Abstract
Background
ECMO is increasingly used for patients with critical illnesses. This study examines ECMO use in patients with cardiogenic shock in US hospitals and associated outcomes (mortality, hospital length of stay, and total hospital charges).
Methods
A matched cohort retrospective study was conducted using the 2013 Nationwide Emergency Department Sample. Cardiogenic shock visits were matched (1 : 1) and compared based on ECMO use.
Results
Patients with ECMO (N = 802) were compared to patients without ECMO (N = 805). Mortality was higher in the ECMO group (48.9% versus 4.0%, p < 0.001). Visits with ECMO use also had higher average hospital charges ($580,065.8 versus $156,436.5, p < 0.001) and average hospital LOS (21.3 versus 11.6 days, p < 0.001). After adjusting for confounders, mortality (OR = 8.52 (95% CI: 2.84–25.58)) and charges (OR = 1.03 (95% CI: 1.02–1.05)) remained higher in the ECMO group, while LOS was similar (OR = 1.01 (95% CI: 0.99–1.02)).
Conclusions
Patients with cardiogenic shock who underwent ECMO had increased mortality and higher cost of care without significant increase in LOS when compared to patients with cardiogenic shock without ECMO use. Prospective evaluation of this observed association is needed to improve outcomes and resources' utilization further.
1. Introduction
Extracorporeal membrane oxygenation (ECMO) is a method of mechanical cardiorespiratory support used in critical cases, usually in intensive care units or emergency department (ED) settings [1]. Early evidence for ECMO efficacy was discouraging and the adoption of ECMO in the medical field was initially slow. A randomized controlled trial conducted in 1979 showed that ECMO did not increase long-term survival and resulted in a 90% mortality rate among adult patients with acute respiratory failure [2].
More recently and according to a recent study based on the Nationwide Inpatient Sample (2000–2011), ECMO use increased significantly (mainly after 2007) and was associated with an increase in healthcare associated costs including increased length of hospital stay (LOS) without improvement in survival [3]. The evidence for ECMO benefits was however becoming more evident especially after influenza H1N1 epidemic. Improved survival was documented with ECMO use among patients with acute respiratory distress syndrome (ARDS) [4–8]. This benefit extended to different clinical settings including the prehospital setting [9] and EDs [10] and to other clinical conditions such as near drowning [11], myocarditis [12], hypothermia [13], overdose [14], and pulmonary embolism [15]. Patients suffering from acute cardiac diseases, including cardiogenic shock, also had improved outcomes after ECMO use [16–20].
Cardiogenic shock is a critical condition characterized by low cardiac output and organ hypoperfusion with hypotension for 30 minutes and elevated left ventricular pressures [21]. Mortality rates for cardiogenic shock can reach up to 40% [22]. According to Extracorporeal Life Support Organization's data registry, cardiogenic shock was the most common cardiac indication for ECMO use in 2015 [23]. Indications for ECMO use usually include persons with severe, acute cardiac and/or respiratory failure who have failed to respond to conventional medical management [24]. A standard set of criteria for ECMO use for patients presenting with cardiogenic shock does not however exist. Cardiac indications for ECMO use typically include low cardiac output and hypotension despite adequate intervention (intravascular volume replacement, inotropic pharmacotherapy, and use of other forms of mechanical circulatory support) [25]. The guidelines for management of heart failure by The American College of Cardiology Foundation/American Heart Association also provide more detailed patient selection criteria for mechanical circulatory support such as ECMO. These criteria consist of “patients with LVEF < 25% and NYHA (New York Heart Association) class III-IV functional status despite guideline-directed medical therapy, including, when indicated, cardiac resynchronization therapy, with either high predicted 1- to 2-year mortality (e.g., as suggested by markedly reduced peak oxygen consumption and clinical prognostic scores) or dependence on continuous parenteral inotropic support” [26].
ECMO has also been shown to be an effective method for supporting hemodynamics in patients with cardiogenic shock due to myocarditis, myocardial infarction, and postcardiotomy [12, 27, 28]. The impact of ECMO use on mortality remains however controversial in this subpopulation: Diddle et al. reported a survival rate to hospital discharge of 61% for patients with acute myocarditis with ECMO use [12]. Kim et al. described ECMO use for patients with cardiogenic shock postmyocardial infarction with reported survival rate to hospital discharge of 59.3% [27]. On the other hand, a high hospital mortality of 67% was reported for patients with refractory cardiogenic shock postcardiotomy [28].
The increasing evidence for ECMO use in cardiogenic shock is showing promise; however its impact is not clear, especially with its associated increase in cost of care and resource utilization. This study examined ECMO use and outcomes of patients with cardiogenic shock (mortality, hospital length of stay, and total hospital charges) in US hospitals.
2. Methods
2.1. Study Design and Setting
This matched retrospective cohort study used discharge data from the Nationwide Emergency Department Sample (NEDS) database. NEDS represents the largest all-payer ED database in the United States and is a Healthcare Cost and Utilization Project (HCUP) database that is sponsored by the Agency for Healthcare Research and Quality (AHRQ) [29].
NEDS combines both clinical and nonclinical variables from both national and state sources, specifically 947 hospitals that represent a 20% stratified sample of hospital-based EDs across 30 participating states in the US. HCUP recommendations and instructions were followed for data weighting using the following stratification variables: US Census region, urban-rural location, ownership, and teaching status of the hospital and trauma center designation [30].
An institutional review board exemption from the American University of Beirut was obtained for the use of this deidentified database. Additionally, data on any variable with size less than or equal to 10 were excluded in order to safeguard patients' privacy and as per HCUP requirements.
2.2. Available Data
NEDS provides data for the following variables: diagnoses and procedural information; demographic patient information; mechanism of injury, intentional harm, and severity of injury; admission and discharge status; payment source; healthcare expenses; and general hospital characteristics. Diagnoses are available as the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes as well as an equivalent and more manageable number of clinically meaningful Clinical Classifications Software (CCS) codes [31].
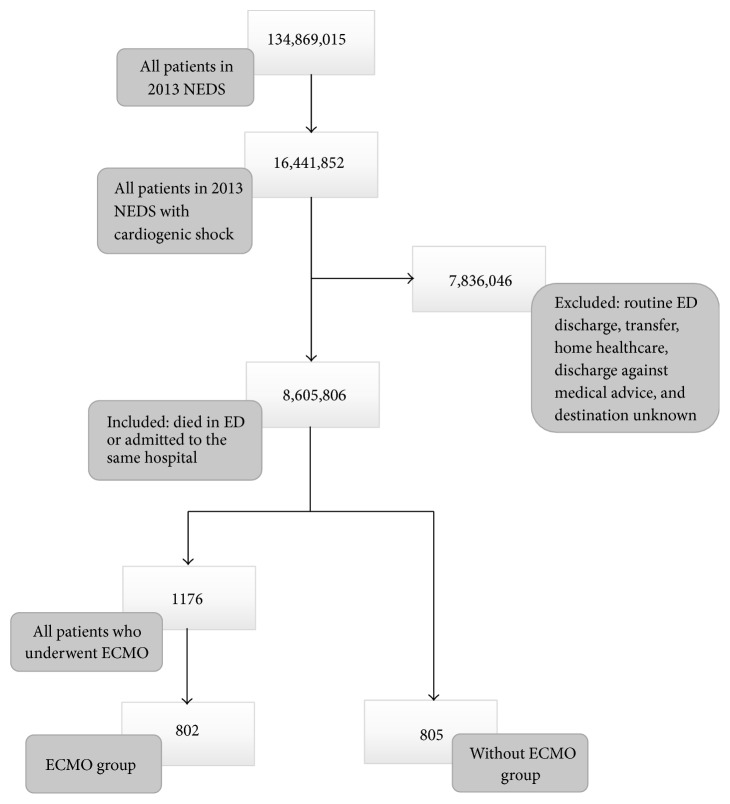
The following CCS codes were adopted from Maxwell et al. to select those presenting with cardiogenic shock: CCS 97, CCS 100, CCS 101, CCS 103, CCS 106, CCS 107, and CCS 108 [32] (a list of equivalent ICD-9-CM codes and variable classification is included as “Supplementary Material (available here)”). ECMO use was selected using the ICD-9-CM 3965 procedure code. Patients who were routinely discharged, transferred, discharged to home healthcare, or discharged against medical advice or whose destination was unknown were excluded from the study. Figure 1 shows a flow chart of patients who met inclusion/exclusion criteria for the study population.
Figure 1.
Flow chart of patients who met inclusion/exclusion criteria for the study population.
A group of patients with cardiogenic shock and reported ECMO use were randomly matched (1 : 1) with another group with cardiogenic shock without ECMO use. The following variables were used for matching: age (match tolerance = 2), sex, season of admission, whether admission day is a weekday or a weekend, presence of chronic conditions, Injury Severity Score (match tolerance = 1), primary expected payer, median household income, hospital urban/rural designation, and the four categories of procedure class (minor diagnostic, minor therapeutic, major diagnostic, and major therapeutic). A procedure is minor or major in terms of invasiveness and/or resource use based on ICD-9-CM procedure codes.
2.3. Statistical Analysis
Descriptive analysis of the study population was done using IBM-SPSS 24. Mean and associated 95% confidence interval (CI) were reported for continuous variables, and frequencies, percentages, and 95% CI were reported for categorical variables. A p value of <0.05 was used to denote statistical significance. HCUPnet, a free online query system based on data from HCUP, was also used to verify and confirm certain analyses.
The Rao-Scott chi-square test, a modified version of Pearson's chi-square test, was used to compare all variables between the two groups at the bivariate level. A logistic regression analysis was used for mortality, while multivariate linear regression was used for LOS and total hospital charge to examine their association with ECMO procedure (yes/no) in the matched data set, adjusting for significant variables. These variables included chronic conditions (infectious and parasitic disease; diseases of blood and blood-forming organs; mental disorders; diseases of the respiratory system; diseases of the genitourinary system; diseases of the skin and subcutaneous tissue; diseases of the musculoskeletal system; symptoms, signs, and ill-defined conditions; injury and poisoning; factors influencing health status; and contact with health services) and selected procedures (temporary and permanent tracheostomy; diagnostic bronchoscopy and biopsy of bronchus; incision of pleura; thoracentesis; chest drainage; other operating room Rx procedures on respiratory system and mediastinum; coronary artery bypass graft; percutaneous transluminal coronary angioplasty; other Operating Room heart procedures; other vascular catheterization, not heart; other non-Operating Room therapeutic cardiovascular procedures; respiratory intubation and mechanical ventilation; blood transfusion; conversion cardiac rhythm). Crude and adjusted odds ratios along with their corresponding 95% CI were calculated. To adjust for the NEDS survey design in developing estimates, the CSDESCRIPTIVES, CSTABULATE, and CSLOGISTIC procedures were used.
3. Results
A total of 134,869,015 weighted ED visits were available in 2013 in the NEDS database. Of those, 16,441,852 were visits that included cardiogenic shock as a diagnostic code. Only 1,176 weighted visits had ECMO procedure documented. After matching for the above-described variables, 802 visits with ECMO were successfully matched with 805 visits without ECMO. Table 1 shows a list of matched characteristics and demographic variables. Patients who underwent ECMO had an average age of 49.9 years (95% CI: 47.1–52.5). They were mostly males (68.4%, 95% CI: 61.6–74.4). A higher frequency of visits (36.3%, 95% CI: 30.1–43.1) was noted to have a corresponding high household median income ($64,000 or more). More admissions occurred during weekdays (73.7%, 95% CI: 67.1–79.3). Patients underwent mainly major therapeutic procedures (ECMO included).
Table 1.
Matched variables for the study groupsa.
| Without ECMO | With ECMO | |||
|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | |
|
| ||||
| Age | 50.0 | (47.7–52.3) | 49.9 | (47.1–52.5) |
|
| ||||
| N | % (95% CI) | N | % (95% CI) | |
|
| ||||
| Injury Severity Score (0–15) a | 800 | 99.4 (96.9–99.9) | 796 | 99.3 (96.0–99.0) |
| Sex | ||||
| Male | 551 | 68.5 (61.6–74.6) | 548 | 68.4 (61.6–74.4) |
| Female | 254 | 31.5 (25.4–38.4) | 254 | 31.6 (25.6–38.4) |
| Season of admission | ||||
| Winter | 199 | 24.7 (19.5–30.8) | 204 | 25.5 (20.2–31.7) |
| Spring | 195 | 24.3 (18.8–30.8) | 185 | 23.0 (17.9–29.2) |
| Summer | 203 | 25.3 (19.8–31.7) | 202 | 25.2 (19.8–31.5) |
| Autumn | 207 | 25.7 (20.1–32.3) | 210 | 26.2 (20.9–32.3) |
| Admission day | ||||
| Monday–Friday | 596 | 74.0 (67.6–79.6) | 591 | 73.7 (67.1–79.3) |
| Saturday-Sunday | 209 | 26.0 (20.4–32.4) | 211 | 26.3 (20.7–32.9) |
| Median household income b | ||||
| $1–$37,999 | 113 | 14.1 (10.0–19.6) | 115 | 14.3 (10.2–19.7) |
| $38,000–$47,999 | 202 | 25.1 (19.7–31.4) | 210 | 26.2 (20.6–32.7) |
| $48,000–$63,999 | 184 | 22.9 (17.6–29.2) | 185 | 23.1 (17.8–29.4) |
| $64,000 or more | 305 | 37.9 (31.6–44.7) | 292 | 36.3 (30.1–43.1) |
| Primary expected payer | ||||
| Medicare & Medicaid | 385 | 47.9 (41.0–54.9) | 384 | 47.8 (41.0–54.7) |
| Private including HMO | 384 | 47.8 (40.9–54.7) | 383 | 47.8 (41.0–54.7) |
| Chronic condition | 805 | 100 | 802 | 100 |
| Procedure class | ||||
| Minor diagnostic | 438 | 54.5 (47.9–60.8) | 448 | 55.9 (49.1–62.4) |
| Minor therapeutic | 725 | 90.1 (85.3–93.4) | 722 | 90.1 (85.5–93.3) |
| Major therapeutic | 800 | 99.4 (96.4–99.9) | 796 | 99.3 (95.9–99.9) |
aPer agreement with HCUP, certain categories of primary expected payer, major diagnostic procedure class, and major trauma category Injury Severity Score were omitted from the table due to a variable count less than 10. bAccording to national quartile for patient ZIP Code derived from ZIP Code-demographic data obtained from Claritas.
Table 2 shows characteristics and demographics that are significantly different between the two groups. While both groups were matched for presence of a chronic condition, the prevalence of subtypes of chronic conditions was different. Diseases of the cardiovascular system were most frequent in both ECMO and non-ECMO groups (100% and 96.8%).
Table 2.
Unmatched variables between the two groupsa.
| Without ECMO | With ECMO | p value | |||
|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | ||
| Chronic condition body system indicator | |||||
| Infectious and parasitic disease | 207 | 25.7 (20.1–32.3) | 315 | 39.3 (32.8–46.1) | 0.004 |
| Blood and blood-forming organs | 257 | 31.9 (25.8–38.6) | 519 | 64.8 (57.8–71.2) | <0.001 |
| Mental disorders | 264 | 32.8 (26.6–39.6) | 128 | 16.0 (11.5–21.7) | <0.001 |
| Circulatory system | 779 | 96.8 (93.0–98.5) | 802 | 100 | 0.014 |
| Respiratory system | 261 | 32.4 (26.2–39.3) | 655 | 81.7 (76.0–86.4) | <0.001 |
| Genitourinary system | 343 | 42.6 (35.9–49.6) | 580 | 72.3 (65.6–78.2) | <0.001 |
| Skin and subcutaneous tissue | 130 | 16.2 (11.7–21.9) | 48 | 6.1 (3.5–10.3) | 0.001 |
| Musculoskeletal system | 233 | 28.9 (23.1–35.5) | 60 | 7.4 (4.7–11.7) | <0.001 |
| Symptoms/signs/ill-defined conditions | 307 | 38.1 (31.7–45.0) | 604 | 75.3 (68.8–80.8) | <0.001 |
| Injury and poisoning | 285 | 35.5 (29.0–42.5) | 478 | 59.6 (52.6–66.2) | <0.001 |
| Health status/contact with health services factors | 537 | 66.7 (59.9–72.9) | 320 | 39.9 (33.6–46.5) | <0.001 |
| Procedures b | |||||
| Extracorporeal membrane oxygenation (ECMO) | 0 | 0 | 802 | 100 | <0.001 |
| Tracheostomy: temporary and permanent | 20 | 2.5 (1.0–5.9) | 81 | 10.2 (6.6–15.4) | 0.002 |
| Diagnostic bronchoscopy and biopsy of bronchus | 25 | 3.0 (1.4–6.5) | 95 | 11.8 (8.2–16.8) | 0.001 |
| Incision of pleura; thoracentesis; chest drainage | 27 | 3.3 (1.5–7.2) | 82 | 10.3 (6.8–15.2) | 0.008 |
| Other Operating Room Rx procedures on respiratory system and mediastinum | 16 | 2.0 (0.7–5.6) | 154 | 19.2 (14.3–25.2) | <0.001 |
| Coronary artery bypass graft (CABG) | 29 | 3.7 (1.9–6.9) | 118 | 14.8 (10.8–19.9) | <0.001 |
| Percutaneous transluminal coronary angioplasty (PTCA) | 185 | 23.0 (17.8–29.3) | 109 | 13.6 (9.5–19.1) | 0.015 |
| Other Operating Room heart procedures | 39 | 4.9 (2.8–8.4) | 401 | 50.0 (43.0–57.0) | <0.001 |
| Other vascular catheterization, not heart | 189 | 23.5 (18.1–29.9) | 288 | 35.9 (29.8–42.6) | 0.006 |
| Other non-Operating Room therapeutic cardiovascular procedures | 239 | 29.8 (23.9–36.3) | 110 | 13.7 (9.6–19.2) | <0.001 |
| Amputation lower extremity | 42 | 5.2 (2.8–9.6) | 11 | 1.4 (0.4–4.6) | 0.04 |
| Respiratory intubation and mechanical ventilation | 101 | 12.5 (8.7–17.8) | 456 | 56.9 (49.9–63.6) | <0.001 |
| Blood transfusion | 118 | 14.7 (10.5–20.3) | 195 | 24.3 (19.0–30.6) | 0.014 |
| Conversion cardiac rhythm | 28 | 3.5 (1.7–7.1) | 146 | 18.2 (13.6–24.0) | <0.001 |
aThe following variables were not significantly different between cases and controls: chronic conditions of endocrine/nutritional/metabolic and immunity disorders, nervous system and sense organs, digestive system, and involving congenital anomalies; presence of injury diagnosis on record; or presence of unintentional injury. bAll procedures are based on Clinical Classification Software (CCS) procedures except ECMO which is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes.
The ED and inpatient procedures recorded for visits in both groups were also different. Only procedures that had significantly different frequencies between the two groups were included in Table 2 (total of 14 procedures). “Respiratory intubation and mechanical ventilation” was the second most frequently performed procedure for patients who underwent ECMO (56.9%, 95% CI: 49.9–63.6). In contrast, only 12.5% of patients in the group without ECMO underwent “respiratory intubation and mechanical ventilation.” The procedures used most often in this latter group were “other nonoperating room therapeutic cardiovascular procedures” (29.8%; 95% CI: 23.9–36.3). Two other procedures were more frequent in the group without ECMO use compared to the group with ECMO use; these were “percutaneous transluminal coronary angioplasty” and “amputation of the lower extremity.”
Table 3 shows main outcome differences between the two groups. Significantly higher mortality was noted in the group with ECMO use (48.9% versus 4%). Visits with ECMO use also had significantly higher average total charges incurred for both ED and inpatient services ($580,065.8 versus $156,436.50) as well as increased average LOS (21.3 versus 11.3 days). Lastly, Table 4 shows odds ratios (OR) of both primary and secondary outcomes before and after adjusting for all variables previously mentioned. Patients with cardiogenic shock and for whom ECMO was used had 8.52 higher likelihood of mortality when compared to those without ECMO use. ECMO use was also associated with higher charges (mean difference = $228,896.0 (standard error of the mean = 55403.8)) but no significant change in hospital LOS (mean difference = 0.8 (standard error of the mean = 4.9)).
Table 3.
Comparison of outcomes between the two groups.
| Without ECMO | With ECMO | p value | |||
|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | ||
|
| |||||
| Primary outcome | |||||
| Survived | 773 | 96.0 (92.5–98.0) | 410 | 51.1 (44.4–57.8) | <0.001 |
| Died in the ED/hospital | 32 | 4.0 (2.0–7.5) | 392 | 48.9 (42.2–55.6) | |
|
| |||||
| Mean | (95% CI) | Mean | (95% CI) | ||
|
| |||||
| Secondary outcomes | |||||
| Total chargesa | $156,436.50 | (123792.1–189080.9) | $580,065.80 | (482527.3–677604.2) | <0.001 |
| LOSb | 11.6 | (8.2–15.1) | 21.3 | (17.3–25.3) | <0.001 |
aTotal charges in US dollars for combined ED and inpatient services. bLength of hospital stay in days.
Table 4.
Unadjusted and adjusted outcomes in the matched dataset.
| Unadjusted | Adjusted a | |||||
|---|---|---|---|---|---|---|
| Mean difference | Standard error | p value | Mean difference | Standard error | p value | |
|
| ||||||
| Total chargesb | $423,629.3 | 52,436.6 | <0.001 | $228,896.0 | 55,403.8 | <0.001 |
| LOSc | 9.6 | 2.7 | <0.001 | 0.8 | 4.9 | 0.870 |
|
| ||||||
| OR | 95% CI | p value | OR | 95% CI | p value | |
|
| ||||||
| Died in the ED/hospital | 23.2 | 11.1–48.4 | <0.001 | 8.5 | 2.8–25.6 | <0.001 |
aAbove outcome adjusted for the following variables: significant chronic conditions (infectious and parasitic disease, diseases of blood and blood-forming organs, mental disorders, diseases of the respiratory system, diseases of the genitourinary system, diseases of the skin and subcutaneous tissue, diseases of the musculoskeletal system, symptoms, signs, and ill-defined conditions, injury and poisoning, factors influencing health status, and contact with health services), and selected procedures (temporary and permanent tracheostomy, diagnostic bronchoscopy and biopsy of bronchus; incision of pleura; thoracentesis; chest drainage, other Operating Room Rx procedures on respiratory system and mediastinum; coronary artery bypass graft, percutaneous transluminal coronary angioplasty; other Operating Room heart procedures; other vascular catheterization, not heart; other non-Operating Room therapeutic cardiovascular procedures; respiratory intubation and mechanical ventilation; blood transfusion; conversion cardiac rhythm). bTotal charges in US dollars for combined ED and inpatient services. cLength of hospital stay in days.
4. Discussion
This study used a retrospective matched cohort design from large national US database of ED visits to examine clinical and financial impact of ECMO use in patients with cardiogenic shock. ECMO use was associated with significantly higher mortality and higher charges in this population.
The mortality rate in the group with ECMO use was 48.9%. This rate is lower than previously reported rates in studies for ECMO use in cardiogenic shock patients and in patients with other medical conditions (66.2% for years 2000–2007 and 63.7% for years 2007–2011) [32]. Survival related to ECMO use may be increasing over time due to improved equipment, more experience and larger caseloads, more specialized centers, more stringent patient selection, improved multidisciplinary team approach, or a combination of any or all the former factors.
The mortality rate in the cardiogenic shock group without ECMO on the other hand was strikingly low (4.0%). The study adopted CCS codes for cardiogenic shock that were previously used to define cardiogenic shock in studies originating from NEDS [32]. This low mortality rate may be related to the different proportions of cardiogenic shock etiologies. In fact, specific etiologies such as acute coronary syndrome are independent predictors of mortality, while other etiologies of cardiogenic shock are associated with lower mortality [22]. Etiologies other than acute coronary syndrome may be predominant in this study population. This variable was however missing in the dataset. Inclusion and exclusion criteria might also be different than other studies reporting higher mortality rates for patients with cardiogenic shock.
Patients who used ECMO in our study had a mean age of 49.9 years. A previous study by Maxwell et al. using NEDS (1998–2009) reported an overall mean age of 53.9 (±0.4) years and an average age of 48.9 (±0.8) for patients with cardiogenic shock with ECMO use [32]. The majority of people who underwent ECMO in our population were also males (68.4%). This is also comparable to findings by Maxwell et al. where 57.5% of patients in the cardiogenic shock group were males [32]. Other studies have also reported similar findings related to male gender and ECMO use in cardiogenic shock and this may be partly related to the higher prevalence of cardiac diseases among males [3].
Visits for cardiogenic shock with ECMO use had much higher charges than visits for cardiogenic shock alone. The average combined hospital and ED charge was $580,065.8 (95% CI: 482,527.3–677,604.2) per visit in the group with ECMO use compared to an average of $156,436.5 (95% CI: 123,792.1–189,080.9) per visit in the group without ECMO use. This average for charges per visit for cardiogenic shock with ECMO use is higher than what was previously reported in the literature during the years 1998–2009 ($344,009 (±$30,707)) [32]. ECMO associated costs are therefore on the rise. ECMO is a complicated procedure that is not easy to implement in a hospital and requires extensive resources and the formation of a comprehensive team of physicians, nurses, and staff [33]. The Extracorporeal Life Support Organization guidelines for ECMO implementation aim at maximizing efficiency and effectiveness of ECMO [33]. This initiative when coupled with advancements in ECMO machines and technology can reduce charges associated with ECMO use [34].
This study also examined the impact of ECMO use on hospital LOS. After adjusting for confounders, the increase in LOS was not significant. The average LOS of 21.3 days for visits with cardiogenic shock with ECMO is consistent finding from a previous study (19.9 days (2000–2007) and 22.6 days (2007–2011)) [3].
Our study has some limitations. One limitation stems from this study's inherent retrospective nature. Visits were identified using discharge diagnoses coding (CCS codes and ICD-9-CM codes) which is dependent on the quality of data, on the expertise and proficiency of the coder, and on the completeness of the patients' records. It is possible that some cases were not included because of coding deficiencies; however, NEDS has been shown to be one of the most robust and inclusive ED datasets available [35]. Additionally, both ICD-9-CM codes and CCS codes do not differentiate between venovenous and venoarterial ECMO. Venovenous ECMO provides respiratory support while venoarterial ECMO compensates for both the respiratory and the hemodynamic supports [25]. Cardiogenic shock is currently an indication only for venoarterial ECMO since it is characterized by cardiac insufficiency [25]. Future use of ICD-10-CM codes would mitigate this limitation.
Another limitation is related to missing important clinical variables from NEDS. Earlier cohort studies on venoarterial ECMO use among cardiogenic shock patients (ENCOURAGE and SAVE) have described significant clinical variables that impact outcomes [36, 37]. NEDS mainly collects administrative data and does not include specific clinical variables (e.g., SOFA score, pre-ECMO use hemodynamics and metabolic parameters, length of ECMO use, and indications for ECMO use) that reflect case mix or clinical severity. As a result, certain confounding variables such as clinical severity and critical nature of each visit were not controlled for. This study however used different methods (matching and logistic regression) to control for several variables considered to be proxies of clinical severity including specific major diagnostic and therapeutic procedures related to cardiac and pulmonary failure (e.g., temporary and permanent tracheostomy, incision of pleura, other Operating Room Rx procedures on respiratory system and mediastinum, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and other Operating Room heart procedures) as well as the presence of chronic medical conditions among different body systems.
Despite these limitations, this study examined the association between a resource intensive clinical intervention and clinical and financial outcomes using the largest ED database from the United States. Its findings are important for assessing expansion or reduction of clinical applications of ECMO and can be easily generalized to other similar acute care settings in the US.
5. Conclusion
Patients with cardiogenic shock who underwent ECMO were found to have increased mortality and higher cost of care without significant increase in length of stay when compared to patients with cardiogenic shock without ECMO use. Future research consisting of randomized clinical trials should prospectively evaluate this observed association between ECMO use in cardiogenic shock patients and outcomes in order to improve further patient care and resources utilization.
Abbreviations
- ECMO:
Extracorporeal membrane oxygenation
- ED:
Emergency department
- ARDS:
Acute respiratory distress syndrome
- CS:
Cardiogenic shock
- NEDS:
Nationwide Emergency Department Sample
- HCUP:
Healthcare Cost and Utilization Project
- AHRQ:
Agency for Healthcare Research and Quality
- ICD-9-CM:
International Classification of Diseases, Ninth Revision, Clinical Modification
- CCS:
Clinical Classifications Software
- CI:
Confidence intervals
- LOS:
Length of hospital stay.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
A list of equivalent ICD-9-CM codes and variable classification.
References
- 1.Marasco S. F., Lukas G., McDonald M., McMillan J., Ihle B. Review of ECMO (Extra Corporeal Membrane Oxygenation) Support in Critically Ill Adult Patients. Heart, Lung and Circulation. 2008;17(4):S41–S47. doi: 10.1016/j.hlc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Zapol W. M., Snider M. T. Extracorporeal Membrane Oxygenation in Severe Acute Respiratory Failure: A Randomized Prospective Study. Journal of the American Medical Association. 1979;242(20):2193–2196. doi: 10.1001/jama.1979.03300200023016. [DOI] [PubMed] [Google Scholar]
- 3.Gerke A. K., Tang F., Cavanaugh J. E., Doerschug K. C., Polgreen P. M. Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007 Pulmonary Medicine. BMC Research Notes. 2015;8(1, article no. 686) doi: 10.1186/s13104-015-1678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwischenberger J. B., Lynch J. E. Will CESAR answer the adult ECMO debate? The Lancet. 2009;374(9698):1307–1308. doi: 10.1016/S0140-6736(09)61630-5. [DOI] [PubMed] [Google Scholar]
- 5.Davies A., Jones D., Bailey M. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. The Journal of the American Medical Association. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 6.Luyt C.-E., Combes A., Becquemin M.-H., et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. CHEST. 2012;142(3):583–592. doi: 10.1378/chest.11-2196. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt M., Hodgson C., Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: A systematic review. Critical Care. 2015;19(1, article no. 99) doi: 10.1186/s13054-015-0806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zangrillo A., Biondi-Zoccai G., Landoni G., et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: A systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Critical Care. 2013;17(1, article no. R30) doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamhaut L., Jouffroy R., Soldan M., et al. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84(11):1525–1529. doi: 10.1016/j.resuscitation.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Mosier J. M., Kelsey M., Raz Y., et al. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: History, current applications, and future directions. Critical Care. 2015;19(1, article no. 431) doi: 10.1186/s13054-015-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K. I., Lee W. Y., Kim H. S., Jeong J. H., Ko H. H. Extracorporeal membrane oxygenation in near-drowning patients with cardiac or pulmonary failure. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine . 2014;22(1, article no. 77) doi: 10.1186/s13049-014-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diddle J. W., Almodovar M. C., Rajagopal S. K., Rycus P. T., Thiagarajan R. R. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Critical Care Medicine. 2015;43(5):1016–1025. doi: 10.1097/CCM.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 13.Jarosz A., Darocha T., Kosiński S., Ziętkiewicz M., Drwiła R. Extracorporeal membrane oxygenation in severe accidental hypothermia. Intensive Care Medicine. 2015;41(1):169–170. doi: 10.1007/s00134-014-3543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Onge M., Fan E., Mégarbane B., Hancock-Howard R., Coyte P. C. Venoarterial extracorporeal membrane oxygenation for patients in shock or cardiac arrest secondary to cardiotoxicant poisoning: A cost-effectiveness analysis. Journal of Critical Care. 2015;30(2):437–437.e14. doi: 10.1016/j.jcrc.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Northey L. C., Shiraev T., Omari A. Salvage intraosseous thrombolysis and extracorporeal membrane oxygenation for massive pulmonary embolism. Journal of Emergencies, Trauma, and Shock. 2015;8(1):55–57. doi: 10.4103/0974-2700.145395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bednarczyk J. M., White C. W., Ducas R. A., et al. Resuscitative extracorporeal membrane oxygenation for in hospital cardiac arrest: A Canadian observational experience. Resuscitation. 2014;85(12):1713–1719. doi: 10.1016/j.resuscitation.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Lin J., Yu H., et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. The Lancet. 2008;372(9638):554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 18.Shin T. G., Choi J.-H., Jo I. J., et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: A comparison with conventional cardiopulmonary resuscitation. Critical Care Medicine. 2011;39(1):1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Deballon I., Hornby L., Shemie S. D., Bhanji F., Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation. 2016;101:12–20. doi: 10.1016/j.resuscitation.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Stub D., Bernard S., Pellegrino V., et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Levy B., Bastien O., Karim B., et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Annals of Intensive Care. 2015;5(1):p. 17. doi: 10.1186/s13613-015-0063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harjola V. P., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. European Journal of Heart Failure. 2015;17(5):501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 23.Thiagarajan R. R., Barbaro R. P., Rycus P. T., et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO Journal. 2017;63(1):60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 24. Extracorporeal Life Support: The ELSO Red Book. 5th Edition: Extracorporeal Life Support Organization (ELSO)
- 25.Makdisi G., Wang I.-W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. Journal of Thoracic Disease. 2015;7(7):E166–E176. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancy C. W., Jessup M., Bozkurt B., et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2017 [Google Scholar]
- 27.Kim H., Lim S.-H., Hong J., et al. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation. 2012;83(8):971–975. doi: 10.1016/j.resuscitation.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Hsu P.-S., Chen J.-L., Hong G.-J., et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. European Journal of Cardio-Thoracic Surgery. 2010;37(2):328–333. doi: 10.1016/j.ejcts.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Boslaugh S. E. HCUP Nationwide Emergency Department Sample (NEDS). 2013, Healthcare Cost and Utilization Project (HCUP) [DOI]
- 30.Barrett M., et al. Population Denominator Data for use with the HCUP Databases (Updated with 2013 Population data) Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 31.HCUP CCS. Healthcare Cost and Utilization Project (HCUP). December 2009. Rockville, MD, USA: U.S. Agency for Healthcare Research and Quality; [PubMed] [Google Scholar]
- 32.Maxwell B. G., Powers A. J., Sheikh A. Y., Lee P. H. U., Lobato R. L., Wong J. K. Resource use trends in extracorporeal membrane oxygenation in adults: An analysis of the Nationwide Inpatient Sample 1998-2009. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(2):416–e1. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 33. ELSO Guidelines For ECMO Centers. 2014: https://www.elso.org/Resources/Guidelines.aspx.
- 34.MacLaren G., Combes A., Bartlett R. H. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Medicine. 2012;38(2):210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 35.Owens P. L., Barrett M. L., Gibson T. B., Andrews R. M., Weinick R. M., Mutter R. L. Emergency department care in the United States: A profile of national data sources. Annals of Emergency Medicine. 2010;56(2):150–165. doi: 10.1016/j.annemergmed.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Muller G., Flecher E., Lebreton G., et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Medicine. 2016;42(3):370–378. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M., Burrell A., Pilcher D., et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. European Heart Journal. 2015;36(33):2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of equivalent ICD-9-CM codes and variable classification.