Abstract
The acrolein derived cyclic 1,N2-propanodeoxyguanosine adduct (Acr-dG), formed primarily from ω-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA) under oxidative conditions, while proven to be mutagenic, is potentially involved in DHA-induced apoptosis. The latter may contribute to the chemopreventive effects of DHA. Previous studies have shown that the levels of Acr-dG are correlated with apoptosis induction in HT29 cells treated with DHA. Because Acr-dG is shown to be repaired by the nucleotide excision repair (NER) pathway, to further investigate the role of Acr-dG in apoptosis, in this study, NER-deficient XPA and its isogenic NER-proficient XAN1 cells were treated with DHA. The Acr-dG levels and apoptosis were sharply increased in XPA cells, but not in XAN1 cells when treated with 125 μM of DHA. Because DHA can induce formation of various DNA damage, to specifically investigate the role of Acr-dG in apoptosis induction, we treated XPA knockdown HCT116 + ch3 cells with acrolein. The levels of both Acr-dG and apoptosis induction increased significantly in the XPA knockdown cells. These results clearly demonstrate that NER deficiency induces higher levels of Acr-dG in cells treated with DHA or acrolein and sensitizes cells to undergo apoptosis in a correlative manner. Collectively, these results support that Acr-dG, a ubiquitously formed mutagenic oxidative DNA adduct, plays a role in DHA-induced apoptosis and suggest that it could serve as a biomarker for the cancer preventive effects of DHA.
Keywords: DNA adduct, Docosahexaenoic acid, Acrolein, Nucleotide excision repair, Apoptosis
1. Introduction
The chemopreventive potential of docosahexaenoic acid (DHA) and other ω-3 polyunsaturated fatty acids (PUFAs) has been shown in animal studies and suggested by epidemiological studies [1–5]. DHA induces apoptosis and has synergetic effects in sensitizing cellular apoptotic responses to anticancer drugs [6,7]. The induction of apoptosis by ω-3 PUFAs such as DHA is believed to be an important mechanism underlying its chemopreventive activities. Although the molecular basis remains unclear [5,8–11], DNA damage derived from the oxidation of ω-3 PUFAs may contribute to the apoptotic effects [12]. We have reported that the acrolein-derived cyclic 1,N2-propano-2′-deoxyguanosine (Acr-dG) is a major endogenous mutagenic DNA lesion derived from peroxidation of DHA and other ω-3 PUFAs [13,14]. Acr-dG adducts are ubiquitously detected in tissues of rodents and humans and they are formed when DNA reacts with acrolein generated through endogenous lipid oxidation as well as from exogenous sources such as cigarette smoking, high temperature oil cooking and fossil fuel combustion [15]. The potential roles of Acr-dG in mutagenesis and carcinogenesis have been extensively studied [16–26]; however, only limited information is available on its role in apoptosis. A previous study demonstrated that Acr-dG formation, when it reaches certain threshold levels, is correlated with apoptotic responses in human colon cancer HT29 cells treated with DHA [12], suggesting that Acr-dG may play a role in DHA-induced apoptosis. Acr-dG adducts are repaired by the nucleotide excision repair (NER) pathway [27]. Xeroderma Pigmentosum Group A (XPA) protein is a key component involved in the initial DNA damage recognition and recruitment of other NER repair proteins [28–30]. XPA protein was also shown to interact with checkpoint machinery in response to DNA damage [31,32].
In this study using NER-deficiency to reduce the repair of Acr-dG and increase its levels in cells, we examined the role of Acr-dG in triggering apoptosis by determining Acr-dG levels and the apoptotic responses in an NER-deficient XPA cells and its isogenic NER-proficient XAN1 cells treated with DHA, and in XPA knockdown HCT116 + ch3 cells treated with acrolein.
2. Material and methods
2.1. Materials
Human skin cancer XPA cells (GM04429, Coriell Cell Repositories) are NER-deficient. XAN1 cells (kindly provided by Dr. J. Christopher States of University of Louisville School of Medicine, Louisville, KY) have a stably transformed XPA minigene to restore NER function [33]. HCT116 + ch3 cells were kindly provided by Dr. Jean Y.J. Wang from of University of California at San Diego. The siRNA of XPA and the Darmacon Smartpool siRNA system were from Fisher Scientific. WST-1 was from Roche Diagnostics.
2.2. Cell culture and treatment
XPA and XAN1 cells were routinely cultured at 37 °C with 5% carbon dioxide in an α-modified minimum essential medium with 10% fetal bovine serum (Mediatech Inc., Herndon, VA). Cells were treated with DHA when they reached about 50% confluence.
For the siRNA interference and transfections experiments, HCT116 + ch3 cells cultured at 37 °C in Dulbecco’s modified Eagle’s medium DMEM were transfected with XPA or nonspecific control siRNA and then treated with 0 or 200 μM of acrolein for 16 h. The XPA levels were monitored before and after the acrolein treatment by western blotting using the total protein quantitation method based on the Bio-Rad stain-free V3 System [34].
2.3. Cell viability and apoptosis assays
The WST-1 assay was performed using the protocol from the manufacturer. The sub-G1 cell cycle analysis was done using the standard protocol of fixation with 75% ethanol and stained with PI, followed by the FACS assay done on a Becton Dickinson FACSort system and the data analysis with MODFIT. The caspase-3 activities and PARP cleavage assays were previously published [12].
2.4. Detection and quantification of Acr-dG by LC–MS/MS-MRM and immunofluorescence assays
The DNA samples were isolated and Acr-dG levels were determined with a previously published LC–MS/MS method [35]. The immunohistochemical staining of Acr-dG in cells was performed with a newly developed anti-Acr-dG monoclonal antibody [36]. Five micron sections from formalin fixed, paraffin embedded cells were de-paraffinized with xylenes and rehydrated through a graded alcohol series. Heat induced epitope retrieval (HIER) was performed by immersing the tissue sections at 98 °C for 20 min in citrate buffer (pH 6.0). Immunofluorescence staining was performed using a horseradish peroxidase-labeled polymer from Dako (K4001) according to manufacturer’s instructions. Briefly, slides were treated with 3% hydrogen peroxide and 10% Normal Goat Serum for 10 min each and exposed to primary antibody for Acr-dG (1:10,000) for 1 h at room temperature. Slides were exposed to the HRP labeled polymer for 30 min and Cyanine 5 TYRAMIDE REAGENT for 10 min. Slides were counterstained and mounted with Prolong Gold antifade reagent with DAPI. Consecutive sections with the primary antibody omitted were used as negative controls and washing buffer was 1X TBS with 0.05% Tween 20.
3. Results and discussion
3.1. DHA treatment induces higher apoptosis and Acr-dG levels in XPA cells than XAN1 cells
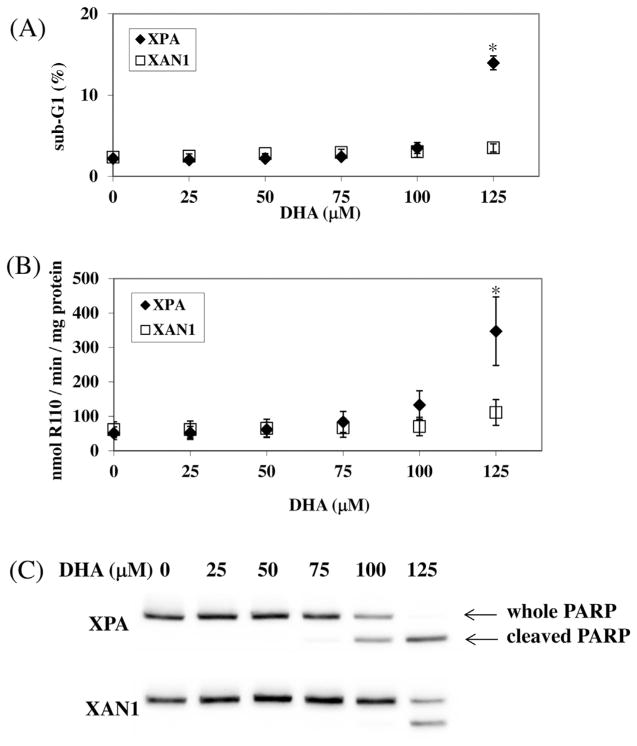
After 24 h incubation with 100 μM DHA, XPA cells displayed morphology changes such as cell rounding, shrinkage and blebbing, whereas XAN1 cells only showed these changes at above 125 μM DHA. These morphological changes were much more pronounced in XPA cells than XAN1 cells. As shown in Fig. 1A, the sub-G1 analysis showed that there was no statistically significant difference between the two cell lines treated with DHA up to 100 μM. At 125 μM, however, the sub-G1 cell population increased significantly only in XPA cells but not in XAN1 cells (p = 0.03).
Fig. 1.
Apoptosis in XPA vs XAN-1 cells treated with DHA. (A) Sub-G1 percentage: XPA vs. XAN1 at 125 μM (*p = 0.03). (B) Caspase-3 activities: XPA vs. XAN1 at 100 μM (p = 0.1) and 125 μM (*p = 0.04); (C) PARP cleavage. Statistical analysis is based on triplicate experiments, and the t-test was done by comparing results from two cell lines at a specific DHA concentration and p values were obtained for that particular concentration.
Caspase-3 activities were measured to quantify DHA-induced apoptosis. As shown in Fig. 1B, caspase-3 activities remained at background levels in XAN1 cells and only increased slightly in cells treated with 125 μM DHA. In XPA cells, however, caspase-3 activities began to increase at 100 μM DHA and rose sharply at 125 μM DHA (p = 0.04). Additionally, PARP cleavage was determined by western blotting (Fig. 1C) with the Bio-Rad stain-free V3 Western system. The cleaved PARP was observed in XAN1 cells treated with 125 μM, but not with 100 μM DHA, whereas a similar degree of cleavage was observed in XPA cells at 100 μM DHA. In fact, the PARP in XPA cells was completely cleaved at 125 μM DHA. These results suggest that the loss of XPA protein sensitizes the cells to DHA-induced apoptosis.
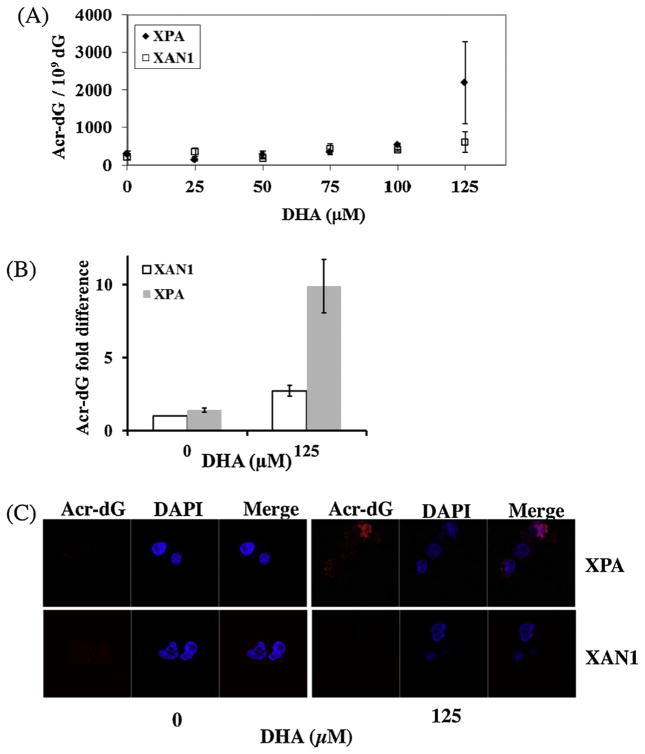
The Acr-dG levels in DNA from these samples were determined using an LC–MS/MS-MRM method [35]. As shown in Fig. 2A, Acr-dG levels in XPA and XAN1 cells did not increase from background (300.1 ± 69.5 and 214.7 ± 70.1 Acr-dG/109 dG, respectively) when treated with 0, 25, 50 and 75 μM DHA. At 100 μM DHA, Acr-dG levels in both cell lines began to rise above the background, however to a different extent: 543.8 ± 40.0 Acr-dG/109 dG for XPA cells and 391.7 ± 30.7 Acr-dG/109 dG for XAN1 cells (p = 0.03). A dramatic increase of Acr-dG was observed in XPA cells at 125 μM DHA, but not in XAN1 cells, with the levels of Acr-dG in XPA cells more than 3-fold higher (2191.8 ± 1087.7 Acr-dG/109 dG) than in XAN1 cells (600.3 ± 273.7 Acr-dG/109 dG, p = 0.09). While the increase was apparent, the statistical significance was compromised because of the relatively high standard deviations, probably due to variations from different batches of samples. For example, the background levels of Acr-dG for XAN1 cells varied from 165.1 to 263.3 Acr-dG/109 dG in different batches, whereas the Acr-dG levels for 125 μM DHA treated samples in the corresponding assay batches varied from 406.8 to 793.8 Acr-dG/109 dG. The statistical analysis showed that the p value was 0.016 when comparing the difference in fold changes (Fig. 2B), indicating that the Acr-dG levels were higher in XPA cells than in XAN1 cells treated with 125 μM DHA. This difference was also confirmed with the immunohistochemical staining assay. As shown in Fig. 2C, little or no Acr-dG staining was observed in both cell lines treated with 0 μM DHA. At 125 μM, both the number of anti-Acr-dG positive stained cells and the staining intensities increased more significantly in XPA cells than in XAN1 cells. These findings indicate that, consistent with the notion that Acr-dG is repaired by the NER pathway, the NER-deficient cells show greater accumulation of Acr-dG. Previous studies showed that acrolein can inhibit NER and base excision repair (BER) activities to various degrees in cells [26]. In this study, we found that DHA might not completely inhibit the NER activity in XAN1 cells as there was no observed increase of Acr-dG in XAN1 cells treated with DHA, even at the highest concentration. The lack of increase in Acr-dG formation at DHA concentrations below 75 μM in XAN1 and XPA cells may be attributed to its efficient repair by NER and other unidentified repair pathways.
Fig. 2.
Acr-dG levels in XPA vs XAN-1 cells treated with DHA. (A) Acr-dG levels measured by LC–MS/MS in two cells treated with various concentrations of DHA for 16 h. (B) Fold changes of Acr-dG levels in XPA and XAN1 cells treated with 125 μM DHA (p = 0.016). To eliminate the batch variations, we calculate the fold changes of Acr-dG levels by comparing the adduct levels in untreated XPA and treated XAN1and XPA cells to the Acr-dG level in untreated XAN1 cells in each assay batch. (C) Immunohistochemical staining with anti-Acr-dG monoclonal antibodies. Statistical analysis was done as described in Fig. 1.
These studies show that Acr-dG levels in XPA and XAN1 cells are correlated with apoptosis. Both caspase-3 activity and PARP cleavage rose above background at 100 μM DHA for XPA cells and 125 μM for XAN1 cells when the levels of Acr-dG reached 543.8 and 600.3 Acr-dG/109 dG, respectively. These levels may represent a threshold for the apoptotic responses in XPA and XAN1 cells. More importantly, Acr-dG formation and caspase-3 activity were higher in XPA cells than in XAN1 cells at 125 μM DHA, indicating that NER deficiency is responsible for increased Acr-dG adducts levels as well as apoptotic response in XPA cells. It is interesting to note that cells of skin origin (XPA and XAN1) appear to be more sensitive to DHA-induced apoptosis than colonic cells (HT29 and HCT116), as the former cells underwent apoptosis at 100 and 125 μM DHA, whereas the latter only became apoptotic at 200 μM or higher [12]. Many factors that could contribute to the sensitivity of DHA-induced apoptosis include repair efficiency and other cell-specific differences in apoptotic pathways, and these factors need to be further studied.
3.2. XPA knockdown sensitizes cells to acrolein-induced apoptosis and increases Acr-dG levels
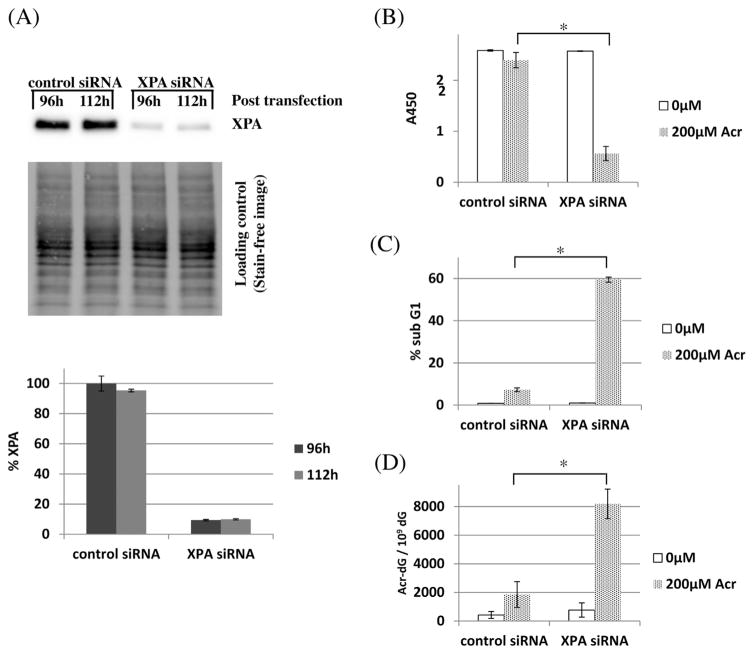
Besides Acr-dG, DHA could potentially modulate the levels of several types of DNA damage [11,37,38] that may be involved in apoptosis. To investigate more specifically whether Acr-dG plays a role in apoptosis induction, we treated XPA and XAN1 cells directly with acrolein. Unfortunately, the sensitivity of both cells to acrolein-induced cytotoxicity in a very narrow acrolein concentration range prohibited their use in these studies. We then decided to use human colon cancer HCT116 + ch3 cells because they are more resistant to acrolein treatment. HCT116 + ch3 cells were transfected with XPA or control siRNA using a Darmacon Smartpool siRNA system for 6 h. At 96 h after transfection, cells were then treated with 0 or 200 μM of acrolein for 16 h. For the acrolein treatment period at 96 h and 112 h post-transfection, the XPA protein levels in both the control and XPA siRNA transfected cells were measured using the total protein quantitation method based on the Bio-Rad stain-free V3 System. As seen in Fig. 3A, the XPA levels in the knockdown cells were about 10% of those in control cells. Because acrolein inhibits caspase-3 activity [39,40], WST-1 and sub G1 assays were chosen to measure cell viability and apoptosis. As shown in Fig. 3B, C and D, the control and XPA siRNA transfected cells without the acrolein treatment showed 100% and 99.5% viability, 0.81% and 1.02% sub G1 population, and the background levels of Acr-dG at 422 and 767 Acr-dG/109 dG (p = 0.15), respectively. There was no difference between the control and XPA siRNA transfected cells and the results were very similar to those observed in HCT116 + ch3 cells alone, indicating that siRNA transfection had a minimal effect on the cells. After treating cells with 200 μM of acrolein for 16 h, the viability slightly decreased to 92.6%, sub G1 increased to 7.3%, and Acr-dG levels increased to 1848 Acr-dG/109 dG in the control siRNA transfected cells. The changes are much more pronounced in the XPA siRNA transfected cells with the viability decreased to 21.7% (p = 0.003), sub G1 increased to 59.5% (p = 6 × 10−5), and Acr-dG levels increased to 8186 Acr-dG/109 dG (p = 0.009). These results again confirm that the elevated levels of Acr-dG due to the lack of NER repair is correlated with the increased apoptosis.
Fig. 3.
Apoptosis and Acr-dG levels in XPA knock-down vs control siRNA transfected HCT116 + ch3 cells treated with acrolein (Acr). (A) The XPA protein levels in HCT116 + ch3 cells transfected with XPA siRNA were about 10% of those of cells with control siRNA at both 96 h and 112 h post transfection. The middle section shows the blot used for measuring total proteins in each sample according to the Bio-Rad protocol; (B) Cell viability with WST-1 assay; (C) Sub G1 cell population with PI staining assay; (D) Acr-dG levels for cells treated with and without acrolein for 16 h. Compared with control siRNA transfected cells, the XPA siRNA transfected cells showed significant lower viability (*p = 0.003), higher sub G1 (*p = 6 × 10−5) and Acr-dG levels (*p = 0.009), when both were treated with 200 μM DHA. Statistical analysis is based on triplicate experiments. The t-test was done to compare apoptosis and adduct levels between non-specific siRNA and XPA siRNA transfected cells with the same treatments.
Collectively, our data confirmed that DHA and acrolein can induce higher levels Acr-dG adducts and the elevated Acr-dG levels correlated with increased apoptosis, implicating a potential role of Acr-dG in DHA-induced apoptosis. The data also confirmed that NER is responsible for repair of Acr-dG.
The molecular basis for Acr-dG-induced apoptosis has yet to be fully investigated. Processing of DNA damage into DNA double strand breaks (DSBs) via DNA repair pathways or stalled replication fork is a common pathway for inducing apoptosis. Our previous studies [12] have demonstrated that increased DSBs levels are correlated with high levels of Acr-dG in HT29 cells treated with DHA. However, more studies are needed to dissect Acr-dG formation, DSBs, and apoptosis, for example, using cells deficient in DSB repair pathway, such as homologous recombination or Fanconi Anemia pathway.
DNA repair pathways have been shown to affect cell viability and apoptotic response to certain DNA-damaging agents [41,42]. For example, mismatch repair is required for apoptosis signaling by alkylating agents and cisplatin [43,44], and overexpression of BER sensitizes ovarian cancer cells to alkylating agent temozolomide treatment [45]. These studies indicate that the DNA repair proteins can activate pro-death signals by interacting with DNA damage or generating toxic repair intermediates to initiate apoptotic responses [43,45]. On the contrary, deficiency of DNA repair proteins can sometimes sensitize cells to apoptosis by the accumulation of unrepaired damage, which could further lead to strand breaks and trigger apoptosis. Studies have shown that NER deficiency is associated with the increased apoptosis in cell and animal models in both p53 independent [46] and dependent manners [47,48], and mitochondria BER deficiency was found to correlate with increased apoptosis in neurons [49]. The results of our study show that the NER pathway responsible for removing Acr-dG protects cells from programmed death triggered by accumulation of Acr-dG.
The mutagenicity of Acr-dG has not been conclusively [16–26]. Acr-dG was shown to cause frameshift and base substitution mutations. On the other hand, the γ isomer Acr-dG, the predominant isomer detected in tissue and cell DNA, was shown to be passed by polymerase without causing mutation. Regardless of its mutagencity, it is believed that Acr-dG at low levels it may cause mutations, whereas at high levels that reach a threshold its predominant effect is to induce apoptosis as DNA damage response.
Although the repair and mutagenesis of Acr-dG have been well-documented, little is known about the relationship between its formation in cellular DNA and apoptosis. The present study supports the role of Acr-dG in apoptosis. However, we cannot exclude the possibility that other yet to be identified oxidative DNA damage derived from DHA repaired by NER could also be involved. The fact that Acr-dG is a major DNA adduct of DHA detected at relatively high levels underlines its potential role in apoptosis and suggests it may serve as a biomarker for the cancer preventive activity of DHA.
Acknowledgments
The authors thank the Flow Cytometry and Cell Sorting, and Tissue Culture Shared Resources at the Lombardi Comprehensive Cancer Center of Georgetown University for their technical assistance. We thank Drs. Monika Aggarwal and Yongwei Zhang for helpful discussion and Dr. Ning Liu from Bio-Rad for support in using stain-free technology-based V-3 system. This work was supported by NCI grant CA043159.
References
- 1.Caygill CPJ, Charlett A, Hill MJ. Fat fish, fish oil and cancer. Br J Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang WCL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Serini S, Fasano E, Celleno L, Cittadini A, Calviello G. Potential of long-chain n-3 polyunsaturated fatty acids in melanoma prevention. Nutr Rev. 2014;72:255–266. doi: 10.1111/nure.12093. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 6.Maheo K, Vibet S, Steghens JP, Dartigeas C, Lehman M, Bougnoux P, Gore J. Differential sensitization of cancer cells to doxorubicin by DHA: A role for lipoperoxidation. Free Radic Biol Med. 2005;39:742–751. doi: 10.1016/j.freeradbiomed.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan BA, Narayanan NK, Reddy BS. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol. 2001;19:1255–1262. doi: 10.3892/ijo.19.6.1255. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui RA, Jenski LJ, Neff K, Harvey K, Kovacs RJ, Stillwell W. Docosahexaenoic acid induces apoptosis in Jurkat cells by a protein phosphatase-mediated process. Biochim Biophys Acta Mol Cell Res. 2001;1499:265–275. doi: 10.1016/s0167-4889(00)00128-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZY, Istfan NW. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2000;63:301–308. doi: 10.1054/plef.2000.0218. [DOI] [PubMed] [Google Scholar]
- 11.Hong MY, Lupton JR, Morris JS, Wang NY, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomark Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 12.Pan J, Keffer J, Emami A, Ma X, Lan R, Goldman R, Chung FL. Acrolein-derived DNA adduct formation in human colon cancer cells: its role in apoptosis induction by docosahexaenoic acid. Chem Res Toxicol. 2009;22:798–806. doi: 10.1021/tx800355k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung FL, Chen HJC, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 14.Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem Res Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 15.Stevens JF, Maier CS. Acrolein: sources metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang IY, Miller H, Wang ZG, Frank EG, Ohmori H, Hanaoka F, Moriya M. Mammalian translesion DNA synthesis across an acrolein-derived deoxyguanosine adduct—participation of DNA polymerase eta in error-prone synthesis in human cells. J Biol Chem. 2003;278:13989–13994. doi: 10.1074/jbc.M212535200. [DOI] [PubMed] [Google Scholar]
- 17.Minko IG, Washington MT, Kanuri M, Prakash L, Prakash S, Lloyd RS. Translesion synthesis past acrolein-derived DNA adduct gamma-hydroxypropanodeoxyguanosine, by yeast and human DNA polymerase eta. J Biol Chem. 2003;278:784–790. doi: 10.1074/jbc.M207774200. [DOI] [PubMed] [Google Scholar]
- 18.Benamira M, Singh U, Marnett LJ. Site-Specific frameshift mutagenesis by a propanodeoxyguanosine adduct positioned in the (Cpg) 4Hot-Spot of salmonella-typhimurium Hisd3052Carried on an M13 vector. J Biol Chem. 1992;267:22392–22400. [PubMed] [Google Scholar]
- 19.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Protein-template-directed synthesis across an acrolein-derived DNA adduct by yeast rev1 DNA polymerase. Structure. 2008;16:239–245. doi: 10.1016/j.str.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Minko IG, Kozekov ID, Harris TA, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N-2-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderVeen LA, Hashim MF, Nechev LV, Harris TM, Harris CM, Marnett LJ. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J Biol Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 22.Yang IY, Hossain M, Miller H, Khullar S, Johnson F, Grollman A, Moriya M. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J Biol Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 23.Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, Moriya M. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 24.Wang HT, Zhang S, Hu Y, Tang MS. Mutagenicity and sequence specificity of acrolein-DNA adducts. Chem Res Toxicol. 2009;22:511–517. doi: 10.1021/tx800369y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng ZH, Hu WW, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HT, Hu Y, Tong D, Huang J, Gu LY, Wu XR, Chung FL, Li GM, Tang MS. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem. 2012;287:12379–12386. doi: 10.1074/jbc.M111.329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhury S, Dyba M, Pan J, Roy R, Chung FL. Repair kinetics of acrolein-and (E)-4-hydroxy-2-nonenal-derived DNA adducts in human colon cell extracts 1. Mutat Res Fundam Mol Mech Mutagen. 2013;751:15–23. doi: 10.1016/j.mrfmmm.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Laat WL, Jaspers NGJ, Hoeijmakers JHJ. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 29.Kamileri I, Karakasilioti I, Garinis GA. Nucleotide excision repair: new tricks with old bricks. Trends Genet. 2012;28:566–573. doi: 10.1016/j.tig.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Miura N, Satokata I, Miyamoto I, Yoshida MC, Satoh Y, Kondo S, Yasui A, Okayama H, Okada Y. Analysis of a human dna excision repair gene involved in group-A xeroderma-pigmentosum and containing a zinc-finger domain. Nature. 1990;348:73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- 31.Marini F, Nardo T, Giannattasio M, Minuzzo M, Stefanini M, Plevani P, Falconi MM. DNA nucleotide excision repair-dependent signaling to checkpoint activation. Proc Natl Acad Sci U S A. 2006;103:17325–17330. doi: 10.1073/pnas.0605446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer J, Hoeijmakers JHJ. Nucleotide excision repair and human syndromes. Carcinogenesis. 2000;21:453–460. doi: 10.1093/carcin/21.3.453. [DOI] [PubMed] [Google Scholar]
- 33.Myrand SP, Topping RS, States JC. Stable transformation of xeroderma pigmentosum group A cells with an XPA minigene restores normal DNA repair and mutagenesis of UV-treated plasmids. Carcinogenesis. 1996;17:1909–1917. doi: 10.1093/carcin/17.9.1909. [DOI] [PubMed] [Google Scholar]
- 34.Gurtler A, Kunz N, Gomolka M, Hornhardt S, Friedl AA, McDonald K, Kohn JE, Posch A. Stain-free technology as a normalization tool in Western blot analysis. Anal Biochem. 2013;433:105–111. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Chung FL, Wu MY, Basudan A, Dyba M, Nath RG. Regioselective formation of acrolein-derived cyclic 1,N-2-propanodeoxyguanosine adducts mediated by amino acids, proteins, and cell lysates. Chem Res Toxicol. 2012;25:1921–1928. doi: 10.1021/tx3002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan J, Awoyemi B, Xuan Z, Vohra P, Wang HT, Dyba M, Greenspan E, Fu Y, Creswell K, Zhang L, Berry D, Tang MS, Chung FL. Detection of acrolein-derived cyclic DNA adducts in human cells by monoclonal antibodies. Chem Res Toxicol. 2012;25:2788–2795. doi: 10.1021/tx3004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umegaki K, Hashimoto M, Yamasaki H, Fujii Y, Sugisawa A, Shinozuka K. Docosahexaenoic acid supplementation-increased oxidative damage in bone marrow DNA in aged rats and its relation to antioxidant vitamins. Free Radic Res. 2001;34:427–435. doi: 10.1080/10715760100300361. [DOI] [PubMed] [Google Scholar]
- 38.Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanel A, Averill-Bates DA. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim Biophys Acta Mol Cell Res. 2005;1743:255–267. doi: 10.1016/j.bbamcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Kern JC, Kehrer JP. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139:79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res Rev Mutat Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 42.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Hickman MJ, Samson LD. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc Natl Acad Sci U S A. 1999;96:10764–10769. doi: 10.1073/pnas.96.19.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topping RP, Wilkinson JC, Scarpinato KD. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. J Biol Chem. 2009;284:14029–14039. doi: 10.1074/jbc.M809303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishel ML, He Y, Smith ML, Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin Cancer Res. 2007;13:260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- 46.Stubbert LJ, Smith JM, Mckay BC. Decreased transcription-coupled nucleotide excision repair capacity is associated with increased p53-and MLH1-independent apoptosis in response to cisplatin. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc Natl Acad Sci U S A. 2007;104:1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muenyi CS, Pinhas AR, Fan TW, Brock GN, Helm CW, States JC. Sodium Arsenite +/− hyperthermia sensitizes p53-expressing human ovarian cancer cells to cisplatin by modulating platinum-DNA damage responses. Toxicol Sci. 2012;127:139–149. doi: 10.1093/toxsci/kfs085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, Ledoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]