Abstract
Background
Chronic alcohol exposure causes lipid dyshomeostasis at the adipose-liver axis, reducing lipid storage in white fat and increasing lipid deposit in the liver. Previous studies have shown that visceral fat, rather than subcutaneous fat, is a risk factor for metabolic diseases. This study was conducted to determine whether chronic alcohol exposure differentially affects lipid metabolism in visceral (epididymal) and subcutaneous fat, and the mechanisms underlying the alcohol effects.
Methods
Male Wistar rats were pair-fed the Lieber-DeCarli control or alcohol liquid diet for 12 weeks to determine the effects of alcohol on the white fat. Tissue explants culture and 3T3-L1 culture were conducted to define the role of acetaldehyde in alcohol-induced adipose tissue dysfunction.
Results
Chronic alcohol feeding significantly reduced visceral fat mass and down-regulated peroxisome proliferator activator receptor-γ and CCAAT/enhancer binding protein-α, 2 important transcription factors in regulation of lipogenesis. The protein levels of lipogenic enzymes including phospho-ATP-citrate lyase, acetyl-CoA carboxylase, fatty acid synthase, lipin1, and diacylglycerol acyltransferase 2 in the visceral fat were reduced. In contrast, chronic alcohol exposure did not affect subcutaneous fat mass and had less effect on the protein levels of lipogenic enzymes and regulators. Accordingly, the visceral fat showed a lower protein level of aldehyde detoxification enzymes compared to the subcutaneous fat. Acetaldehyde treatment to either visceral fat explants or 3T3-L1 adipocytes produced similar effects on lipogenic enzymes and regulators as observed in animal model.
Conclusions
These results demonstrated that visceral fat is more susceptible to alcohol toxicity compared to subcutaneous fat, and disruption of adipose lipogenesis contributes to the pathogenesis of alcoholic lipodystrophy.
Keywords: White Adipose Tissue, Visceral Fat, Subcutaneous Fat, Lipid Dyshomeostasis, Lipogenesis, Alcohol
Alcohol consumption HAS long been known as a risk factor for both disease and death. Excessive alcohol consumption is the third leading lifestyle-related cause of death in the United States (Mokdad et al., 2004). Alcohol misuse has been causally related to more than 60 different medical conditions (Room et al., 2005), including neurological impairments (dementia and neuropathy), cardiovascular problems (hypertension, strokes, and renal failure), and liver diseases (fatty liver, hepatitis, and cirrhosis) (Corrao et al., 2004). The estimated economic cost of excessive drinking was $223.5 billion in 2006 or approximately $1.90 per alcoholic drink (Bouchery et al., 2011).
White adipose tissue (WAT) plays several key roles in mammalian physiology, including energy storage, inflammatory processes, and glucose homeostasis (Trayhurn and Beattie, 2001). WAT is a major energy storage organ which stores energy in the form of triglycerides. It also functions as an endocrine organ, controlling energy homeostasis and metabolism through secretion of adipokines such as adiponectin and leptin (Otto and Lane, 2005; Rosen, 2005). WAT develops in distinct intra-abdominal depots, that is, visceral fat, including epididymal (EWAT), mesenteric, and perirenal depots, and in the subcutaneous layer (SWAT) (Seale et al., 2011). Published data showed that subcutaneous and visceral WAT express unique gene signatures and have distinct metabolic effects (Fox et al., 2007; Gesta et al., 2006; Miyazaki et al., 2002; Porter et al., 2009; Rexrode et al., 1998).
Chronic alcohol exposure has been shown to affect energy metabolism in both humans and animals (Addolorato et al., 1997, 1998, 2000; Greco et al., 2000). It has also been suggested that chronic alcohol exposure could result in the disruption of lipid homeostasis, which is likely a mediator of alcohol-related disease progression (Kang et al., 2007). More specifically, our group has shown that chronic alcohol exposure disturbs lipid homeostasis at the WAT-liver axis in mice (Wei et al., 2013; Zhong et al., 2012), which contributes to hepatic fat gain and progression of alcoholic fatty liver.
Most of alcohol consumed is metabolized in the liver. Alcohol is first oxidized by alcohol dehydrogenase (ADH) to generate acetaldehyde, a highly promiscuous intermediate (Haseba and Ohno, 2010). Acetaldehyde can be detoxified to acetic acid by the aldehyde dehydrogenase (ALDH) family. It is known that mitochondrial ALDH2 plays a central role in detoxification of acetaldehyde into acetate (Ohsawa et al., 2003). ALDH1A1 also plays a significant role in the oxidation of acetaldehyde (Vasiliou et al., 1999). Another enzyme, ALDH3A1, catalyzes the oxidation of lipid peroxidation-derived aldehydes such as 4-hydroxynonenal (Pappa et al., 2003).
Visceral and subcutaneous fat depots display not only distinct localizations but also differential metabolic characteristics. Increased visceral fat has been shown to be a major player in the development of metabolic syndrome and insulin resistance compared to the subcutaneous fat. Although it has been shown that chronic alcohol exposure affects adipose lipid metabolism, it remains unclear that whether the EWAT and SWAT are differentially affected. Therefore, this study focused on the effect of chronic alcohol exposure on lipid metabolism in EWAT and SWAT, respectively.
MATERIALS AND METHODS
Animals and Alcohol Feeding
Male Wistar rats were obtained from Charles River (Durham, NC). The animal protocol was approved by the Institutional Animal Care and Use Committee of the North Carolina Research Campus (Permit Number: 13-016). Eight-week-old rats were pair-fed a modified Lieber-DeCarli alcohol (alcohol-fed [AF]) or isocaloric maltose dextrin control (pair-fed [PF]) liquid diet for 12 weeks (n = 8 for each group) with a stepwise feeding procedure as described previously (Zhong et al., 2012). The ethanol (EtOH) content (%, w/v) in the diet was 4.8% (34% of total calories) for the first 2 weeks and gradually increased to 5.4% (38% of total calories) for the last 2 weeks. The amount of food given to the PF rats was that the AF rats consumed in the previous day. At the end of 12-week feeding, rats were anesthetized with inhalational isoflurane, and blood, EWAT, inguinal WAT, that is, bilateral superficial SWAT depots between the skin and muscle fascia just anterior to the upper segment of the hind limbs were collected.
Histopathological Analysis
WAT tissues were fixed in 10% formalin. Paraffin sections were cut at 5 µm and processed for hematoxylin and eosin staining to assess the histological features of EWAT and SWAT. A minimum of 10 random microscopic fields (20× objective) were taken from each sample using Nikon eclipse 55i microscope (Nikon, Melville, NY). The images were acquired using NIS-Elements F software (Nikon).
Ex Vivo Study
Ex vivo culture of rat adipose tissue was carried out as previously described (Zhong et al., 2012). Briefly, EWAT was removed, washed twice with prewarmed Dulbecco’s phosphate buffered saline (PBS) containing 100 U/ml penicillin and 100 mg/ml streptomycin, and finely minced with scissors after removing of connective tissue and blood vessels. Fifty milligrams of adipose tissue explants was placed into 50-ml conical tubes and cultured in 50 ml of Dulbecco’s modified Eagle’s medium (DMEM) with 2 mmol/l l-glutamine, 50 U/ml penicillin, and 50 mg/ml streptomycin for 72 hours with rotation in the presence or absence of 100 µmol/l of acetaldehyde. Then, adipose tissue explants were collected for later analysis.
Cell Culture and Treatments
3T3-L1 preadipocytes obtained from American Type Culture Collection (Rockville, MD) were cultured in DMEM (Life Technologies, Grand Island, NY) containing 10% bovine calf serum (BCS; Life Technologies), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Grand Island, NY), at 37°C in a humidified atmosphere of 5% CO2. Differentiation of 3T3-L1 preadipocytes into mature adipocytes was conducted as reported previously (Kanda et al., 2012). Briefly, 2 days after reaching confluence, 3T3-L1 preadipocytes grown in individual wells of collagen-coated 6-well cell culture plates (BD Falcon, Franklin Lakes, NJ) were subjected to induction by replacing the culture medium with the MDI induction media (DMEM medium containing 10% fetal bovine serum [FBS], 5 µg/ml of insulin [Sigma, St. Louis, MO], 0.5 mM IBMX [Sigma], and 10 µM DEX [Sigma]), and the induction proceeded for 2 days. Subsequently, culture media were changed to insulin media (DMEM containing 10% FBS and 5 µg/ml of insulin) for 2 days. Two days later, change media to DMEM containing 10% FBS (Atlanta Biologicals, Lawrenceville, GA). Acetaldehyde was treated to 3T3-L1 cells at 100 µmol/l in the presence or absence of ALDH inhibitor, cyanamide, at a concentration of 5 µmol/l for 3 days. EtOH was also used to treat 3T3-L1 cells at 200 mmol/l for 3 days. Then, 3T3-L1 cells were washed 2 times with PBS and collected for later use.
Immunoblot Analysis
Whole protein lysates of both EWAT and SWAT were extracted using 10% Nonidet P-40 lysis buffer supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma). Protein concentrations were measured with a protein assay reagent based on the Bradford method (Bio-Rad, Hercules, CA). Aliquots containing 50 µg of proteins were loaded onto 8 to 15% SDS-PAGE, trans-blotted onto PVDF membrane, blocked with 5%nonfat dry milk in Tris-buffered saline solution with 0.1% Tween-20 (TBST) for 1 hour at room temperature, and incubated with anti-PPARγ (peroxisome proliferator-activated receptor-γ), C/EBPα (CCAAT/enhancer binding protein), β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), phospho-hormone-sensitive lipase (p-HSL), adipose triglyceride lipase (ATGL), ATP-citrate lyase (ACL), phospho-ATP-citrate lyase (p-ACL), fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC) (Cell Signaling Technology, Danvers, MA), diacylglycerol acyltransferases 2 (DGAT2) (Acris Antibodies Inc., San Diego, CA), lipin1 (LPIN1), cytochrome P450 2E1 (CYP2E1), aldehyde dehydrogenase 2 family (mitochondrial) (ALDH2) (Abcam, Cambridge, MA), ADH, aldehyde dehydrogenase 1 family, member A1 (ALDH1A1) (Novus Biologicals, Littleton, CO), and aldehyde dehydrogenase 3 family member A1 (ALDH3A1) antibody (Thermo Scientific, Rockford, IL), respectively. The dilution was 1:200 or 1:1,000 for primary antibodies from Santa Cruz Biotechnology or other companies, respectively. The membranes were then washed 3 times with TBST and then incubated using horseradish peroxidase-conjugated donkey anti-rabbit, goat anti-mouse, or rabbit anti-goat IgG (Thermo Scientific). The bound complexes were detected via enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). The immunoblot bands were quantified by densitometry analysis, and the ratio to β-actin was calculated and is given as fold changes, setting the values of PF or control at 1.
Measurement of ALDH Activity
ALDH activity in the liver, EWAT, or SWAT was measured using the ALDH activity colorimetric assay kit (BioVision, Milpitas, CA) following the manufacturer’s instructions.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). The results were analyzed using the 1-sample t-test or 1-way analysis of variance (ANOVA) followed by least significant difference. In all tests, p-values <0.05 were considered statistically significant.
RESULTS
Chronic Alcohol Feeding Significantly Reduced the Mass and Adipocyte Size of EWAT, But Not SWAT
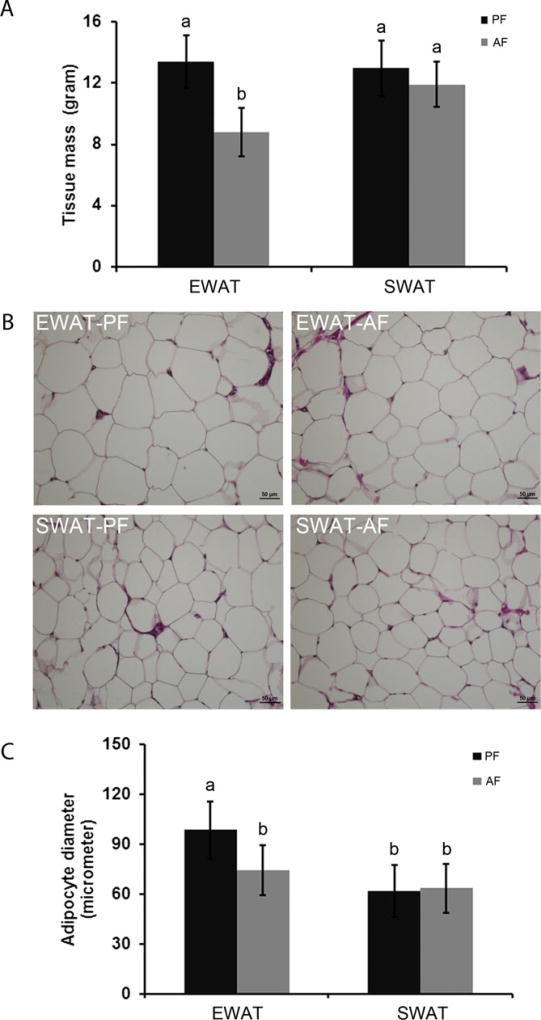
After 12-week alcohol feeding, rats were anesthetized. Body weight, liver weight, and WAT weight were measured. No significant change was found in body weight (PF vs. AF: 452.00 ± 21.25 vs. 438.72 ± 17.71 g) or liver weight (PF vs. AF: 12.53 ± 1.88 vs. 13.67 ± 0.94 g). As depicted in Fig. 1, the average EWAT masses of AF rats and PF rats were 8.78 ± 1.56 g and 13.40 ± 1.72 g, respectively. The average SWAT masses of AF rats and PF rats were 11.91 ± 1.46 g and 12.97 ± 1.83 g, respectively. Statistical analysis showed that 12-week alcohol feeding significantly lowered EWAT mass, but not SWAT mass. Moreover, EtOH feeding significantly decreased EWAT adipocyte size, from 98.28 ± 17.37 µm in PF rats to 74.36 ± 14.98 µm in AF rats. In contrast, alcohol feeding did not affect the adipocyte sizes of SWAT. The SWAT adipocyte sizes from PF and AF rats were 61.62 ± 15.52 µm and 63.25 ± 14.53 µm, respectively.
Fig. 1.
Chronic alcohol feeding significantly reduced the mass and adipocyte size of EWAT. Male Wistar rats were fed liquid diets containing alcohol (AF) or maltose dextrin (PF) for 12 weeks. EWAT and SWAT were taken. Tissue masses (A) were weighed, adipose tissue morphology (B), and adipocyte sizes (C) were measured at 20× objective using Nikon Eclipse 55i. All values are denoted as means ± SD. Significant differences are indicated by different letters (ANOVA, p < 0.05).
Chronic Alcohol Feeding Significantly Reduced the Protein Levels of Lipogenic Enzymes and Regulators in EWAT, But Not in SWAT
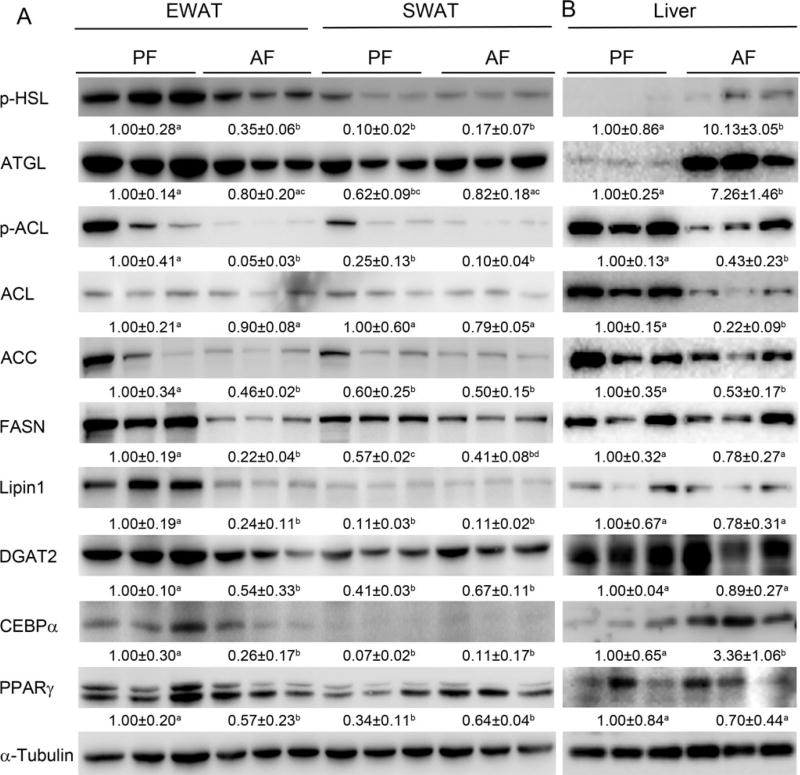
To find out the reason why EWAT mass is reduced after alcohol feeding, the expression levels of lipolytic enzymes, p-HSL and ATGL, and 2 important transcription factors in lipogenesis, PPARγ and C/EBPα, were examined by Western blot analysis. As shown in Fig. 2A, in EWAT, chronic alcohol exposure down-regulated p-HSL expression to 35% of the controls, but did not have significant effect on ATGL expression. However, chronic alcohol exposure did not affect either p-HSL or ATGL in SWAT. Alcohol feeding also significantly lowered the protein level of PPARγ and C/EBPα by 43% and 74% respectively, compared to that of PF rats. In contrast, alcohol feeding did not have significant effect on the protein level of PPARγ or C/EBPα in SWAT. Accordingly, lipogenic enzymes including p-ACL, ACC, FASN, LPIN1, and DGAT2 were significantly down-regulated by 95%, 54%, 78%, 76%, and 46% in EWAT, respectively, by chronic alcohol feeding. In contrast, no significant change in the protein level of those proteins mentioned above was found in SWAT after chronic alcohol feeding. The protein levels of lipolytic enzymes, lipogenic enzymes, and lipogenic transcription factors in the liver were also measured by Western blot analysis. As shown in Fig. 2B, chronic alcohol feeding up-regulated the expression of p-HSL, ATGL, and C/EBPα, and down-regulated the expression of p-ACL, ACL, and ACC, and had no effect on the expression of FASN, LPIN1, DGAT2, and PPARγ.
Fig. 2.
Chronic alcohol feeding significantly reduced the protein levels of lipogenic enzymes and regulators in EWAT. Male Wistar rats were fed liquid diets containing alcohol (AF) or maltose dextrin (PF) for 12 weeks. (A) The expressions of lipogenic enzymes and regulators in both EWAT and SWAT were measured by Western blotting. (B) The expressions of lipogenic enzymes and regulators in liver were measured by Western blotting. Expression levels of indicated proteins were quantitated by the NIH ImageJ software (Bethesda, MD). All values are denoted as means ± SD. Significant differences are indicated by different letters (ANOVA, p < 0.05).
Chronic Alcohol Feeding Significantly Elevated ALDH Activity in Both EWAT and SWAT and Differentially Increased Aldehyde Metabolic Enzymes
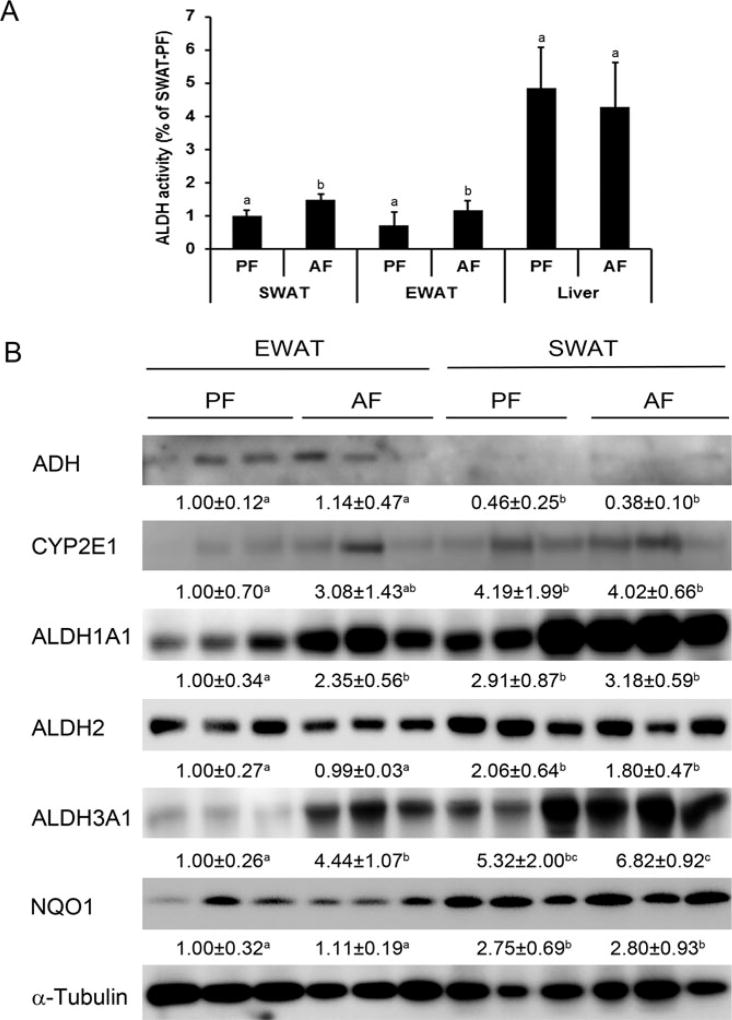
As shown in Figure 3A, ALDH activity in the liver was higher than that of the WATs, 6.73 times higher than EWAT, and 4.68 times higher than SWAT, respectively. Chronic alcohol feeding significantly increased ALDH activity in both EWAT and SWAT, but not in the liver. To further elucidate the potential mechanism of how WATs differentially respond to chronic alcohol feeding, we measured the protein levels of enzymes involved in alcohol metabolism and the clearance of alcohol metabolite-acetaldehyde. As depicted in Fig. 3B, ADH, the enzyme that catalyzes the major pathway of oxidative metabolism of EtOH, had higher protein level in EWAT compared to that of SWAT, and showed no change to alcohol exposure. CYP2E1, another enzyme contributes to oxidative metabolism of alcohol, had higher protein levels in SWAT compared to that of EWAT, and also showed no change to alcohol exposure. The overall expression level of ADH and CYP2E1 in EWAT and SWAT had no difference. Two enzymes involved in the clearance of aldehydes, ALDH1A1 and ALDH3A1, had significant higher expression levels after alcohol exposure in EWAT. The expression levels of ALDH1A1 and ALDH3A1 in SWAT showed no change after alcohol exposure. ALDH2 showed no change after alcohol exposure, but had higher expression levels in SWAT compared to EWAT. Taken together, the overall expression levels of ALDH1A1, ALDH2, and ALDH3A1 in SWAT were higher than that in EWAT.
Fig. 3.
Chronic alcohol feeding significantly elevated aldehyde dehydrogenase (ALDH) activity in both EWAT and SWAT, and differentially increased aldehyde metabolic enzymes. Male Wistar rats were fed liquid diets containing alcohol (AF) or maltose dextrin (PF) for 12 weeks. (A) ALDH activity in WATs and liver was measured by colorimetric kit. (B) Protein levels of enzymes involved in alcohol and acetaldehyde metabolism in both EWAT and SWAT were measured by Western blotting. Protein levels of indicated proteins were quantitated by the NIH ImageJ software. All values are denoted as means ± SD. Significant differences are indicated by different letters (ANOVA, p < 0.05).
Acetaldehyde Treatment Significantly Reduced the Protein Levels of Lipogenic Enzymes and Regulators in EWAT Explants and in 3T3-L1 Adipocytes
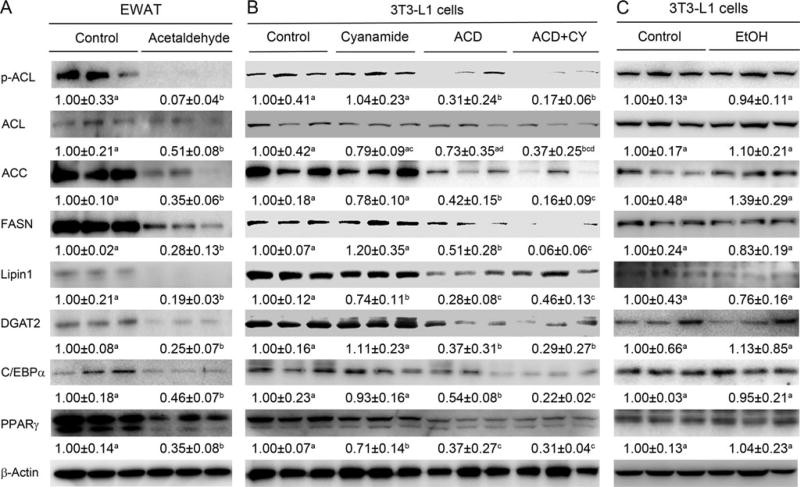
To elucidate the mechanistic link between acetaldehyde and down-regulation of lipogenic transcription factors and genes, EWAT and 3T3-L1 adipocytes were treated with acetaldehyde and the expressions of lipogenic enzymes and regulators were determined. Treatment of EWAT with 100 µmol/l acetaldehyde for 3 days caused significant reduction of PPARγ, C/EBPα, p-ACL, ACC, FASN, LPIN1, and DGAT2 (Fig. 4A). Acetaldehyde treatment also significantly reduced the protein level of the lipogenic enzymes and regulators in 3T3-L1 adipocytes (Fig. 4B). Moreover, administration of ALDH inhibitor further decreased some of the lipogenic enzymes and regulators (Fig. 4B). But EtOH treatment to 3T3-L1 adipocytes did not have significant effect on the protein levels of the lipogenic enzymes and regulators (Fig. 4C).
Fig. 4.
Acetaldehyde treatment significantly reduced the expression of lipogenic enzymes and regulators in EWAT explants and in 3T3-L1 adipocytes. EWAT explants were from male Wistar rats treated in the presence or absence of 100 µmol/l of acetaldehyde for 3 days. 3T3-L1 cells were treated with 100 µmol/l acetaldehyde in the presence or absence of aldehyde dehydrogenase inhibitor, cyanamide, at concentration of 5 µmol/l for 3 days also. Ethanol (EtOH) was also used to treat 3T3-L1 cells at 200 mmol/l for 3 days. The protein levels of lipogenic enzymes and regulators in both EWAT (A), 3T3-L1 cells treated with acetaldehyde (B), or 3T3-L1 cells treated with EtOH (C) were measured by Western blotting. Band intensity was quantitated by the NIH ImageJ software. All values are denoted as means ± SD. Significant differences are indicated by different letters (t-test, p < 0.05).
DISCUSSION
In this study, we investigated the effects of chronic alcohol feeding on lipogenesis in rat EWAT and SWAT. We demonstrated that chronic alcohol exposure significantly reduced EWAT mass, whereas no significant mass change was found in SWAT upon chronic alcohol exposure. In consistence with the decreased tissue mass, expression levels of lipogenic transcription factor, that is, PPARγ and C/EBPα, and genes involved in lipogenesis, that is, p-ACL, ACC, FASN, LPIN1, and DGAT2 in EWAT were also down-regulated by chronic alcohol exposure when compared to that of PF rats. In sharp contrast, neither SWAT tissue mass nor expression levels of lipogenic enzymes and regulators had significant change upon chronic alcohol exposure compared to that of controls. We demonstrated that the overall protein levels of ADH and CYP2E1, 2 important enzymes in oxidative metabolism of ethanol, was almost the same in both EWAT and SWAT. However, SWAT has higher levels of aldehyde clearance enzymes, ALDH1A1, ALDH2, and ALDH3A1, than EWAT, which might explain why SWAT is not susceptible to chronic alcohol exposure. Next, we clearly showed that acetaldehyde, a toxic metabolite of alcohol, treatment to explants of EWAT or 3T3-L1 adipocytes substantially decreased the expression levels of PPARγ, C/EBPα, p-ACL, ACC, FASN, LPIN1, and DGAT2, which confirms that different levels of aldehyde metabolic enzymes in EWAT and SWAT contributes to the observed responses of 2 WATs to chronic alcohol exposure.
WAT, 1 of the 2 types of adipose tissue found in mammals, plays an important role in regulation of whole-body energy homeostasis. WAT stores energy in the form of triglyceride under positive energy balance condition and releases fatty acids under energy deprivation condition (Sethi and Vidal-Puig, 2007). Energy imbalance, for example, excess free fatty acid flux or excess body adiposity, especially abdominal obesity and ectopic fat accumulation, has been considered as a key risk factor in the development of a number of chronic diseases, including type 2 diabetes and cardiovascular disease (Britton and Fox, 2011; Delarue and Magnan, 2007; Thomas et al., 2012). It has been reported that alcohol exposure has diverse effects on EWAT, such as reducing lipid storage (Wei et al., 2013; Zhong et al., 2012), increasing the rate of triglyceride degradation (Kang et al., 2007), and increasing the expression of inflammatory cytokines and chemokines (Chen et al., 2009). However, the effects of chronic alcohol exposure on subcutaneous fat remain unclear. The present study demonstrated that chronic alcohol exposure significantly reduced EWAT mass and decreased EWAT adipocyte size. In contrast, it had no significant effect on SWAT mass or SWAT adipocyte size (Fig. 1).
It is known that whole-body fat mass and lipid turnover in adipose tissue are determined by the relative rates of lipolysis and lipogenesis in adipose tissue (McTernan et al., 2002). Thus, different rates of lipolysis and lipogenesis in EWAT and SWAT might be the reasons of observed different effects of chronic alcohol exposure on EWAT and SWAT. In the present study, we analyzed the genes and transcription factors involved in lipolysis and lipogenesis to investigate whether chronic alcohol exposure differentially regulated lipolysis and lipogenesis in EWAT and SWAT. ATGL and HSL are 2 key enzymes responsible for intracellular degradation of triglyceride. ATGL is an adipose-enriched protein with triglyceride-specific lipase activity (Kershaw et al., 2006), which catalyzes the first step of triglyceride hydrolysis. HSL exhibits a much broader substrate spectrum, with preference for diacylglycerols (Morak et al., 2012). As shown in Fig. 2, chronic alcohol exposure decreased p-HSL in EWAT but had no effect on the expression level of p-HSL in SWAT compared to PF rats. However, chronic alcohol exposure did not have any effect on the expression level of ATGL in both EWAT and SWAT when compared to controls. These results indicated that the aforementioned EWAT mass loss was not due to enhanced lipolysis. It is worth to mention that our group showed an increased lipolytic enzyme expression in mice after chronic alcohol feeding (Zhong et al., 2012). The difference seen might be caused by different severities of adipose dysfunction in these 2 animal models. In the mouse model, the EWAT weight loss was 69%, whereas in rat model, EWAT weight loss was 34%. Next, we measured genes or transcription factors involved in lipogenesis. Data in Fig. 2A depicted that chronic alcohol exposure dramatically decreased expression levels of lipogenic transcription factors, that is, PPARγ and C/EBPα, and genes involved in lipogenesis, that is, p-ACL, ACC, FASN, LPIN1, and DGAT2 in EWAT. In contrast, chronic alcohol exposure did not show any significant effect on expression levels of lipogenic enzymes or regulators in SWAT. The protein levels of lipolytic enzymes, lipogenic transcription factors, and lipogenic enzymes in liver were also measured by Western blot analysis. ATGL and p-HSL were significantly up-regulated by chronic alcohol feeding, while p-ACL, ACL, and ACC were significantly down-regulated. The up-regulation of lipolytic enzymes and down-regulation of lipogenic enzymes might be adaptive responses of the liver against excessive lipid deposition due to the decreased capacity of WATs to hold lipids. Taken together, these findings suggest that chronic alcohol exposure differentially affects lipid metabolism in EWAT and SWAT.
Alcohol is eliminated from the body by diverse metabolic pathways. Liver is the primary organ responsible for ethanol oxidation. The major pathway of oxidative metabolism of ethanol in the liver involves ADH and CYP2E1 (Haseba and Ohno, 2010; Zakhari, 2006). The oxidation of ethanol leads to the production of acetaldehyde, a highly promiscuous intermediate, that is able to interact with various proteins or other complex molecules to form modified molecules known as adducts at concentrations as low as 5 µM (Salmela et al., 1997). It has been well documented that ethanol-induced damage is largely attributed to its toxic metabolite, acetaldehyde (Stagos et al., 2010) and the formation of adducts is believed to be a key event leading to alcohol-induced liver injury (Niemela, 2001). Indeed, chronic alcohol feeding significantly increased serum acetaldehyde level to 0.95 mg/l. Therefore, we hypothesized that different capabilities of alcohol detoxification in EWAT and SWAT resulted in the different regulation of lipid metabolism in EWAT and SWAT. To address this possibility, we investigated the expression levels of enzymes involved in alcohol metabolism, ADH and CYP2E1, and enzymes involved in acetaldehyde detoxification, including ALDH2, ALDH1A1, and ALDH3A1. ADH converts alcohol into acetaldehyde. Figure 3B clearly showed that ADH had higher expression in EWAT, whereas CYP2E1 had higher expression in SWAT. Therefore, the overall expression of ethanol metabolic enzymes remains the same. After chronic alcohol exposure, neither ADH nor CYP2E1 showed significant change. Next, the protein level and activity of enzymes (ALDH2, ALDH1A1, and ALDH3A1) involved in detoxification of acetaldehyde were measured. As shown in Fig. 3B, the expression levels of ALDH2, ALDH1A1, and ALDH3A1 in SWAT were significantly higher than those in EWAT, suggesting that SWAT has higher capability of aldehyde detoxification which might explain why alcohol exposure did not decrease mass and adipose size of SWAT. ALDH activity in EWAT, SWAT, and liver was also measured. ALDH activity in the liver was higher than that of WATs. Chronic alcohol feeding significantly increased ALDH activity in both EWAT and SWAT. Because WATs have limited capacity to oxidize alcohol, the increased ALDH activity in WATs could be induced by increased acetaldehyde level in the circulation. Failure of induction of hepatic ALDH activity could be due to the fact that chronic alcohol feeding causes liver mitochondrial dysfunction. Taken together, these findings demonstrate that different capabilities of acetaldehyde detoxification in EWAT and SWAT contribute to different regulation of lipid metabolism in epididymal and subcutaneous WAT.
Adipose tissue consists of a heterogeneous cell population, including adipocytes, preadipocytes, fibroblasts, macrophages, vascular endothelial cells, and a variety of immune cells. Adipocytes are the cells that primarily compose adipose tissue (Rondinone, 2006). Therefore, we also introduced 3T3-L1 adipocytes and tested the effect of acetaldehyde on expression levels of lipogenic enzymes and regulators in 3T3-L1 adipocytes in the presence or absence of ALDH inhibitor, cyanamide. We found that acetaldehyde challenge significantly down-regulated expression levels of PPARγ, C/EBPα, p-ACL, ACC, FASN, LPIN1, and DGAT2 in 3T3-L1 adipocytes. Moreover, administration of ALDH inhibitor further decreased the expression of some of those lipogenic enzymes and regulators (Fig. 4B), indicating that acetaldehyde, an intermediate of ethanol metabolism, regulates lipid metabolism in 3T3-L1 adipocytes. But ethanol treatment of 3T3-L1 adipocytes did not have significant effect on those lipogenic enzymes and regulators (Fig. 4C); this might be due to limited (could not be detected by Western blot analysis) ethanol metabolizing enzymes (i.e., ADH and CYP2E1) in 3T3-L1 adipocytes and the ethanol could not be metabolized into acetaldehyde to affect those lipogenic enzymes and regulators.
To further confirm that acetaldehyde does play a role in the observed different responses of EWAT and SWAT to chronic alcohol feeding, we took advantage of EWAT explant to determine the effects of acetaldehyde. Then, the expression levels of lipogenic enzymes and regulators were measured by Western blotting analysis. As shown in Fig. 4A, acetaldehyde treatment to EWAT significantly decreased expression levels of lipogenic transcription factors, that is, PPARγ and C/EBPα, and enzymes involved in lipogenesis, that is, p-ACL, ACC, FASN, LPIN1, and DGAT2 in EWAT. These findings strongly argue that acetaldehyde, an intermediate product in ethanol metabolism, contributes to chronic alcohol exposure induced different regulations of lipid metabolism in EWAT and SWAT.
In summary, the present study demonstrated that chronic alcohol exposure for 12 weeks reduces EWAT mass, but not SWAT mass in rats. The reduction of EWAT mass in AF rats was associated with disruption of adipose lipogenesis. Reduced EWAT mass caused the decrease of lipid storage in EWAT. Triglycerides stored in EWAT were reverse transported to the liver after alcohol exposure, leading to increased ectopic lipid accumulation in the liver and lipodystrophy at the adipose-liver axis. Furthermore, the capacity of acetaldehyde detoxification was lower in EWAT compared to SWAT. Acetaldehyde treatment of primary EWAT adipocytes or adipocyte cell line suppressed the proteins involved in lipogenesis. These results suggest that EWAT is more susceptible than SWAT to alcohol-induced lipodystrophy due to less capacity in acetaldehyde detoxification.
Acknowledgments
This research was supported by the National Institutes of Health (R01AA018844 and R01AA020212).
References
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res. 1997;21:962–967. [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on bodyweight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med. 1998;244:387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, Messineo D, Sasso GF, Gasbarrini G, Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95:2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554–1562. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco AV, Mingrone G, Favuzzi A, Capristo E, Gniuli D, Addolorato G, Brunani A, Cavagnin F, Gasbarrini G. Serum leptin levels in post-hepatitis liver cirrhosis. J Hepatol. 2000;33:38–42. doi: 10.1016/s0168-8278(00)80157-9. [DOI] [PubMed] [Google Scholar]
- Haseba T, Ohno Y. A new view of alcohol metabolism and alcoholism – role of the high-Km Class III alcohol dehydrogenase (ADH3) Int J Environ Res Public Health. 2010;7:1076–1092. doi: 10.3390/ijerph7031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Nishi K, Kadota A, Nishimoto S, Liu MC, Sugahara T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim Biophys Acta. 2012;1820:461–468. doi: 10.1016/j.bbagen.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- McTernan PG, Harte AL, Anderson LA, Green A, Smith SA, Holder JC, Barnett AH, Eggo MC, Kumar S. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes. 2002;51:1493–1498. doi: 10.2337/diabetes.51.5.1493. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Morak M, Schmidinger H, Riesenhuber G, Rechberger GN, Kollroser M, Haemmerle G, Zechner R, Kronenberg F, Hermetter A. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol Cell Proteomics. 2012;11:1777–1789. doi: 10.1074/mcp.M111.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med. 2001;31:1533–1538. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Yasuda C, Kamino K, Ohta S. Deficiency in a mitochondrial aldehyde dehydrogenase increases vulnerability to oxidative stress in PC12 cells. J Neurochem. 2003;84:1110–1117. doi: 10.1046/j.1471-4159.2003.01619.x. [DOI] [PubMed] [Google Scholar]
- Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Pappa A, Estey T, Manzer R, Brown D, Vasiliou V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003;376:615–623. doi: 10.1042/BJ20030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81–90. doi: 10.1385/endo:29:1:81. [DOI] [PubMed] [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73:31–34. doi: 10.1016/j.plefa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Salmela KS, Sillanaukee P, Itala L, Vakevainen S, Salaspuro M, Roine RP. Binding of acetaldehyde to rat gastric mucosa during ethanol oxidation. J Lab Clin Med. 1997;129:627–633. doi: 10.1016/s0022-2143(97)90197-9. [DOI] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Investig. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagos D, Chen Y, Brocker C, Donald E, Jackson BC, Orlicky DJ, Thompson DC, Vasiliou V. Aldehyde dehydrogenase 1B1: molecular cloning and characterization of a novel mitochondrial acetaldehyde-metabolizing enzyme. Drug Metab Dispos. 2010;38:1679–1687. doi: 10.1124/dmd.110.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Frost G, Taylor-Robinson SD, Bell JD. Excess body fat in obese and normal-weight subjects. Nutr Res Rev. 2012;25:150–161. doi: 10.1017/S0954422412000054. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Bairoch A, Tipton KF, Nebert DW. Eukaryotic aldehyde dehydrogenase (ALDH) genes: human polymorphisms, and recommended nomenclature based on divergent evolution and chromosomal mapping. Pharmacogenetics. 1999;9:421–434. [PubMed] [Google Scholar]
- Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, Zhou Z, Zhang X. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS ONE. 2013;8:e55382. doi: 10.1371/journal.pone.0055382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]