Abstract
Purpose
Registries provide a unique tool for tracking quality of life in rare cancer survivors, whose survivorship experience is less known than for common cancers. This paper reports on these outcomes in 321 patients enrolled in the Rare Cancer Genetics Registry diagnosed with rare gastrointestinal, genitourinary, gynecologic, sarcoma, head/neck, or hematologic cancers.
Methods
Four outcomes were assessed, reflecting registrants’ self-reported physical and mental health, psychological distress, and loneliness. Combining all patients into a single analysis, regression was used to evaluate the association between outcomes and socio-demographic and clinical factors.
Results
Median time since diagnosis was 3 years (range 0–9); 69% were no longer in treatment. Poorer physical health was reported in registrants who were older at diagnosis, unmarried, and still in treatment. Poorer mental status was associated with younger diagnosis age and unmarried status. Psychological distress varied by cancer type, and was higher among currently treated and unmarried registrants. Greater loneliness was reported in registrants with gynecological cancers, and those who were less educated or unmarried. The physical and mental health profile of rare cancer survivors is similar to what is reported for common cancers.
Conclusions
Unmarried participants reported poorer outcomes on all measures of quality of life. Furthermore, physical and mental health were not significantly different by cancer type after adjustment for diagnosis age, whether currently in treatment, and marital status. Thus, the combined analysis performed here is a useful way to analyze outcomes in less common diseases. Our findings could be valuable in guiding evaluation and intervention for issues impacting quality of life.
Keywords: quality of life, SF-12, Brief Symptom Survey, loneliness, rare cancer
BACKGROUND
Cancer is the second most common cause of death in the US, accounting for nearly 1 of every 4 deaths. The National Cancer Institute estimates that approximately 15.5 million Americans with a history of cancer were alive on January 1, 2016 [1]. Each year, about one-half million Americans die of cancer and over 1.5 million more new cancer cases are diagnosed. Over 25% of these diagnoses will be rare cancers, defined in the US Rare Diseases Act of 2002 as those with a prevalence of less than 200,000 people in the US (less than 0.07% the population in 2015). Rare cancers account for about a fourth of cancer mortality, yet because each specific cancer affects such a small number of people, it is difficult to conduct research to advance treatment or understanding of the underlying causes and prognostic characteristics of these diseases. To remedy this research gap, the National Institutes of Health funded a Rare Cancer Genetics Registry in 2009. Registries provide a unique opportunity to collect information on patient reported outcomes of disease and treatment in survivors over the years after their diagnosis. The importance of this research has drawn attention in the recent literature [2].
The physical and mental health of cancer patients has been a concern of clinicians and researchers for many years and tools to evaluate quality of life have been developed and come into widespread use in clinical trials for cancer therapies [3–5]. Prior research has shown that clinically significant levels of emotional distress and physical impairments are common long-term effects of cancer treatment. For example, Carlson et al confirmed the prevalence of clinical levels of distress, including anxiety and depression, in 38% of all patients they assessed and found that the most common symptoms endorsed by patients were fatigue and pain [6]. Additionally, Gao et al reported that the overall prevalence of psychological distress in cancer patients ranged from 24.5% during or soon after treatment, dropping to 16.5% among cancer patients in the general community and rising markedly to 59.3% for those in specialist palliative care [7]. The National Comprehensive Cancer Network has established guidelines for management of cancer-related distress, recommending that distress be promptly identified and treated throughout the stages of the disease [8].
While previous publications have mostly focused on more common cancers such as breast, colorectal, lung and prostate cancer, there are limited data on physical and mental health status among patients with rare cancers. These patients face unique challenges as diagnosis can be difficult and thus delayed and treatment options can be limited and unclear. These challenges can affect the physical and emotional status of rare cancer patients and also result in a sense of isolation and hopelessness [9, 10].
It is challenging to gather a cohort with a specific rare cancer that is large enough to assess predictors of poor quality of life. However, since the strongest predictors of outcomes like physical and mental health are likely to be disease stage, age at diagnosis, sex, whether treatment is still underway, and social factors (like marriage), it is not unreasonable to study quality of life in an aggregated cohort containing multiple types of cancers. By combining patients with several different rare cancer diagnoses, identified from multiple cancer centers, it is possible to perform analyses that would not have been possible in each of the sparse subgroups defined by specific cancers.
The purpose of our study was to investigate self-reported quality of life outcomes of rare cancer diagnosis and treatment in a cohort of patients diagnosed with one of several rare cancer types using three well-established assessment tools. We focused on describing the patient-reported physical and mental health status, as well as psychological distress and loneliness in patients enrolled in a multi-center rare cancer registry and determining what characteristics (including primary site of disease) drive the differences we found. Such an analysis could identify which patients are at greatest risk for lower quality of life in order to guide evaluation and intervention for these issues.
METHODS
Population
The Rare Cancer Genetics Registry (RCGR) is a National Cancer Institute supported national registry of individuals with rare cancers with an accompanying family and medical history database, DNA and tumor tissue bank developed to enable research (www.rcgr.org). Since it was established in 2009, the RCGR has enrolled over 600 participants at six U.S. academic research centers, and is currently available as a resource for recruitment, planning, and analysis of rare cancer studies. Registrants were recruited using hospital tumor registries to identify individuals diagnosed with rare hematologic, genitourinary, gastrointestinal, head/neck, gynecologic cancers or sarcoma within the previous ten years. In general, a cancer was considered rare based on the incidence (<20 per hundred thousand population) rather than prevalence because some of the cancers studied have high cure rates (such as testicular, kidney, leukemia, lymphoma) so the prevalence does not meet the definition of a rare disease, but relative to the common cancers (such as breast cancer with an incidence of 125 per hundred thousand), these diseases are not common. Further, the treatment of these diseases often has a profound effect on quality of life.
At enrollment, registrants completed a mail or telephone survey, providing information on socio-demographic characteristics, personal and family cancer history, and cancer risk factors. In 2011, the RCGR began requesting that current and future members complete an assessment of their physical and mental health status, psychological distress, and loneliness. Physical and mental health summary scores were computed from registrants’ domain-specific responses to the SF-12v2 Health-Related Quality of Life Survey, using scoring software provided by QualityMetric [5, 11, 12] These norm-based T scores are characterized by a distribution with a mean of 50, a standard deviation (SD) of 10, and possible scores ranging from 0 to 100 with higher scores indicating better physical or mental health. Psychological distress scores were calculated according to Brief Symptom Inventory 18 (BSI-18) manual[13], with raw scores converted to standardized T scores (mean=50, SD=10, range: 33–81) using gender-specific community norms. Higher distress scores indicate greater psychological distress. Self-reported loneliness was assessed using a three-item loneliness scale developed by Hughes et al [14]. Loneliness scores were computed as the sum of each registrant’s responses to three loneliness questions which ranged from 1–3, with higher scores indicating a greater degree of loneliness (range: 3–9) [15, 14].
Statistical Methods
Univariate and multivariate regression models were used to assess associations between each patient-reported outcome (as a continuous variable) and socio-demographic characteristics (including gender, race/ethnicity, education level, marital status), and cancer-related variables (including cancer type, stage, age at diagnosis, years since diagnosis, family history of cancer and whether or not the registrant was currently receiving treatment). We group subjects who were married or living as married and refer to them as “married.” White and Asian races are combined throughout due to similar results and sparse Asian participation. Cancer stage is treated as a categorical variable with four levels: 0/I, II, III, and IV. Finally, we group cancers by anatomical site. For each outcome, a single analysis, combined across cancer types, was performed. The combined analysis allows comparisons within and between the various cancer sites and ensures greater power for the analysis. Variation due to type of cancer (e.g. genitourinary, hematologic, gynecologic, etc) was assessed as a categorical covariate in the models. Backwards selection was used to derive the final multivariate model for each outcome, by starting with variables that were significant (p<0.10) in the univariate analysis and removing them until only significant (p<0.05) variables remained. Registrants with missing or unknown values for a characteristic were excluded from analyses of the given characteristic. F-tests were carried out to evaluate whether mean scores varied by sub-groups defined by participant characteristics, with two-sided p-values less than 0.05 considered statistically significant. Estimated means and 95% confidence intervals for the means, obtained from univariate regression, were plotted and compared to the mean in the general population.
RESULTS
Registrants
Of the more than 600 enrollees in the Rare Cancer Genetics Registry (RCGR) in 2011, 321 completed the three quality of life assessments. These participants were diagnosed with hematologic (31%), genitourinary (21%), gastrointestinal (18%), sarcoma (15%), head/neck (11%) or gynecologic (4%) cancers. Registrant characteristics are presented in Table 1, and details of the types of diagnoses are provided in Table 2. The median age at cancer diagnosis was 58 years (range: 18–86) and the median time since diagnosis was three years (range: 0–9). Most registrants were not currently receiving treatment (69%), reported white race (88%), had at least a college degree (59%), were married (82%), and reported at least one first-degree relative affected with cancer (65%). The assessments were completed by most participants who entered after 2011 when this study began, but the study participation for subjects enrolled prior to 2011 was only 51%. The registrants who never completed the questionnaires were more likely to be more recently diagnosed, unmarried, non-white, and less likely to have a family history of cancer than those who completed the quality of life assessment (p < 0.05 via chi-square test for all comparisons). The completion rate was not related to age, stage at diagnosis, sex, or education level.
Table 1.
Participant Characteristics (N = 321)
| Characteristic | N (%) |
|---|---|
|
| |
| Cancer Type | |
| Sarcoma | 49 (15) |
| Hematologic | 99 (31) |
| Genitourinary | 68 (21) |
| Head & neck | 34 (11) |
| Gynecologic | 13 (4) |
| Gastrointestinal | 57 (18) |
| Bone | 1 |
|
| |
| Cancer Stage at Diagnosis | |
| 0/I | 51 (26) |
| II | 47 (24) |
| III | 51 (26) |
| IV | 48 (24) |
| Don’t know/missing | 124 |
|
| |
| Age at Diagnosis (years) | |
| < 50 | 71 (23) |
| 50 – 65 | 159 (51) |
| > 65 | 81 (26) |
| Unknown/missing | 10 |
|
| |
| Time since Diagnosis (years) | |
| < 2 | 45 (15) |
| 2 – 4 | 156 (52) |
| > 4 | 98 (33) |
| Unknown/missing | 22 |
|
| |
| Currently Receiving Treatment | |
| Yes | 94 (31) |
| No | 211 (69) |
| Unknown/missing | 16 |
|
| |
| Gender | |
| Male | 188 (59) |
| Female | 133 (41) |
|
| |
| Race | |
| White | 281 (88) |
| African American | 16 (5) |
| Asian | 8 (2) |
| Other | 14 (4) |
| Unknown/missing | 2 (1) |
|
| |
| Education | |
| College graduate or beyond | 187 (59) |
| Some college or technical school | 73 (23) |
| High school graduate or less | 59 (19) |
| Not sure/missing | 2 |
|
| |
| Marital Status | |
| Married or living as married | 260 (82) |
| Unmarried (never married, separated, divorced or widowed) | 57 (18) |
| Not sure/missing | 4 |
|
| |
| Family Cancer History | |
| No first degree relatives affected | 111 (35) |
| One or more affected first degree relatives | 210 (65) |
Table 2.
Details for Type of Rare Cancer Diagnosis
| Cancer Group & Type | N (% of group) |
|---|---|
|
| |
| Gastrointestinal (n=57) | |
| Esophagus | 34 (60) |
| Pancreas | 12 (21) |
| Stomach | 11 (19) |
|
| |
| Genitourinary (n=68) | |
| Kidney | 59 (87) |
| Testis | 9 (13) |
|
| |
| Gynecologic (n=13) | |
| Peritoneum | 6 (46) |
| Fallopian tube | 4 (31) |
| Ovary | 3 (23) |
|
| |
| Head/neck (n=34) | |
| Head/neck | 32 (94) |
| Thyroid | 2 (6) |
|
| |
| Hematologic (n=99) | |
| Myeloma | 74 (75) |
| Leukemia | 15 (15) |
| Lymphoma | 10 (10) |
|
| |
| Sarcoma | 50 (100) |
Patient-Reported Physical Health
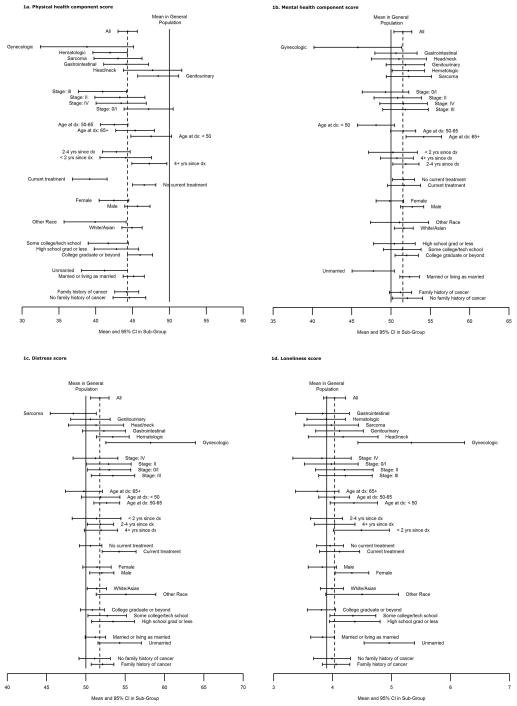
Univariate confidence bounds on the mean score for physical health broken out by participant characteristics are presented graphically in Figure 1a, and F-test p-values from univariate and multivariate analyses are shown in Table 3. Overall, the mean SF-12 physical health summary score among rare cancer registrants was 44.3 (95% CI: 43.0–45.6, range: 10.0–67.0), which is significantly lower than the average of 50 reported for the general population [11]. Univariate results indicated that mean physical health score was significantly better for subjects who were younger at diagnosis, had a greater time since diagnosis, not currently receiving treatment, male gender, white/Asian race, higher education level, and currently married. Physical health tended to be better among subjects with genitourinary and head/neck cancers and poorest among those with gynecologic cancers. The strongest univariate predictor of better physical health was not currently being treated for cancer (p<.0001). The results of the multivariate analysis showed that the significant independent predictors of better physical health were younger age at diagnosis (p=.02), being currently married (p=.02), and no longer receiving treatment (p<.0001). Cancer site did not significantly add to the model after these variables were included.
Figure 1.
Table 3.
Univariate and Multivariate Results for Characteristics Associated with Quality of Life Outcomes (N=321)
| Characteristic | SF-12 Physical Health | SF-12 Mental Health | BSI-18 Distress | Loneliness | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate P-value | Multivariate P-value | Univariate P-value | Multivariate P-value | Univariate P-value | Multivariate P-value | Univariate P-value | Multivariate P-value | |
| Cancer type | 0.002 | - | 0.4 | - | 0.02 | 0.04 | 0.08 | 0.05 |
| Stage at diagnosis | 0.07 | - | 0.6 | - | 0.7 | - | 0.6 | - |
| Age at diagnosis | 0.01 | 0.02 | 0.001 | 0.002 | 0.1 | - | 0.08 | - |
| Time since diagnosis | 0.01 | - | 0.5 | - | 0.9 | - | 0.1 | - |
| Current treatment | <.0001 | <.0001 | 0.9 | - | 0.006 | 0.04 | 0.4 | - |
| Gender | 0.02 | - | 0.01 | - | 0.6 | - | 0.009 | - |
| Race | 0.03 | - | 0.8 | - | 0.07 | - | 0.1 | - |
| Education | 0.02 | - | 0.6 | - | 0.2 | - | 0.02 | 0.02 |
| Marital status | 0.02 | 0.02 | 0.002 | 0.004 | 0.05 | 0.04 | <.0001 | <.0001 |
| Family cancer history | 0.8 | - | 0.5 | - | 0.4 | - | 0.7 | - |
Patient-Reported Mental Health
Univariate results for the association between participant characteristics and mental health score are presented in Figure 1b, and associated F-test p-values are shown in Table 3. The mean mental health summary score was 51.5 (95% CI: 50.4–52.6, range: 17.8–67.0) in rare cancer registrants, which is higher than the general population mean of 50 [11]. In the univariate analysis, mean mental health summary scores were positively associated with age at cancer diagnosis, and were higher among men and in those who were married. Although the mean mental health score was substantially lower among registrants with gynecologic cancers compared to the other cancer types, the variation in mental health score by cancer type was not statistically significant in the univariate comparison. Multivariate analysis showed that the significant independent predictors of better mental health were older age at diagnosis (p=.002) and married status (p=.004). Cancer site did not significantly add to the model after these variables were included.
Psychological Distress
Results for the analysis of psychological distress are shown in Figure 1c and Table 3. In general, registrants’ mean psychological distress score (51.8, 95% CI: 50.6–52.9, range: 33–78) was higher than in the general population. In both the univariate and multivariate analyses, mean psychological distress score varied significantly by cancer type (p=.04), with sarcoma patients reporting the least distress (significantly less than the population mean of 50) and registrants with gynecologic cancers reporting the highest distress levels (significantly more than the population mean of 50). Additional significant predictors of lower distress included whether the participant was not currently receiving treatment (p=.04) and being currently married (p=.04). The difference by cancer site could reflect the greater proportion of late stage diagnosis among the registrants with gynecologic cancer compared to registrants with other cancer types.
Loneliness
Results for the analysis of loneliness are plotted in Figure 1d and univariate and multivariate p-values are presented in Table 3. In general, rare cancer registrants did not have significantly elevated loneliness scores (mean: 4.0, 95% CI: 3.8–4.2, range: 3–9) compared to a large reference population of ambulatory individuals aged 55 years and older (mean=3.9). Multivariate findings suggest that mean loneliness score was significantly associated with cancer type (p=.05), with women diagnosed with gynecologic cancers reporting higher levels of loneliness compared to the other cancer types; the mean loneliness score among registrants with gynecologic cancers (5.3) was also significantly higher than the mean in the general population (3.9). Other significant predictors of higher loneliness from the multivariate analysis were lower educational level (p=.02) and not being married (p<.0001).
DISCUSSION
The study of outcomes in patients with rare cancer is challenging as there is sparseness in these populations and few opportunities to contact the survivors years after their diagnosis. We overcame this issue by building a rare cancer registry and mounting a study to obtain patient reported outcomes from three published measures of quality of life. In addition, by combining the data from registrants with several types of cancer it was possible to compare within and between the various cancer sites and ensure greater power for the analysis. This population was several years from diagnosis (and usually from treatment), and thus it is reasonable to assume that functional and psychological outcomes of disease and treatment would be predicted by clinical and social/demographic characteristics (years since diagnosis etc) more than by the original site of the tumor. This has been reported in the literature. For example, a study of cancer survivors two years from diagnosis found that while cancer type impacted survivors’ physical health-related quality of life, type of cancer was not associated with mental health score [16].
The combined analysis showed that while the rare cancer registrants reported worse physical health (mean=41) and greater psychological distress (mean=52) than the general population (as expected), their overall mental health (mean=51) was better [17]. We speculate that the mental health scores in our study were slightly higher than in the general population because the RCGR population has several characteristics that are associated with better quality of life—e.g. greater proportions of college graduates, married, and white/Asian race than in the general population. However, our results were similar to those reported for the general cancer population (mean of 41 for physical health and 47 for mental health) [12]. Similarly, another study assessing these measures in a mixed cancer population at a mean of 3.3 years since diagnosis reported comparable results (mean physical health score of 43 and mean mental health score of 52) [17]. This has also been reported for other more common cancers. For example, [18] indicates that among colorectal cancer survivors in their survey, the mean mental health score was 52 while the mean physical health score was 41 from the SF-12 survey, and [17] found that breast cancer survivors had a mean physical health score of 43 and a mean mental health score of 51 from the SF-36 measure (which has the same norms as the SF-12). These mean scores are provided in a table in the supplement.
We found that currently unmarried registrants, including those never married, separated, divorced or widowed, had significantly poorer scores for all four outcomes. This has been reported in studies of long-term survivors (5+ years) of common cancers, which have reported that lack of social support in general is associated with poorer quality of life [19, 17]. Furthermore, this result suggests that increased psychological surveillance may be warranted for unmarried rare cancer patients.
Distress and loneliness varied significantly by type of cancer, even after controlling for differences in the clinical and demographic characteristics between cancer type groups. This difference was primarily driven by gynecologic cancer patients who reported markedly higher levels of distress and loneliness compared to the other cancer types, and the association remained significant in multivariate analysis despite the relatively small number of gynecologic cancers and after adjustment for the generally higher disease stage in the subgroup. Other studies have reported higher levels of distress in gynecologic cancers compared to other cancers, and findings on the association between distress and disease stage are mixed, with some studies reporting higher distress in patients with more advanced disease and others finding no association [20, 9].
We found that physical health scores were worse for patients who were older at diagnosis, while mental health scores were better for older patients. Registrants who were aged 65 or older at diagnosis reported mental health scores (mean=54) that were significantly higher compared to the younger patients in our study (mean=48 for patients under 50 at diagnosis) as well as the general population (mean=50). Distress and loneliness scores were also lowest in the ≥65 group, with mean values comparable to those in the general population. This is consistent with the current literature, which has shown that older survivors are likely to be more affected by cancer in terms of physical function but seem to be coping better than their younger counterparts [21].
Our study had many limitations. The cross-sectional design does not allow us to examine temporal trends in quality of life following a rare cancer diagnosis. The response rate among our registrants was a little over half of the 600 RCGR registrants. However, this was primarily due to the timing of the conduct of this study (after they had enrolled in RCGR); the completion rate was nearly 100% in subjects who entered after 2011 and this was probably not a source of bias since we do not think there is an era effect in the registry. Nevertheless, as is the case with most studies conducted in cancer survivor populations, survivorship bias cannot be ruled out since patients had to survive long enough to be enrolled in the registry and, for those who were asked to complete the quality of life assessment after initial enrollment, long enough to enter this study. In addition, the combined multivariate analysis of these registrants, while necessary for the power to detect predictors of these outcomes, could be criticized for combining patients who are different due to the symptoms of the original disease as well as the specific treatments they were given. However, the site of cancer was included in the model, and we note that psychiatric interventions, physical therapy etc are often provided to patients over a spectrum of primary cancer sites. Finally, although we found statistically significant differences in these outcomes, it is arguable whether they represent clinically meaningful differences, given that none of the overall or sub-group means was further than one standard deviation from the mean in the general population.
CONCLUSIONS
The Rare Cancer Genetics Registry provided an opportunity to get a view into the quality of life of survivors of rare cancers. The method we chose, to analyze the aggregated data, was reasonable since cancer site was at most a weak predictor in the measures of quality of life that we studied. This method could be useful in studies of rarer cancers (with even sparser data) as well. We found that the physical and mental health profiles of rare cancer survivors are similar to what is reported for common cancers. Participants without spousal support (especially those currently in treatment) reported poorer outcomes on all measures, suggesting the need for monitoring psychological status in this subgroup. Our findings could be useful in guiding assessment, clinical evaluation and intervention for issues impacting quality of life in cancer survivors.
Supplementary Material
Implications for cancer survivors.
Rare cancer survivors, particularly those without spousal support, should be monitored for challenges to the physical as well as psychological aspects of quality of life.
Acknowledgments
the authors would like to thank the Rare Cancer Genetics Registry participants and project managers, and the NIH for funding this project.
Funding: NIH grants RC1 CA 144706 and R01 CA160233
Footnotes
Authors’ Contributions NH, DMF data analysis, NH, AM, DMF manuscript writing, JL, SD, PM, CG, CI, AYK, DMF recruitment and development of idea and tools, KV manuscript review
Conflict of Interest: the authors declare no potential conflict of interests.
Dedication: The article is dedicated to Dr. Connie Griffin who did not live to see the importance of her contributions to the Rare Cancer Genetics Registry.
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Thong MS, Mols F, Stein KD, Smith T, Coebergh JW, van de Poll-Franse LV. Population-based cancer registries for quality-of-life research: a work-in-progress resource for survivorship studies? Cancer. 2013;119(Suppl 11):2109–23. doi: 10.1002/cncr.28056. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–9. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Testa MA, Simonson DC. Assesment of quality-of-life outcomes. N Engl J Med. 1996;334(13):835–40. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 5.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90(12):2297–304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao W, Bennett MI, Stark D, Murray S, Higginson IJ. Psychological distress in cancer from survivorship to end of life care: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46(11):2036–44. doi: 10.1016/j.ejca.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 8.NCCN.org. 2015. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): NCPGiON. Distress Management. [Google Scholar]
- 9.Roland KB, Rodriguez JL, Patterson JR, Trivers KF. A literature review of the social and psychological needs of ovarian cancer survivors. Psychooncology. 2013;22(11):2408–18. doi: 10.1002/pon.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross AH, Cromwell J, Fonteyn M, Matulonis UA, Hayman LL. Hopelessness and complementary therapy use in patients with ovarian cancer. Cancer Nurs. 2013;36(4):256–64. doi: 10.1097/NCC.0b013e31826f3bc4. [DOI] [PubMed] [Google Scholar]
- 11.Ware J, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12 Health Survery Lincoln, RI. QualityMetric Incorporated; Lincoln MA: 2004. [Google Scholar]
- 12.Maruish M, editor. User’s manual for the SF-36v Health Survery. 3. Lincoln, MA: QualityMetric Incorporated; 2011. [Google Scholar]
- 13.Derogatis L, editor. Minneapolis NCS Pearson. 2000. BSI 18 Brief Symptom Inventory 18, administration, scoring, and procedures manual. [Google Scholar]
- 14.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A Short Scale for Measuring Loneliness in Large Surveys: Results From Two Population-Based Studies. Res Aging. 2004;26(6):655–72. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39(3):472–80. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- 16.Le Corroller-Soriano AG, Bouhnik AD, Preau M, Malavolti L, Julian-Reynier C, Auquier P, et al. Does cancer survivors’ health-related quality of life depend on cancer type? Findings from a large French national sample 2 years after cancer diagnosis. Eur J Cancer Care (Engl) 2011;20(1):132–40. doi: 10.1111/j.1365-2354.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 17.Parker PA, Baile WF, de Moor C, Cohen L. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003;12(2):183–93. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- 18.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16(8):691–706. doi: 10.1002/pon.1208. [DOI] [PubMed] [Google Scholar]
- 20.Norton TR, Manne SL, Rubin S, Carlson J, Hernandez E, Edelson MI, Rosenblum N, Warshal D, Bergman C. Prevalence and predictors of psychological distress among women with ovarian cancer. J Clin Oncol. 2004;22(5):919–26. doi: 10.1200/JCO.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113:3519–3529. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.