occludin levels and increased inflammatory mediators [18]. Thus, there is ample evidence from multiple organs that increased TLR4 levels lead to increased permeability and reduced levels of key barrier proteins.
Focusing on the role of TLR4 in the retina, one study showed that TLR4 in bone marrow-derived cells contributes to the progression of diabetic retinopathy, with the inhibition of TLR4 protecting the retina [19]. Other study groups showed that the pharmacological inhibition of TLR4 in diabetic rats significantly reduced retinal barrier permeability as well as reducing TLR4 signaling [9]. A study on systemic TLR4 KO showed that the loss of TLR4 resulted in a significant reduction in inflammatory proteins as well as the increased survival of neurons in the ganglion cell layer (GCL) [20]. Other groups reported that TLR4 KO mice are protected from LPS-induced neuronal loss [21]. Since we recently reported that TLR4 was altered in the diabetic retina, specifically in retinal endothelial cells (REC) and Müller cells, we generated an endothelial cell (EC)-specific TLR4 KO mouse to explore the role of TLR4 in the retina, focusing on the vasculature.
These studies led us to hypothesize that EC-specific TLR4 KO mice would have reduced permeability and increased levels of ZO-1 and occludin. These hypotheses were tested in mouse retina as well as in REC in culture.
Experimental Procedures
Mice
All animal procedures were done according to the Association for Research in Vision and Ophthalmology requirements, were approved by the Institutional Animal Care and Use Committee of Wayne State University, and conform to NIH guidelines.
EC-Specific TLR4 KO Mice
TLR4 floxed mice, i.e., B6(Cg)-Tlr4tm1.1Karp/J mice, and B6 FVB-Tg, i.e., (cdh5-Cre)7Mlia/J Cre mice, were purchased from Jackson Laboratories. After 2 generations, the TLR4 floxed mice with the cdh5-Cre mice were bred to generate conditional KO mice, where TLR4 is knocked out in vascular EC. At 3 months of age, the TLR4 floxed and TLR4 Cre-Lox mice were used for experiments. Genotyping and Western blot results for TLR4 from these mice have been recently published [22].
Immunohistochemical Staining
To verify that TLR4 was reduced in the EC-specific KO mice, we performed fluorescence staining. Three-month-old male and female TLR4 floxed and TLR4 cdh5 Cre-Lox mice (3 in each group) were euthanized by CO2 followed by cervical dislocation. Their eyes were removed and immediately placed into 4% paraformaldehyde in PBS for 4 h at 4 °C. Retinas were then dissected out and rinsed in PBS overnight. Whole retinas were put in 5% normal goat serum for 3 h at room temperature for blocking any nonspecific staining, followed by incubation with isolectin GS-IB4 (Alexa Fluor 488 conjugate, 1: 100, Life Technologies) and TLR4 (1: 100, Abcam, Cambridge, MA, USA) at 4 °C for 2 days. After rinsing, the retinas were incubated with a secondary antibody conjugated to Alexa Fluor 594 (1: 1000, Life Technologies) overnight at 4 °C. Retinas were then rinsed in PBS and counterstained with DAPI, and then mounted and examined using a Leica confocal microscope.
Ischemia/Reperfusion
Some animals were anesthetized with an intraperitoneal injection of ketamine and xylazine. Once these animals lacked a toepinch reflex, the anterior chamber of the eye was cannulated with a 32-gauge needle attached to an infusion line of sterile saline. The eye was subjected to 90 min of hydrostatic pressure (80–90 mm Hg, TonoPen, Medtronic, Jacksonville, FL, USA) to induce retinal ischemia as demonstrated by the blanching of the iris and the loss of red reflex [6, 23]. After 90 min, the needle was withdrawn and the intraocular pressure was normalized, causing reperfusion. The contralateral eye served as an intra-animal control.
Fluorescein Angiography
Eight mice (4 exposed to I/R and 4 as a control) from each group (TLR4 floxed and TLR4 Cre-Lox) were used for retinal vascular leakage analysis. The right eye of each mouse was exposed to the I/R lesion as described above. Twenty-four hours after I/R, the pupils were dilated with tropicamide ophthalmic solution. Fifteen minutes later, the mice were anesthetized by ketamine and xylazine. After they had been deeply anesthetized (no toe-pinch reflex), 150 μL of AK-FLUOR (1% W/V, AKORN, INC, Lake Forest, IL, USA) was injected intraperitoneally. Retinal vessel leakage was analyzed and photographed using the Micron IV (Phoenix Research Labs, Pleasanton, CA, USA). Images were obtained <5 min after injection of the dye.
Analyses
Two days after I/R exposure, a subset of each group of mice was sacrificed for measurements of neuronal thickness, as we have done previously [24]. Briefly, the whole globe is removed and placed into formalin overnight. The eyeballs were washed in PBS and embedded in O.C.T. freezing medium. Sections (10-μm) were taken from throughout the retina, followed by hematoxylin and eosin staining. Analyses were done for retinal thickness and the number of cells in the GCL on 10 sections from throughout the retina of 5 animals in each group as we have done in the past [7, 24].
For the vascular analyses, additional mice (5 from each group) were sacrificed 10 days after I/R exposure, in order to measure degenerate capillaries as we have done previously [23, 25].
REC Culture
Primary human REC were acquired from the Cell Systems Corporation (CSC, Kirkland, WA, USA). Cells were grown in Cell Systems medium supplemented with microvascular growth factors (MVGS), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B (Invitrogen, Carlsbad, CA, USA) on attachment factor-coated dishes. Once cells reached confluence, some dishes were moved to the Cell Systems medium supplemented with D-glucose to 25 mM. Only cells prior to passage 6 were used. Cells were quiesced by incubating in high- or normal-glucose medium without MVGS for 24 h prior to experimentation.
Treatments
REC in normal- (5 mM) and high-glucose (25 mM) medium were transfected with TLR4 siRNA (SMARTpool: ON-TARGET-plus TLR4 siRNA, Dharmacon, Lafayette, CO, USA) or scrambled siRNA at a final concentration of 20 n M using GenMute transfection reagent (Signagen Laboratories, Rockville, MD, USA), based upon the manufacturer’s instructions (except that siRNA was incubated with the cells for 12 h). Analyses were done on REC samples after 3–4 days in normal- or high-glucose medium and 12 h after transfection.
Permeability Assay
REC were grown in normal- and high-glucose medium in Transwell chambers. Once 100% confluent and treated with hydrocortisone, some cells in each condition were transfected with TLR4 or scrambled siRNA. On the day of the experiments, 70 kD of rhodamine isothiocyanate dextran was added to the upper chamber of the Transwell insert. Aliquots from the basolateral chamber were taken every 30 min for 2 h and placed into the 96- well plate. At 2 h (the final time point), a sample was also taken from the apical chamber to ensure that fluorescence in this chamber was unchanged. All aliquots were placed into a 96-well black plate for measurement using a fluorescence plate reader (SynergyHT, Biotek Instruments, Winnoski, VT, USA) at 570/590. The diffusive flux (Po) was calculated as reported previously [26, 27].
Western Blot
Whole-retina lysates from mice or REC lysates were collected into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein were separated onto a precast Tris-glycine gel (Invitrogen, Carlsbad, CA, USA), and blotted onto a nitrocellulose membrane. After blocking in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, the membranes were treated with TLR4, ZO-1, and occludin (all from Abcam) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibodies, followed by incubation with secondary antibodies labeled with horseradish peroxidase. Antigen-antibody complexes were detected with a chemiluminescence reagent kit (Thermo Fisher Scientific, Pittsburgh, PA, USA) and data were acquired using an Azure C500 (Azure Biosystems, Dublin, CA, USA). Western blot data were assessed using Image Studio Lite software.
ELISA
The TNFα ELISA was done according to the manufacturer’s instructions (Thermo Fisher Scientific), with the exception that sample exposure to primary antibody occurred for 24 h. One hundred micrograms of protein were used to ensure equal protein amounts in all wells.
Statistics
The Kruskal-Wallis and Dunn post hoc tests were done on cell culture data. A one-way ANOVA with the Student Newman-Keul post hoc test was used for animal data. p < 0.05 was considered statistically significant. A representative Western blot is shown where appropriate.
Results
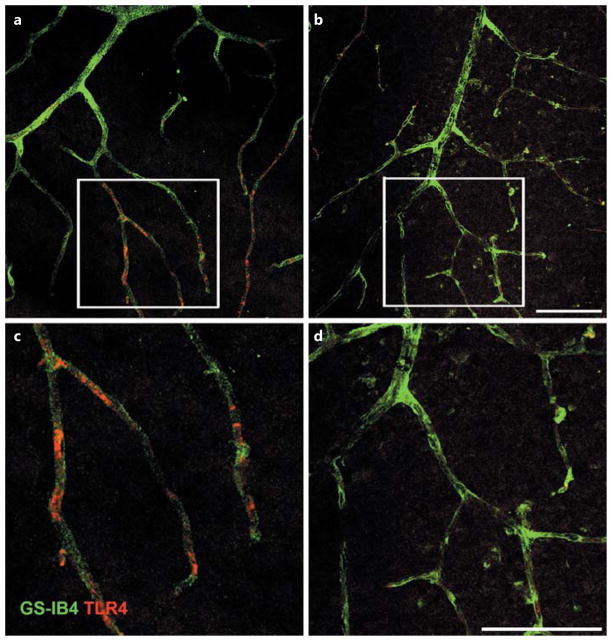
TLR4 Staining Is Reduced in the Vasculature of the TLR4 Cre-Lox Mice
Figure 1a, c shows significantly more staining for TLR4 (red) in the REC in the TLR4 floxed mice than in the TLR4 Cre-Lox mice (Fig. 1b, d). These data agree with the genotyping and Western blot results for whole retina reported previously [22].
Fig. 1.
Staining of TLR4 in whole-retina vascular mounts. TLR4 staining in the TLR4 floxed mice (a, c) versus the TLR Cre-Lox mice (b, d). Higher magnification images are provided for TLR4 floxed (c) and TLR4 Cre-Lox (d) mice. n = 3 in each group. Scale bar, 50 μm.
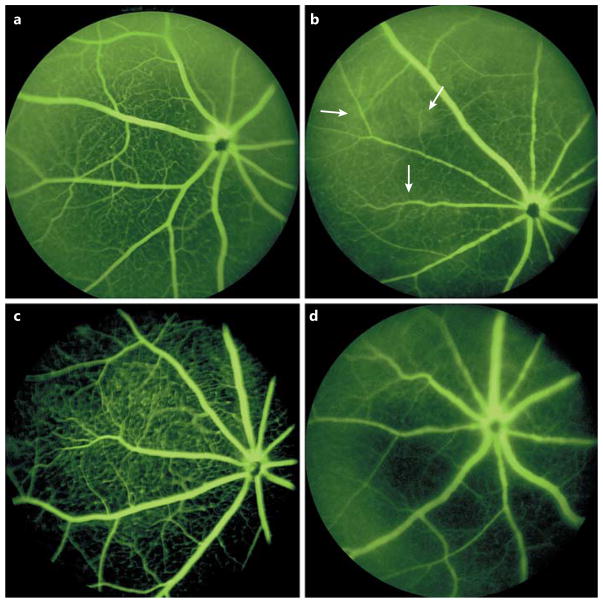
The Loss of TLR4 Decreased Retinal Vascular Permeability
Since inflammation is a key component of retinal stress, we subjected TLR4 floxed and EC-specific KO mice to I/R and measured retinal permeability. Fluorescein angiography was performed on the TLR4 floxed mice (control eye, Fig. 2a; treated eye, Fig. 2b) and the TLR4 Cre-Lox mice (untreated, Fig. 2c; exposed to I/R, Fig. 2d). The arrows show increased vascular leakage in the TLR4 floxed mice exposed to I/R compared to that observed in the TLR4 Cre-Lox mice.
Fig. 2.
Loss of TLR4 decreases retinal vascular permeability. Fluorescein angiography results for TLR4 floxed mice (a) or TLR4 floxed mice exposed to ischemia/repferfusion (I/R, b) and for TLR4 Cre-Lox (c) and TLR4 Cre-Lox (d) mice exposed to I/R. n = 4. Arrows point to areas of vascular leakage.
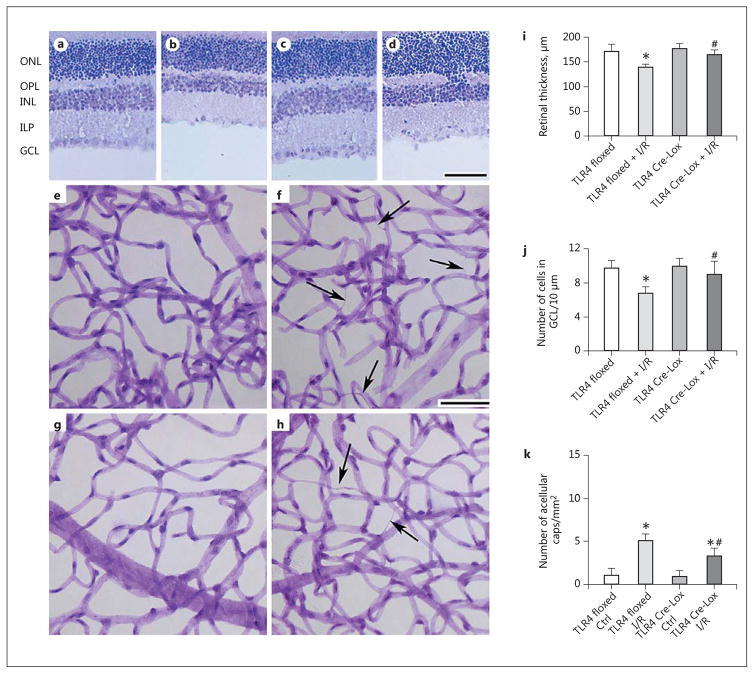
Loss of TLR4 Increases Retinal Thickness and Reduces Vascular Damage in the Mouse I/R Model
To further characterize the vascular-specific TLR4 Cre-Lox mice, we measured neuronal and vascular damage in the TLR4 floxed and Cre-Lox mice after exposure to I/R. Figure 3(a–d, i, j) show neuronal changes at 2 days post-I/R. There are no changes in the retinal thickness (Fig. 3i) or cell numbers in the GCL (Fig. 3j) in either the TLR4 floxed or TLR4 Cre-Lox mouse eyes not exposed to I/R. Following I/R, retinal thickness was reduced in the TLR4 floxed mice, but there was no change in the TLR4 Cre-Lox mice (Fig. 3i). Similar results were observed for the numbers of cells in the GCL (Fig. 3j). There was vascular damage in the control eyes (Fig. 3e, TLR4 floxed; Fig. 3g, TLR4 Cre-Lox) when compared to the eyes subjected to I/R (Fig. 3f, TLR4 floxed; Fig. 3h, TLR4 Cre-Lox). Figure 3k shows that there were significantly more degenerate capillaries in the TLR4 floxed mice than in the TLR4 Cre-Lox mice after I/R.
Fig. 3.
Loss of TLR4 increases retinal thickness and reduces the numbers of degenerate capillaries. a–d Neuronal thickness measurements in TLR4 floxed control (a), TLR4 floxed + I/R (b), TLR4 Cre-Lox (c), and TLR4 Cre-Lox + I/R (d) mice. e–h Degenerate capillary measurements in TLR4 floxed control (e), TLR4 floxed + I/R (f), TLR4 Cre-Lox control (g), and TLR4 Cre-Lox + I/R (h) mice. i Quantification of retinal thickness in a–d. j The number of cells in the GCL in a–d. k The quantification of the number of degenerate capillaries (caps) in e–h. Scale bar, 100 μm (neuronal data). f, h Arrows point at degenerate capillaries. n = 5 for all analyses. Bar graphs represent mean ± SEM. * p < 0.05 versus TLR4 floxed, # p < 0.05 vs. TLR4 floxed + I/R (i, j); # p < 0.05 versus TLR4 Cre-Lox Ctrl (k). ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
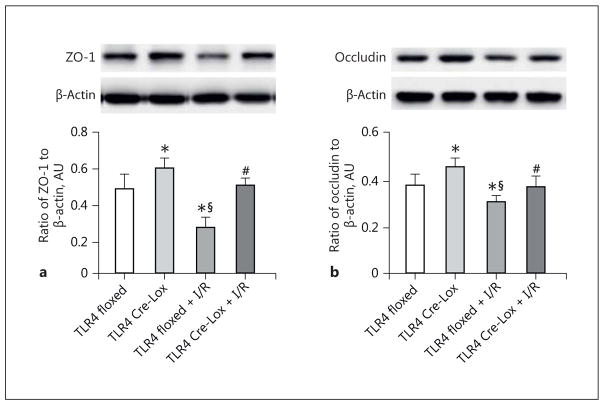
TLR4 Regulates ZO-1 and Occludin Levels in vivo
Since we found that loss of TLR4 in the EC led to reduced retinal permeability after I/R, we wanted to investigate the effects of TLR4 on ZO-1 and occludin levels. ZO-1 (Fig. 4a) and occludin (Fig. 4b) levels were increased in the TLR4 Cre-Lox mice retinas. Exposure to I/R reduced both ZO-1 and occludin levels in the TLR4 Cre-Lox mice, but levels were still significantly higher than in the TLR4 floxed mice exposed to I/R.
Fig. 4.
TLR4 regulates occludin and ZO-1 in vivo. Protein levels of ZO-1 (a) and occludin (b) from TLR4 floxed control, TLR4 Cre-Lox control, TLR4 floxed + I/R, and TLR4 Cre-Lox + I/R whole-retina lysates. * p < 0.05 versus TLR4 floxed, # p < 0.05 versus TLR4 floxed + I/R, § p < 0.05 versus TLR4 Cre-Lox. n = 5 for all groups. Data are mean ± SEM.
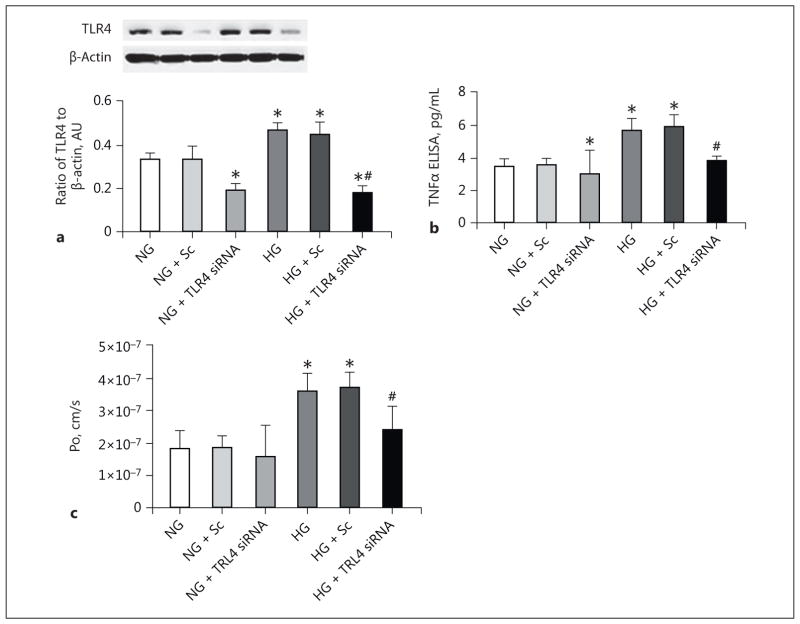
TLR4 Regulates TNFα and Permeability in REC in vitro
To support our data, we grew REC in normal- and high-glucose medium. We then treated a subset with either scrambled or TLR4 siRNA. To verify successful knockdown, we performed Western blotting for TLR4 on the cell lysates from each group (Fig. 5a). TLR4 siRNA had no effect on TNFα levels on REC grown in normal-glucose medium, but significantly reduced the levels in REC grown in high-glucose medium (Fig. 5b). High-glucose culturing conditions significantly increased REC permeability, which was then significantly reduced when TLR4 siRNA was applied (Fig. 5c).
Fig. 5.
Loss of TLR4 reduces TNFα and decreases permeability in retinal endothelial cells (REC) grown in high-glucose (HG) medium. REC were cultured in normal-glucose (NG) or HG medium and treated with scrambled siRNA (Sc) or TLR4 siRNA. a TLR4 levels. b TNFα levels. c HG increased REC permeability, which was blocked by TLR4 siRNA. Po, diffusive flux. * p < 0.05 versus NG, # p < 0.05 versus HG. n = 4 for all groups. Data are mean ± SEM.
Loss of TLR4 Increases ZO-1 and Occludin in REC in vitro
The levels of both ZO-1 (Fig. 6a) and occludin (Fig. 6b) were significantly reduced in REC grown in high-glucose medium. TLR4 siRNA application led to a significant increase in both ZO-1 and occludin levels in REC grown in high-glucose medium. These data, together with the mouse data, suggest that TLR4 regulates retinal permeability both in vitro and in vivo.
Fig. 6.
TLR4 regulates occludin and ZO-1 levels in vitro. Retinal endothelial cells (REC) were cultured in normal-glucose (NG) or high-glucose (HG) medium and treated with scrambled siRNA (Sc) or TLR4 siRNA. a ZO-1 levels. b Occludin levels. * p < 0.05 versus NG, # p < 0.05 versus HG. n = 4 for all groups. Data are mean ± SEM.
Discussion
Increased inflammation is a key complication of retinal damage [28]. Increased inflammatory mediators can lead to increased permeability in a multitude of targets [29]. Studies on the intestine, liver, and brain all suggest that increased TLR4 levels are highly related to increased permeability [14–16]. Additionally, TLR4 can regulate occludin levels in the colon, leading to increased permeability [15]. Similarly, a study on the intestine showed that methotrexate altered the ZO-1/claudin 4 interaction, which was associated with increased TLR4 mRNA [30].
Since it was found that TLR4 can regulate permeability in the diabetic rat retina [9], we wanted to investigate whether TLR4 in REC is a key to the changes in key barrier proteins both in vivo and in vitro. Our data showing that I/R caused an increase in permeability in the TLR4 floxed mice agree well with the diabetic rat retina data [9]. Loss of TLR4 in the EC was able to reduce retinal permeability in response to I/R. These changes in permeability matched the increased occludin and ZO-1 levels in the TLR4 conditional KO mouse retina. In support of our mouse data, our work on REC grown in high-glucose medium showed significantly increased permeability, which could be blocked by TLR4 siRNA. TLR4 also regulated ZO-1 and occludin levels in REC grown in high-glucose medium.
We extended the work on the role of TLR4 in retina permeability to demonstrate that TLR4 also regulates vascular and neuronal damage in response to I/R. Our work on TLR4 KO mice exposed to I/R showed that TLR4-deficient mice have reduced levels of key inflammatory mediators and increased survival of neurons in the GCL [20]. Other study groups have reported similar findings, i.e., that TLR4 knockdown was protective to the retina after LPS-induced damage in an I/R model [21]; work with this LPS model showed that LPS-induced microglia proliferation was reduced in TLR4 KO mice. Our findings demonstrate that I/R caused a significant reduction in retinal thickness and cell numbers in the GCL, but that this was restored to control levels in the EC-specific TLR4 KO mice. In agreement with the neuronal data, I/R caused a significant increase in the numbers of degenerate capillaries, which were reduced in the conditional KO mice. While these findings are specific to TLR4 in the retinal vasculature, it is established that TLR4 in retinal microglia and neurons likely play a role in permeability changes [20, 21]. Since we had reported that TLR4 is present in the diabetic mouse retina, we wanted to determine whether TLR4 could regulate retinal vascular permeability in response to other retinal stressors. Our findings suggest that TLR4 can indeed regulate permeability in the retinal vasculature, in addition to what was reported previously.
We also showed that the knockdown of TLR4 led to a decrease in TNFα levels in REC grown in high-glucose medium. Since TNFα can be activated by downstream signaling of TLR4 [13], we used TNFα as a demonstration that a loss of TLR4 would decrease high-glucose-mediated increases in inflammatory mediators in the REC.
In conclusion, the literature strongly supports a role for a TLR4-induced increase in permeability in a multitude of organs including the retina. Use of EC-specific conditional TLR4 KO mice exposed to I/R showed that TLR4 deficiency led to reduced permeability, which was associated with increased levels of occludin and ZO-1. REC grown in high-glucose medium showed increased permeability, which was blocked with the use of TLR4 siRNA. High-glucose culturing conditions also reduced occludin and ZO-1 levels in REC, which were then restored to normal by TLR4 siRNA. I/R also led to retinal neuronal and vascular damage, which was attenuated in the conditional KO mice. Taken together, these data suggest that TLR4 can mediate retinal damage in response to IR in vivo or (in high-glucose culture) in vitro, suggesting that the inhibition of TLR4 may provide a novel target for therapeutic development.
Acknowledgments
This work was supported by R01EY022330 (J.J.S.), P30EY04068, and an unrestricted grant to the Department of Ophthalmology from the Kresge Eye Institute (Research to Prevent Blindness).
Footnotes
Disclosure Statement
No authors have any conflicts of interest with these studies.
Author Contributions
Y.J. and L.L. were responsible for data collection, L.L. for the maintenance of the mice, and J.J.S. for the experimental design and for writing the paper. All authors reviewed the final text.
References
- 1.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 3.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kern TS, Miller CM, Du Y, et al. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56:373–379. doi: 10.2337/db05-1621. [DOI] [PubMed] [Google Scholar]
- 5.Joussen AM, Doehmen S, Le ML, et al. TNF-α-mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 6.Abcouwer SF, Lin CM, Shanmugam S, et al. Minocycline prevents retinal inflammation and vascular permeability following ischemia-eperfusion injury. J Neuroinflamm. 2013;10:149. doi: 10.1186/1742-2094-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Guy K, Pagadala J, et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest Ophthalmol Vis Sci. 2012;53:3004–3013. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YL, Wang K, Yu SJ, et al. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:389–398. doi: 10.1007/s00417-014-2832-y. [DOI] [PubMed] [Google Scholar]
- 10.Berger EA, Carion TW, Jiang Y, et al. β-Adrenergic receptor agonist, compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol Cell Biol. 2016;94:656–661. doi: 10.1038/icb.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollen KP, Anand RJ, Tsung A, et al. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 12.Leon CG, Tory R, Jia J, Sivak O, Wasan KM. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. Pharm Res. 2008;25:1751–1761. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Wang C, Nie J, et al. Toll-like receptor 4 increases intestinal permeability through upregulation of membrane PKC activity in alcoholic steatohepatitis. Alcohol. 2013;47:459–465. doi: 10.1016/j.alcohol.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Mir H, Meena AS, Chaudhry KK, et al. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim Biophys Acta. 2016;1860:765–774. doi: 10.1016/j.bbagen.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S, Gao W, Xu X, et al. Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res Bull. 2016;127:226–233. doi: 10.1016/j.brainresbull.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Peterson CY, Costantini TW, Loomis WH, et al. Toll-like receptor-4 mediates intestinal barrier breakdown after thermal injury. Surg Infect. 2010;11:137–144. doi: 10.1089/sur.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Shi H, Zhang J, et al. Toll-like receptor 4 in bone marrow-derived cells contributes to the progression of diabetic retinopathy. Mediators Inflamm. 2014;2014:858763. doi: 10.1155/2014/858763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol Vis. 2010;16:1907–1812. [PMC free article] [PubMed] [Google Scholar]
- 21.Halder SK, Matsunaga H, Ishii KJ, et al. Retinal cell type-specific prevention of ischemia-induced damages by LPS-TLR4 signaling through microglia. J Neurochem. 2013;126:243–260. doi: 10.1111/jnc.12262. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Jiang Y, Curtiss E, Fukuchi KI, Steinle JJ. TLR4 regulates insulin-resistant proteins to increase apoptosis in the mouse retina. Inflamm Res. 2017 doi: 10.1007/s00011-017-1080-0. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Jiang Y, Steinle JJ. Compound 49b restores retinal thickness and reduces degenerate capillaries in the rat retina following ischemia/reperfusion. PLoS One. 2016;11:e0159532. doi: 10.1371/journal.pone.0159532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinle JJ, Kern TS, Thomas SA, McFadyen-Ketchum LS, Smith CP. Increased basement membrane thickness, pericyte ghosts, and loss of retinal thickness and cells in dopamine beta hydroxylase knockout mice. Exp Eye Res. 2009;88:1014–1019. doi: 10.1016/j.exer.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Veenstra A, Liu H, Lee CA, et al. Diabetic retinopathy: retina-specific methods for maintenance of diabetic rodents and evaluation of vascular histopathology and molecular abnormalities. Curr Protoc Mouse Biol. 2015;5:247–270. doi: 10.1002/9780470942390.mo140190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC., Jr Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677. doi: 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang YS, Munn LL, Hillsley MV, et al. Effect of vascular endothelial growth factor on cultured endothelial cell monolayer transport properties. Microvasc Res. 2000;59:265–277. doi: 10.1006/mvre.1999.2225. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi K, Poulaki V, Doehmen S, et al. Contribution of TNF-α to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- 29.Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in diabetic retinopathy. Curr Diabetes Rev. 2007;3:3–14. doi: 10.2174/157339907779802139. [DOI] [PubMed] [Google Scholar]
- 30.Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol. 2013;72:757–765. doi: 10.1007/s00280-013-2238-2. [DOI] [PubMed] [Google Scholar]