Abstract
Hyperglycemia is a significant risk factor for diabetic retinopathy and induces multiple biochemical changes, including inflammation and endothelial dysfunction in the retina. Alterations in microRNA expression have been implicated in the pathological responses of diabetic retinopathy and the manipulation of microRNA may provide powerful strategy for therapeutics. Among the predicted targets of miR-15a and -16 are TGF-beta3, SMAD2/3, and VEGF, all of which are known to play a role in vascular endothelial functions. The purpose of this study was to investigate the hypothesis that miR-15a/16 inhibits TGF-beta3/VEGF signaling to maintain retinal endothelial cell barrier protein levels. Human primary retinal endothelial cells (REC) were maintained in normal (5 mM) glucose or transferred to high glucose medium (25 mM) for 3 days. REC were transfected with miRNA mimics (hsa-miR-15a-5p and -16-5p). Retinal lysates from miR-15a-transgenic mice were also analyzed. We demonstrated that overexpression of miR-15a/16 resulted in decreased TGF-beta3 signaling and VEGF levels in cultured REC grown in high glucose conditions. In addition, the levels of tight junction proteins, zonula occludens-1 (ZO-1) and occludin, were elevated in REC following overexpression of miR-15a and -16. Overexpression of miR-15a and - 16 played a role in reducing cellular permeability through inhibition of VEGF signaling in REC cultured under high glucose conditions. Using miR-15a-transgenic mice, we demonstrated the regulatory role of miR-15a on TGF-beta3 signaling and tight junction proteins in vivo. Our outcomes suggest that miR-15a/16 maintain the retinal endothelial cell barrier by reducing TGFbeta3/VEGF signaling and increasing levels of key tight junction proteins.
Keywords: miR-15a/16, TGF-beta3, SMAD2/3, VEGF, Retinal endothelial permeability
1. Introduction
Diabetic retinopathy is the primary cause of visual loss in working age adults (Fante, Durairaj, & Oliver, 2010). Prolonged hyperglycemia is the key factor in the progression of diabetic retinopathy (Engerman & Kern, 1986; Klein, Klein, Moss, & Cruickshanks, 1994; Nyengaard, Ido, Kilo, & Williamson, 2004). Hyperglycemia-induced pathological responses in the retina include pericyte loss, which can result in retinal endothelial cell (REC) dysfunction and breakdown of the blood retinal barrier (BRB) (Shin, Sorenson, & Sheibani, 2014). Molecular mechanisms underlying the pathology of hyperglycemia and diabetic retinopathy have been identified (Devi et al., 2012; Jiang, Pagadala, Miller, & Steinle, 2013; Jiang, Zhang, Soderland, & Steinle, 2012; Ye & Steinle, 2015; Zhang et al., 2013), but the pathological mechanisms are still not completely understood. A better understanding of cellular signaling and associated molecular mediators is required to develop effective and reliable therapeutics for diabetic retinopathy. microRNA (miRNA) are ubiquitously expressed noncoding molecules, with cell- and tissue-specific functions. It is established that miRNA binds to 3′ untranslated regions (3′UTR) of target mRNA to reduce gene translation, however they can also activate translation of target genes under growth arrest (Vasudevan, Tong, & Steitz, 2007). The role of miRNA has come to light in pathophysiology, as increasing numbers of studies reveal the regulatory functions of miRNA in a variety of diseases, including diabetic retinopathy (Fulzele et al., 2015; Hill & Lukiw, 2016; Maciotta, Meregalli, & Torrente, 2013; Yang et al., 2015). miRNA can be a useful and easily accessible biomarker to diagnose diseases, and the expression profiles of miRNA in diabetic retinas have been recently reported (Wu et al., 2012; Xiong et al., 2014). However, we have a limited amount of information which miRNAs are involved in the pathology of diabetic retinopathy and how they may regulate molecular pathways in the retina.
The dysfunction of vascular endothelial cells and inflammatory pathways are clearly affected by diabetes and hyperglycemia (Ceriello, Esposito, Ihnat, Thorpe, & Giugliano, 2010; Joussen et al., 2001; Saker, Stewart, Browning, Allen, & Amoaku, 2014). Increased levels of TGF-beta were shown in human retinal endothelial cells in high glucose conditions (Pascal, Forrester, & Knott, 1999). In addition, rhTGF-beta1 increased the permeability of bovine retinal endothelial cells in vitro by stimulating MMP-9 production (Behzadian, Wang, Windsor, Ghaly, & Caldwell, 2001). The role of TGF-beta in basal lamina thickening was also shown in bovine retinal endothelial cells and pericytes (Van Geest, Klaassen, Vogels, Van Noorden, & Schlingemann, 2010).
TGF-beta3 and SMAD2/3/4 are predicted targets of miR-15a-3p (targetscan). In addition, miR-16 is predicted to target TGF-beta receptor 3 and SMAD3/5 (targetscan). Little is known about the association of miR-15a/16 with TGF-beta3 in the pathology of diabetic retinopathy. Studies on other groups of miRNA have shown that miR-152 (Haque, Hur, Farrell, Iuvone, & Howell, 2015) and miR-200b (Jiang, Zhao, Liu, Li, & Liu, 2015) significantly downregulated TGF-beta1 levels in human REC under high glucose conditions.
TGF-beta3, as well as TGF-beta1/2, are potent inducers of VEGF secretion by human retinal pigmented epithelial cells (Nagineni et al., 2003). VEGFA is also a direct target of miR-15a/16 (Sun et al., 2013). It was shown that TGF-beta signaling mediated an increase in VEGF in ARPE-19 cells under hyperglycemic conditions (Grigsby, Betts, Vidro-Kotchan, Culbert, & Tsin, 2012). Increased levels of VEGF signaling have also been shown in diabetic retina (Witmer et al., 2002) and the blockade of VEGFR1 suppressed retinal leukostasis, vascular leakage, and disorganization of zonula occludens-1 (ZO-1) (He et al., 2015).
We have little understanding about the regulatory roles of miR-15a and -16 on VEGF in diabetic retinopathy. Only one study has reported a regulatory role of miR-15a on VEGF in pathological responses of diabetic retinopathy (Wang et al., 2016). Other types of miRNAs have been identified for their roles on VEGF in diabetic retinopathy. Both miR-152 (Haque et al., 2015) and miR-200b (Jiang et al., 2015) suppressed VEGF expression in human REC under high glucose conditions. In addition, a negative correlation of miR-126 with VEGF signaling was found in the retinas of STZ-induced diabetic rats (Wang & Yan, 2016) and topical delivery of miR-106a mimic reduced VEGF levels in the retina of STZ-diabetic mice (Ling et al., 2013).
The breakdown of BRB is associated with inflammatory responses, including the secretion of vascular permeability factors and pro-inflammatory cytokines, and alterations of tight junction proteins (Rangasamy, McGuire, & Das, 2012). In diabetic retina, increased vascular permeability often involves decreased tight junction proteins, zonula occludens-1 (ZO-1) and occludin (Kim, Kim, Jun, Yu, & Kim, 2010; Leal et al., 2007). Additionally, TGFbeta plays a role in downregulation of tight junction proteins in epithelial-to-mesenchymal transitions (Barrios-Rodiles et al., 2005; Krizbai et al., 2015).
Much less is known of miR15a/16 regulation of TGF-beta3 and VEGF signaling in diabetic retinopathy. Therefore, in the present study we investigated the hypothesis that miR-15a/16 reduces TGF-beta3 and VEGF signaling, thus maintaining the retinal endothelial cell barrier.
2. Materials and methods
2.1. Cell culture
Cell culture was done as stated in our previous study (Ye & Steinle, 2015). Briefly, human primary REC were purchased from Cell Systems Corporation (CSC, Kirkland, WA) and grown in M131 medium containing microvascular growth supplement (Invitrogen), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B. Cells were maintained in normal (5 mM) glucose or transferred to high glucose medium (25 mM) (Cell Systems) for 3 days. Cells were quiesced by incubating in high or normal glucose medium without growth supplementation for 20 h prior to experimentation.
2.2. Cell transfection with microRNA-mimics
As described in our previous study (Ye & Steinle, 2015), transfection was performed on REC with miRNA mimic (hsa-miR-15a-5p and hsa-miR-16-5p) (Invitrogen, Carlsbad, CA) using Oligofectamine (Invitrogen) following manufacturer’s instructions. Cells were transfected 48 h before protein extraction. A final concentration of 30 nM was used when transfected separately (miR-15a and -16) and 15 nM was used in combination (miR-15a + miR-16). A 30 nM Mimic Negative Control (Invitrogen) was transfected into REC cultured in high glucose as a control. Other control groups, normal glucose (NG) and high glucose (HG), were treated with Oligofectamine only.
2.3. Western blot analysis
Changes of protein levels were examined by Western blotting, as described in our previous study (Ye & Steinle, 2015). After transfer, the membrane was incubated with appropriate primary antibodies followed by treatment with secondary antibodies labeled with horseradish peroxidase. Antigen-antibody complexes were detected by chemilluminescence reagent kit (Thermo Scientific, Pittsburgh, PA). Primary antibodies used were TGF beta3, phosphorylated SMAD2/3, and SMAD2/3 (rabbit monoclonal, 1:500; all purchased from Cell Signaling, Danvers, MA), VEGF, ZO-1, and occludin (all from Abcam, Cambridge, MA), and beta actin (Santa Cruz Biotechnology, Santa Cruz, CA). Imaging was performed using C500 system (Azure Biosystems, Dublin, CA) within a linear range of exposure. Beta actin was used to normalize signal intensity of protein bands.
2.4. Transendothelial permeability assay
REC were maintained in normal (5 mM) glucose or transferred to high glucose medium (25 mM) (Cell Systems) and transfected with miRNA mimic (hsa-miR-15a-5p or hsa-miR-16-5p; 100 nM of a final concentration (Wang et al., 2016)) (Invitrogen, Carlsbad, CA) using GeneMute transfection reagents (Signagen Laboratories) following manufacturer’s instructions. The final concentration of miRNA mimic was modified to 100 nM to examine the effects of miR-15a on transendothelial permeability based on a previous study (Wang et al., 2016). The following day, transfected cells were plated onto fibronectin-coated transwell inserts and cultured to form a monolayer. Exogenous rhVEGF was treated onto REC for 2 h at a final concentration of 10 ng/ml. Hydrocortisone (100 nM final concentration) was added to the cells for the following two days, followed by RITC-dextran (10 μM of a final concentration) on the final day. Media was collected from the bottom wells every 30 min for 4 h after adding dextran. The rate of diffusive flux was calculated by the following formula (Antonetti & Wolpert, 2003):
Po = diffusive flux (cm/s), FA = basolateral fluorescence, FL = apical fluorescence,
Δt = change in time, A = surface area of the filter (cm2), VA = volume of the basolateral chamber (cm3). Background was subtracted using a control group that included media only and no RITC-dextran.
2.5. miR-15a-transgenic mice
The Tie2-miR-15a-transgenic (TG) mice and corresponding background control C57BL/6 mice were provided by Dr. Chen’s lab (University of Michigan, Ann Arbor, MI). At 3 months of age, retinas of miR-15a-TG and wild-type mice were used for Western blot analysis. All animal procedures were reviewed and approved by the Institute Animal Care and Use Committees of the Wayne State University School of Medicine (Protocol # A 11-08-14) and conform to NIH guidelines.
2.6. Genotyping analysis
We performed genotyping analysis as stated in our previous study (Ye et al., 2016). Genomic DNA from ear punches of 2-week-old mice was digested with one step tail DNA extract buffer (100 mM Tris, 5 mM EDTA, 200 mM NaCl, 1% Triton) plus proteinase K (10 mg/ml) at 55 °C overnight and then heat-inactivated at 85 °C for 45 min. Sequences of primer pairs used to screen the transgenic mice were as follows: miR-15a forward: GTC CTC ATC GCA TAC CAT ACA, reverse: GCT GAA GTA AGG TTG GCA ATA. Standard PCR reaction protocol was completed using KAPA2G HotStart Genotyping PCR Mix (KK5621, KAPA Biosystems). The steps of the PCR reaction were as follows: denature: 95 °C 5 min, 35 cycles at 94 °C, 30 s, 61 °C 30 s, 72 °C 40 s, then final extension at 72 °C 10 min.
2.7. Statistics
Data were statistically analyzed using Prism software (Graph-Pad, La Jolla, CA). Unpaired Student t test with two-tailed p value was used for analyses. P values less than 0.05 were considered to be significant. Data are presented as mean ± SEM. For Western blot data, a representative blot is presented.
3. Results
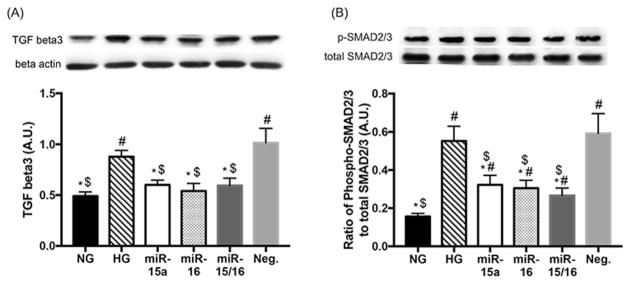
3.1. miR-15a/16 suppressed TGF-beta3 and SMAD2/3 signaling in high glucose conditions
A previous study has shown that TGF-beta levels were elevated in human REC in high glucose conditions (Pascal et al., 1999). It is predicted that TGF-beta3 and SMAD2/3/4 are targets of miR-15a-3p, and miR-16 targets TGF-beta receptor 3 and SMAD3/5 (targetscan). Additionally, our previous studies have demonstrated decreased miR-15a and -16 expression in REC cultured under high glucose conditions (Ye et al., 2016; Ye & Steinle, 2015). Thus, in this study, we increased the expression of miR-15a and -16 through transfection with miRNA mimics to examine the effects of miR-15a/16 on TGF-beta3 signaling in REC under high glucose conditions. Our results showed that overexpression of miR-15a and -16 resulted in decreased levels of TGF-beta3 (Fig. 1A) and SMAD2/3 signaling (Fig. 1B) in REC under high glucose conditions. That suggests that miR-15a/16 inhibit TGF-beta3 and SMAD2/3 actions in REC under high glucose conditions.
Fig. 1.
Effects of miR-15a/16 on TGF-beta3 and SMAD2/3 levels in REC. REC were cultured in normal glucose (5 mM, NG) or high glucose (25 mM, HG). Transfection with miR-15a, -16, and Neg. mimics were performed under HG conditions. HG increased levels of TGF-beta3 (A) and SMAD2/3 phosphorylation (B) as compared to NG. REC transfected with miR-15a and/or -16 mimics (labeled as miR-15a, miR-16, and miR-15a/16) had reduced levels of TGF-beta3 (A) and SMAD2/3 phosphorylation (B), compared to that of control groups (HG and Neg.). Neg. control indicates REC transfected with a negative miR-mimic. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg. control, N = 4; Data are mean ± S.E.M.
3.2. miR-15a/16 decreased VEGF levels in high glucose conditions
Increased levels of VEGF have been shown in diabetic retina (Witmer et al., 2002), and VEGF signaling can be induced by TGF-beta pathway (Grigsby et al., 2012; Nagineni et al., 2003). Our results showed that the levels of VEGF were decreased when miR-15a and -16 were overexpressed in REC cultured under high glucose conditions (Fig. 2). This finding suggests that miR-15a/16 may directly or indirectly target VEGF in REC, which agrees with work by Wang et al., 2016 (Wang et al., 2016).
Fig. 2.
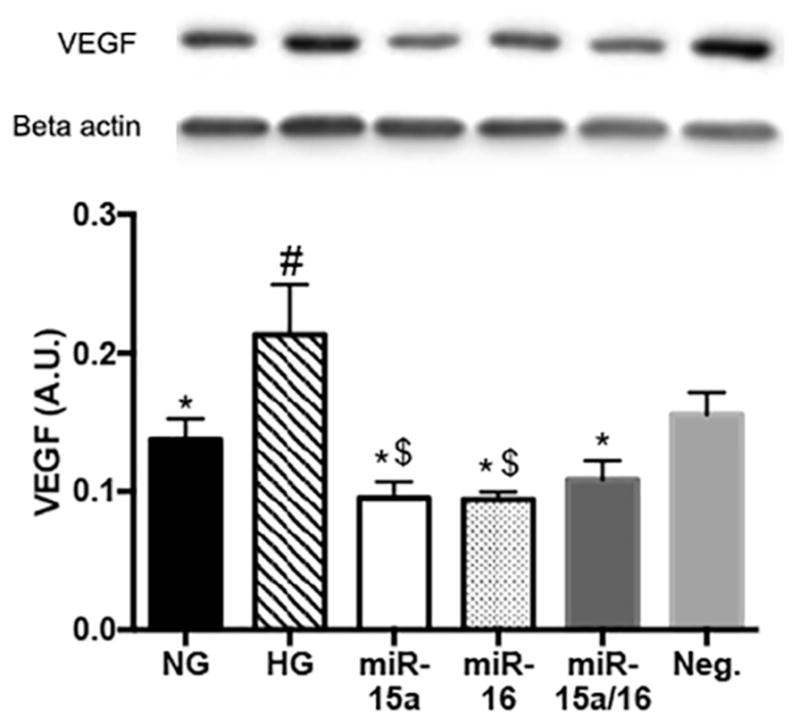
Changes of VEGF levels in high glucose conditions. Western blot results for VEGF on REC. Transfection with miR-15a, -16, and Neg. mimics were performed under HG conditions. VEGF levels, which were elevated in control HG condition, but were reduced when REC was transfected with miR-15a and/or -16 mimics (labeled as miR-15a, miR-16, and miR-15a/16) as compared to that of control groups (HG and Neg.). #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg. control, N = 3; Data are mean ± S.E.M.
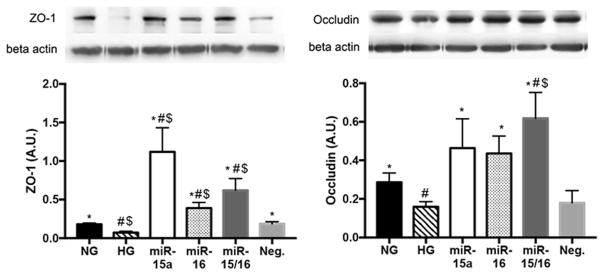
3.3. miR-15a/16 increased the levels of ZO-1 and occludin in REC under high glucose conditions
In diabetic retina, increased vascular permeability often involves a decrease of key tight junction proteins, ZO-1 and occludin (Kim et al., 2010; Leal et al., 2007). Our results showed that overexpression of miR-15a/16 significantly increased the levels of tight junctions proteins ZO-1 (Fig. 3A) and occludin (Fig. 3B) in REC in high glucose conditions. We suggest that miR-15a/16 may play a role in the elevation of ZO-1 and occludin levels, potentially through inhibition of VEGF levels in REC.
Fig. 3.
Elevated levels of ZO-1 and occludin in REC. Western blot results for tight junction proteins on REC. Transfection with miR-15a, -16, and Neg. mimics were performed under HG conditions. HG decreased the levels of ZO-1 (A) and occludin (B) as compared to NG. REC transfected with miR-15a and/or -16 mimics (labeled as miR-15a, miR-16, and miR-15a/16) had increased levels of ZO-1 (A) and occludin (B), compared to that of control groups (HG and Neg.). #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg. control, N = 4; Data are mean ± S.E.M.
3.4. miR-15a/16 reduced endothelial cell permeability through inhibiting VEGF in high glucose conditions
Previous studies have shown a regulatory role for VEGF on endothelial barrier functions in vitro (Fischer et al., 1999; Qin, Liu, Liu, Li, & Ren, 2016). Additionally, the blockade of VEGFR1 suppressed vascular leakage and disorganization of ZO-1 (He et al., 2015). As we found the regulatory role of miR-15a/16 on the levels of TGF-beta3, VEGF, and tight junction proteins, we examined the effects of miR-15a/16 on the permeability of endothelial cells in vitro (Fig. 4). High glucose only significantly increased permeability in REC, and rhVEGF furthered the high glucose-induced increase in permeability. However, REC treated with miR-mimics (miR-15a and -16) and rhVEGF had reduced permeability as compared to HG only and HG + VEGF groups. Therefore, the results suggest that miR-15a/16 decreased retinal endothelial cell permeability under high glucose conditions in vitro, potentially through suppression of VEGF signaling.
Fig. 4.
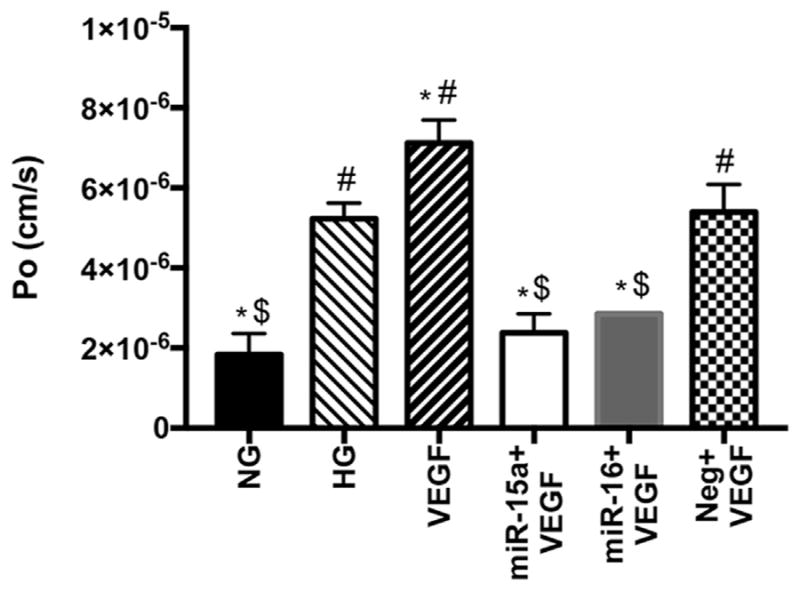
Transendothelial permeability assay using REC. Treatments with miR-mimics and/or VEGF were done in HG conditions. The rate of diffusive flux (Po) was calculated from the absorbance measured at 30, 60, 90, 120, 150, 180, 210, and 240 min after adding RITC-dextran onto REC monolayer. REC transfected with miR-15a and -16 showed reduced levels of Po in high glucose conditions, compared to HG only and VEGF-treated groups. *p < 0.05 versus HG, $p < 0.05 versus Neg., #p < 0.05 versus NG, N = 3; Data are mean ± S.E.M.
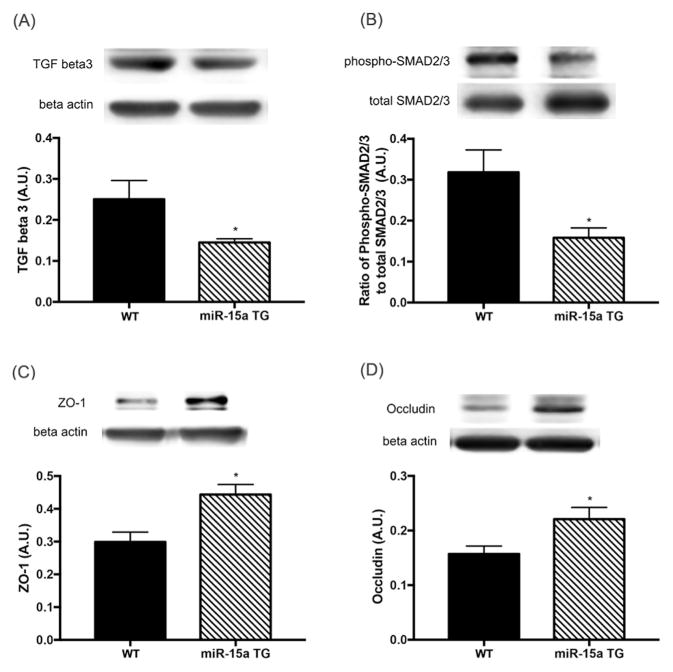
3.5. miR-15a inhibited TGF-beta3/SMAD signaling and increased ZO-1 and occludin levels in the retina
As our in vitro results showed a regulatory role for miR-15a/16 on TGF-beta3 signaling and tight junction proteins in cultured REC, we next investigated whether the miRNA had the same effects on the levels of TGF-beta3, SMAD2/3, ZO-1, and occludin in vivo. We examined retinal lysates collected from miR-15a transgenic mice and wild type mice. Our results demonstrated that overexpression of miR-15a in vascular endothelial cells resulted in decreased TGF-beta3 and SMAD2/3 signaling, with a concurrent increase in ZO-1 and occludin levels in the retina (Fig. 5). Thus, these data indicate that miR-15a regulates TGF beta3 signaling and ZO-1/occludin levels in the retina, potentially leading to the maintenance of retinal endothelial barriers.
Fig. 5.
Changes of TGF-beta3 signaling, ZO-1, and occludin levels in the retina of miR-15a overexpressing TG mice. Retinal lysates were examined by Western blot. The retinas of miR-15a-TG mice showed decreased levels of TGF- beta3 (A) and SMAD2/3 phosphoryation (B), with elevated levels of ZO-1 (C) and occludin (D) compared to control wild type (WT) mice. *p < 0.05 versus control mice, N = 3; Data are mean ± S.E.M.
4. Discussion
TGF-beta played a role in basal lamina thickening in pericytes and retinal endothelial cells (Van Geest et al., 2010). Additionally, it has been shown that VEGF increases endothelial permeability in retinal endothelial cells (Behzadian et al., 2003; Qin et al., 2016; Yun et al., 2016). Our recent study has shown an inhibitory role of miR-15a/16 on the inflammatory signaling of IL-1β, TNFα, NF-κB, as well as retinal leukostasis in vivo (Ye et al., 2016). Moreover, TGF-beta3 and SMAD3 are predicted targets of miR-15a. MiR-16 is predicted to target TGF-beta receptor 3 and SMAD3 (targetscan). Additionally, VEGFA was reported as a direct target of miR-15a/16 (Sun et al., 2013). However, less information is available on miR-15a/16 regulation of TGF-beta3 and VEGF signaling in diabetic retinopathy. Thus, we examined our hypothesis that miR-15a/16 plays a role in suppressing TGF beta3/VEGF signaling and maintaining the retinal endothelial cell barrier. We demonstrated overexpression of miR-15a and -16 significantly reduced levels of TGF-beta3 and SMAD2/3 in REC under high glucose conditions. Our in vivo data supported these findings, demonstrating that overexpression of miR-15a in vascular endothelial cells played a role in the supression of TGF-beta3 and SMAD2/3 levels in the retina. Previous studies have reported that other miRNAs, miR-152 (Haque et al., 2015) and miR-200b (Jiang et al., 2015), can inhibit TGF-beta1 levels in human REC under high glucose conditions. No other studies to our knowledge have investigated the effects of miR-15a/16 on TGF-beta3 in pathological mechanisms of diabetic retinopathy. Previous studies, instead, have shown roles of miR-15 and -16 on TGF-beta signaling in other types of disorders, as miR-15/16 upregulated TGF-beta signaling, leading to progression of prostate cancer (Bonci et al., 2016). miR-16 overexpression suppressed TGF-beta release in human alveolar epithelial cells (Tamarapu Parthasarathy et al., 2012).
Signaling of TGF-beta 1/2/3 can induce VEGF expression (Ferrari et al., 2006; Grigsby et al., 2012; Nagineni et al., 2003). In addition, activated VEGFR2 mediated apoptotic activities of TGF-beta1 in human umbilical vein endothelial cells (HUVEC) (Ferrari et al., 2006), as well as in vivo (Ferrari, Cook, Terushkin, Pintucci, & Mignatti, 2009), suggesting an interrelationship between TGF-beta1 and VEGF. We demonstrated that overexpression of miR-15a and -16 decreased VEGF levels in REC under high glucose conditions. Our results are in agreement with in vivo inhibitory effects of miR-15a on VEGF-A levels in the retinas of diabetic miR-15a-TG mice (Wang et al., 2016). Both miR-152 (Haque et al., 2015) and miR-200b (Jiang et al., 2015), have also been shown to inhibit VEGF expression in human REC under high glucose conditions. Overexpression of miR-126 reduced VEGF under hypoxic conditions (Ye, Liu, He, Xu, & Yao, 2014). Our results also showed that miR-15a/16 reduced endothelial cell permeability through downregulation of VEGF in REC under high glucose conditions. Inductive roles of VEGF on endothelial permeability have been reported in previous studies (Fischer et al., 1999; Qin et al., 2016; Yun et al., 2016).
In addition to our in vitro results, our in vivo data showed elevated expression of ZO-1 and occludin in the retina of miR-15a overexpressing TG mice. A recent study using miR-15a TG mice demonstrated decreased vascular permeability in the diabetic retina of the mice (Wang et al., 2016). We suggest that miR-15a played a role in maintaining vascular endothelial barrier, potentially through 1) inhibition of VEGF signaling and/or 2) elevation of ZO-1/occludin levels.
Our study provides evidence that miR-15a plays a role in regulating tight junction proteins ZO-1 and occludin in retinal endothelium. We showed that levels of ZO-1 and occludin were increased by miR-15a and -16 overexpression in cultured REC, as well as in the retina of miR-15a-TG mice. Regulatory roles of VEGF on tight junction proteins ZO-1 and occludin have been previously reported. VEGF reduced the levels of ZO-1, ZO-2, and occludin, mediating the breakdown of blood-retinal barrier in retinal endothelial cells (Kim et al., 2009). It has been suggested that elevated levels of VEGF downregulate occludin to increase vascular permeability in STZ-diabetic retina (Antonetti et al., 1998). Also, VEGFR2 signaling mediated occludin phosphorylation and vascular permeability in the retina following ischemia-reperfusion (Muthusamy et al., 2014).
Other miRNAs have also been identified for their ability to regulate vascular permeability. miR-147b and miR-650 reduced endothelial permeability in HUVEC (Chatterjee et al., 2014; Guo, Wu, Sun, & Yuan, 2011). miR-200b reduced VEGF levels and endothelial permeability in vitro and in vivo (McArthur, Feng, Wu, Chen, & Chakrabarti, 2011). In addition, topical delivery of miR-106a mimic resulted in decreased levels of VEGF and vascular permeability in the retina of STZ-diabetic mice (Ling et al., 2013). Decreased levels of miR-126 were found in the retinas of STZ-induced diabetic rats, which were accompanied by reduced expression of ZO-1 and occludin genes, with increased levels of VEGF and BRB breakdown (Wang & Yan, 2016).
It has been suggested that the infiltration of inflammatory cells and IL-1beta expression can induce VEGF upregulation and BRB breakdown in the developing mouse retina (Vinores, Xiao, Zimmerman, Whitcup, & Wawrousek, 2003). Our recent study showed an inhibitory role of miR-15a/16 on the levels of IL-1beta and the influx of inflammatory cells into the retinal vasculature of miR-15a/16 Cre-lox mice (Ye et al., 2016). These findings may provide another clue for a regulatory role of miR-15a/16 in the maintenance of the retinal endothelial cell barrier. Further studies are needed to investigate specific mechanisms by which miR-15a/16 regulate the vascular endothelial cell barrier via TGF-beta3/VEGF signaling in the diabetic retina.
5. Conclusions
We demonstrated that miR-15a/16 played a role in inhibiting levels of TGF-beta3, SMAD2/3 phosphorylation, and VEGF in REC cultured under high glucose conditions. The levels of tight junction proteins, ZO-1 and occludin, were increased through overexpression of miR-15a/16 in REC. In addition, overexpression of miR-15a/16 resulted in decreased endothelial permeability through VEGF suppression in high glucose conditions in vitro. In vivo regulatory roles of miR-15a on TGF-beta signaling and tight junction proteins were also demonstrated in the retina of miR-15a-transgenic mice. Therefore, we suggest that miR-15a/16 reduces TGF-beta3/VEGF signaling and increases tight junction proteins, leading to maintenance of retinal endothelial cell barrier. Thus the miRNAs may be a potential molecular target for diabetic retinopathy.
Acknowledgments
This work was supported by NIH R01EY022045 (JJS), P30EY04068 (Hazlett), and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute).
Abbreviations
- REC
retinal endothelial cells
- TGF-beta3
transforming growth factor beta 3
- SMAD2/3
mothers against decapentaplegic homolog 2/3
- VEGF
vascular endothelial growth factor
- ZO-1
zonula occludens-1
Footnotes
Author contributions
EY performed all in vitro studies, collected data, and wrote the manuscript. LL contributed to in vivo studies by maintaining and genotyping transgenic mice. JS guided designing of the study. JS and EY edited the manuscript. All authors have read and approved of this manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content – Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods in Molecular Medicine. 2003;89:365–374. doi: 10.1385/1-59259-419-0:365. [DOI] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: Possible role of glial cells in endothelial barrier function. Investigative Ophthalmology & Visual Science. 2001;42:853–859. [PubMed] [Google Scholar]
- Behzadian MA, Windsor LJ, Ghaly N, Liou G, Tsai NT, Caldwell RB. VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB Journal. 2003;17:752–754. doi: 10.1096/fj.02-0484fje. [DOI] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Patrizii M, Addario A, Cannistraci A, Francescangeli F, et al. A microRNA code for prostate cancer metastasis. Oncogene. 2016;35:1180–1192. doi: 10.1038/onc.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Effect of acute hyperglycaemia, long-term glycaemic control and insulin on endothelial dysfunction and inflammation in Type 1 diabetic patients with different characteristics. Diabetic Medicine. 2010;27:911–917. doi: 10.1111/j.1464-5491.2009.02928.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee V, Beard RS, Reynolds JJ, Haines R, Guo MZ, Rubin M, et al. MicroRNA-147b regulates vascular endothelial barrier function by targeting ADAM15 expression. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi TS, Lee I, Huttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: Implications for diabetic retinopathy. Experimental Diabetes Research. 2012;2012:438238. doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Hyperglycemia as a cause of diabetic retinopathy. Metabolism. 1986;35:20–23. doi: 10.1016/0026-0495(86)90182-4. [DOI] [PubMed] [Google Scholar]
- Fante RJ, Durairaj VD, Oliver SC. Diabetic retinopathy: An update on treatment. American Journal of Medicine. 2010;123:213–216. doi: 10.1016/j.amjmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-beta 1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. Journal of Cellular Physiology. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17260–17265. doi: 10.1073/pnas.0605556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. American Journal of Physiology. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- Fulzele S, El-Sherbini A, Ahmad S, Sangani R, Matragoon S, El-Remessy A, et al. MicroRNA-146 b-3p regulates retinal inflammation by suppressing adenosine deaminase-2 in diabetes. BioMed Research International. 2015;2015:846501. doi: 10.1155/2015/846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby J, Betts B, Vidro-Kotchan E, Culbert R, Tsin A. A possible role of acrolein in diabetic retinopathy: Involvement of a VEGF/TGF beta signaling pathway of the retinal pigment epithelium in hyperglycemia. Current Eye Research. 2012;37:1045–1053. doi: 10.3109/02713683.2012.713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo MZ, Wu MH, Sun CX, Yuan SY. MicroRNA-147b and-650 modulate ADAM15 Expression in the regulation of endothelial cell permeability. Faseb Journal. 2011;25 [Google Scholar]
- Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFbeta1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Molecular Vision. 2015;21:224–235. [PMC free article] [PubMed] [Google Scholar]
- He JB, Wang H, Liu Y, Li W, Kim D, Huang H. Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. Journal of Ophthalmology. 2015 doi: 10.1155/2015/605946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Lukiw WJ. MicroRNA (miRNA)-Mediated Pathogenetic Signaling in Alzheimer’s Disease (AD) Neurochemical Research. 2016;41:96–100. doi: 10.1007/s11064-015-1734-7. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pagadala J, Miller D, Steinle JJ. Reduced insulin receptor signaling in retinal Muller cells cultured in high glucose. Molecular Vision. 2013;19:804–811. [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cellular Signalling. 2012;24:1086–1092. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhao F, Liu XM, Li RR, Liu JM. Effect of miR-200 b on retinal endothelial cell function under high glucose environment. International Journal of Clinical and Experimental Pathology. 2015;8:10482–10487. [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. American Journal of Pathology. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Jun HO, Yu YS, Kim KW. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. American Journal of Pathology. 2010;176:1517–1524. doi: 10.2353/ajpath.2010.090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Lee YM, Ahn EM, Kim KW, Yu YS. Decursin inhibits VEGF-mediated inner blood-retinal barrier breakdown by suppression of VEGFR-2 activation. Journal of Cerebral Blood Flow and Metabolism. 2009;29:1559–1567. doi: 10.1038/jcbfm.2009.78. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Archives of Internal Medicine. 1994;154:2169–2178. [PubMed] [Google Scholar]
- Krizbai IA, Gasparics A, Nagyoszi P, Fazakas C, Molnar J, Wilhelm I, et al. Endothelial-mesenchymal transition of brain endothelial cells: Possible role during metastatic extravasation. PLoS ONE. 2015;10:e0119655. doi: 10.1371/journal.pone.0119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal EC, Manivannan A, Hosoya KI, Terasaki T, Cunha-Vaz J, Ambrosio AF, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- Ling SK, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Ye YM. MicroRNA-dependent cross-talk between VEGF and HIF1 alpha in the diabetic retina. Cellular Signalling. 2013;25:2840–2847. doi: 10.1016/j.cellsig.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Maciotta S, Meregalli M, Torrente Y. The involvement of microRNAs in neurodegenerative diseases. Frontiers in Cellular Neuroscience. 2013;7:265. doi: 10.3389/fncel.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur K, Feng BA, Wu YX, Chen SL, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. Journal of Cerebral Blood Flow and Metabolism. 2014;34:522–531. doi: 10.1038/jcbfm.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, et al. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. Journal of Cellular Physiology. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: Implications for diabetic retinopathy. Diabetes. 2004;53:2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- Pascal MM, Forrester JV, Knott RM. Glucose-mediated regulation of transforming growth factor-beta (TGF-beta) and TGF-beta receptors in human retinal endothelial cells. Current Eye Research. 1999;19:162–170. doi: 10.1076/ceyr.19.2.162.5332. [DOI] [PubMed] [Google Scholar]
- Qin B, Liu J, Liu S, Li B, Ren J. MiR-20 b targets AKT3 and modulates vascular endothelial growth factor-mediated changes in diabetic retinopathy. Acta Biochimica et Biophysica Sinica (Shanghai) 2016;48:732–740. doi: 10.1093/abbs/gmw065. [DOI] [PubMed] [Google Scholar]
- Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East African Journal of Ophthalmology. 2012;19:52–59. doi: 10.4103/0974-9233.92116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saker S, Stewart EA, Browning AC, Allen CL, Amoaku WM. The effect of hyperglycaemia on permeability and the expression of junctional complex molecules in human retinal and choroidal endothelial cells. Experimental Eye Research. 2014;121:161–167. doi: 10.1016/j.exer.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. Journal of Ophthalmic and Vision Research. 2014;9:362–373. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CY, She XM, Qin Y, Chu ZB, Chen L, Ai LS, et al. MiR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis. 2013;34:426–435. doi: 10.1093/carcin/bgs333. [DOI] [PubMed] [Google Scholar]
- Tamarapu Parthasarathy P, Galam L, Huynh B, Yunus A, Abuelenen T, Castillo A, et al. MicroRNA 16 modulates epithelial sodium channel in human alveolar epithelial cells. Biochemical and Biophysical Research Communications. 2012;426:203–208. doi: 10.1016/j.bbrc.2012.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO. Differential TGF-{beta} signaling in retinal vascular cells: A role in diabetic retinopathy? Investigative Ophthalmology & Visual Science. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Xiao WH, Zimmerman R, Whitcup SM, Wawrousek EF. Upregulation of vascular endothelial growth factor (VEGF) in the retinas of transgenic mice overexpressing interleukin-1 beta (IL-1 beta) in the lens and mice undergoing retinal degeneration. Histology and Histopathology. 2003;18:797–810. doi: 10.14670/HH-18.797. [DOI] [PubMed] [Google Scholar]
- Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, et al. Dual anti-inflammatory and anti-angiogenic action of miR-15a in diabetic retinopathy. EBioMedicine. 2016;11:138–150. doi: 10.1016/j.ebiom.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yan H. MicroRNA-126 contributes to Niaspan treatment induced vascular restoration after diabetic retinopathy. Scientific Reports. 2016;6 doi: 10.1038/srep26909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Blaauwgeers HG, Weich HA, Alitalo K, Vrensen GFJM, Schlingemann RO. Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Investigative Ophthalmology & Visual Science. 2002;43:849–857. [PubMed] [Google Scholar]
- Wu JH, Gao Y, Ren AJ, Zhao SH, Zhong M, Peng YJ, et al. Altered MicroRNA expression profiles in retinas with diabetic retinopathy. Ophthalmic Research. 2012;47:195–201. doi: 10.1159/000331992. [DOI] [PubMed] [Google Scholar]
- Xiong F, Du XH, Hu JY, Li TT, Du SS, Wu Q. Altered retinal MicroRNA expression profiles in early diabetic retinopathy: An in silico analysis. Current Eye Research. 2014;39:720–729. doi: 10.3109/02713683.2013.872280. [DOI] [PubMed] [Google Scholar]
- Yang TT, Song SJ, Xue HB, Shi DF, Liu CM, Liu H. Regulatory T cells in the pathogenesis of type 2 diabetes mellitus retinopathy by miR-155. European Review for Medical and Pharmacological Sciences. 2015;19:2010–2015. [PubMed] [Google Scholar]
- Ye P, Liu J, He F, Xu W, Yao K. Hypoxia-induced deregulation of miR-126 and its regulative effect on VEGF and MMP-9 expression. International Journal of Medical Sciences. 2014;11:17–23. doi: 10.7150/ijms.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye EA, Liu L, Jiang Y, Jan J, Gaddipati S, Suvas S, et al. MiR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. Journal of Neuroinflammation. 2016;13:305. doi: 10.1186/s12974-016-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye EA, Steinle JJ. MiR-15 b/16 protects primary human retinal microvascular endothelial cells against hyperglycemia-induced increases in tumor necrosis factor alpha and suppressor of cytokine signaling 3. Journal of Neuroinflammation. 2015;12:44. doi: 10.1186/s12974-015-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, et al. Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: Implications for diabetic retinopathy. Journal of Cellular Physiology. 2016 doi: 10.1002/jcp.25575. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jiang Y, Toutounchian JJ, Soderland C, Yates CR, Steinle JJ. Insulin-like growth factor binding protein-3 inhibits monocyte adhesion to retinal endothelial cells in high glucose conditions. Molecular Vision. 2013;19:796–803. [PMC free article] [PubMed] [Google Scholar]