Abstract
Measurement of serum testosterone (T) levels is recommended in the evaluation of osteoporosis in older men and estradiol (E2) and sex hormone binding globulin (SHBG) levels are associated with the rate of bone loss and fractures, but the clinical utility of sex steroid and SHBG measurements for the evaluation of osteoporosis in men has not been examined. To evaluate whether measurements of T, E2 and/or SHBG are useful for the prediction of fracture risk or the rate of bone loss in older men, we analyzed longitudinal data from 5487 community-based men participating in the MrOS Study in the US, Sweden and Hong Kong. Serum T, E2 and SHBG levels were assessed at baseline; incident fractures were self-reported at 4 month intervals with radiographic verification (US), or ascertained via national health records (Sweden, Hong Kong). Rate of bone loss was assessed by serial measures of hip BMD. We used receiver operating characteristic (ROC) curves, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to assess improvement in prediction. Mean age at baseline was 72-75 years and the prevalence of low T levels (<300 ng/dL) was 7.6-21.3% in the three cohorts. There were 619 incident major osteoporotic and 266 hip fractures during follow up of approximately 10 years. Based on ROC curves, there were no improvements in fracture risk discrimination for any biochemical measure when added to models, including FRAX with BMD. Although minor improvements in NRI were observed for the dichotomous parameters low BioE2 (<11.4 pg/ml) and high SHBG (>59.1 nM), neither sex steroids nor SHBG provided clinically useful improvement in fracture risk discrimination. Similarly, they did not contribute to the prediction of BMD change. In conclusion, there is limited clinical utility of serum E2, T and SHBG measures for the evaluation of osteoporosis risk in elderly men.
Keywords: fracture risk assessment, osteoporosis, aging, epidemiology, DXA
INTRODUCTION
Osteoporotic fractures in men are a major public health problem in the United States.(1,2) Similarly, almost 1.2 million fragility fractures were estimated to occur in men in Europe in 2010,(3) causing more than 20,000 premature deaths. In both men and women, the proximal femur, forearm, and humerus are among the most frequent sites of incident nonvertebral fractures, and a variety of factors amplify the risk of fractures, including increased age, previous fractures, lower bone mineral density (BMD), and falls.(4)
Sex steroids are essential for skeletal integrity in men. For instance, profound hypogonadism induced by androgen deprivation therapy of prostate cancer causes bone loss and an increased fracture rate.(5) Adverse skeletal effects have also been linked to the age-related decline in sex steroid levels that generally occurs in men. Whereas testosterone was long considered to be most critical, recent physiological and epidemiological findings show that estradiol levels are more highly related to the rate of bone loss and fracture in men(4,6,7) and there appears to be a threshold level of estradiol below which they are more probable.(8,9) Increased SHBG is also independently associated with greater bone loss and higher fracture risk.(8,9)
When osteoporosis is a clinical concern, a measurement of serum testosterone is recommended to identify men with sex steroid insufficiency(10,11) but the clinical utility of testosterone, estradiol, or SHBG measurements in this context is unknown. Here we examined the premise that measurements of sex steroids and SHBG provide benefit in the identification of men at higher risk of hip fracture, major osteoporotic fracture (hip, vertebral, humoral or forearm), and bone loss. We used data from a large, multinational, prospective observational study of musculoskeletal health in older men – the MrOS Study – in which baseline measures of sex steroids and SHBG, and long term follow-up for fracture events and bone density change, were available. We evaluated the ability of the biochemical measures to correctly predict the skeletal outcomes using receiver operation characteristic curves, and net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices.
METHODS
MrOS Cohorts
The Osteoporotic Fractures in Men (MrOS) Study Consortium is a collaboration among several large, prospective, longitudinal cohort studies from the United States, Sweden and Hong Kong. Details of the MrOS cohorts have been described.(8,12–15) Men ≥65 yrs old were recruited from local communities. Those who could not ambulate independently, had bilateral hip prostheses, were not expected to survive 6 months or who could not consent were not enrolled. A single protocol for phenotyping was used in all cohorts. For sex steroid measures in the US, 1469 men were randomly selected from the overall US MrOS cohort of 5994. In Sweden (N= 2,542) and Hong Kong (N= 1476) measures were available in all those who had sufficient baseline serum for analyses (from total Swedish and Hong Kong MrOS cohorts of 3014 and 2000, respectively). In all three cohorts the character of those men with sex steroid and SHBG measures was essentially the same as those in the entire cohorts (Supplementary Table 1). Participants with surgical or chemical castration, and androgen or anti-androgen treatment were excluded from these analyses. In the US and Hong Kong cohorts men who received anti-osteoporosis drugs were excluded from these analyses. Information concerning osteoporosis therapy was not available in Sweden. The studies were approved by relevant regulatory bodies and participants provided informed consent.
Hormone assays
Sex steroids were measured using gas chromatography/mass spectrometry, and SHBG by radioimmunoassay. All samples were analyzed in the same lab as described.(9, 16) Bioavailable fractions of testosterone and estradiol were calculated using mass action equations as described by Sodergard et al.(17) using the association constants: testosterone vs SHBG - 5.97; testosterone vs albumin - 4.06; 17b-estradiol vs SHBG - 3.14; 17b-estradiol vs albumin - 4.21. The correlations between calculated bioavailable fractions and calculated free fractions were very high (r~ 0.98), and for the sake of simplicity we report only results for bioavailable fractions.
Incident fractures
We assessed the incidence of hip and major osteoporotic fractures. In the US, participants were contacted every 4 months by mail or telephone to ask about recent fractures; all reported fractures were adjudicated by physician review of radiology reports or x-rays.(18) Fracture follow-up was 99% over 9.5 years. In Sweden, central registers covering all Swedish citizens were used to identify the participants and computerized X-ray archives were searched for new fractures occurring after the baseline visit.(8) Fractures that occurred in Hong Kong were ascertained from q 4-month contacts with participants with verification via medical records/radiographs from the Hong Kong Authority electronic database.(19) Median follow-up time for fractures was 8.6 yrs in the US, 10.6 in Sweden and 9.8 in Hong Kong.
Bone density
In the US, 1068 of the 1469 participants had both baseline and repeat (~4.6 yrs later) hip dual-energy X-ray absorptiometry (DXA) scans using Hologic 4500 scanners (Hologic, Waltham, MA, USA) as described.(20) Repeat BMD measures were not available in 401 men because of death (N= 160), a repeat visit that did not include a BMD measurement (N= 177), refusal or study termination (N= 43), or inadequate scan quality (N= 21). Centralized quality-control procedures, certification of DXA operators, and standardized procedures for scanning were used to ensure reproducibility of DXA measurements. A mixed effects model was used to determine the rate of BMD change between visits.(20)
Statistical Analyses
We evaluated the discriminative ability of baseline sex steroids and SHBG to correctly predict hip fracture and major osteoporotic fracture risk at 10-years of follow up via logistic regression models. Receiver operating characteristic curves (ROC) were produced, and the areas under the ROC curves were compared. We further compared different models by using the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices to assess the improvement in risk prediction over a baseline models containing the corresponding FRAX parameter with or without BMD.(21, 22) To identify the proportion of subjects correctly reclassified by adding sex hormone levels as extra predictors, the incremental discriminative ability of the sex hormone levels compared with FRAX models (10-year probability of fracture, both with and without BMD) were assessed by using category-free NRI, IDI and relative IDI (after rounding predicted values to 4 decimal places). We added interaction terms to models to assess whether the contribution of sex hormone levels to prediction differed by BMD (or FRAX) categories. Country-specific FRAX (version 3.3) estimates of 10-year hip fracture and major osteoporotic fracture risk were calculated at the WHO Collaborating Centre for Metabolic Bone Disease, University of Sheffield, UK.
In the US cohort, annualized percentage change in total hip BMD was analyzed using general linear models. R2 statistics were reported to evaluate how much of the variation in BMD loss rate could be explained by sex hormone and SHBG levels in addition to BMI and age. Fit of the linear regression models were reported by Akaike’s Information Criterion (AIC). Predicted values from models including T, E and SHBG were compared to those from models including only BMI and age.
For each model, continuous baseline sex steroid and SHBG measures were assessed individually and combined. We also examined thresholds to assess predictive utility. We used clinically accepted threshold levels of testosterone(23) and levels of estradiol and SHBG established in our previous work(8,9,19) that demonstrated that lower levels of testosterone and estradiol and higher levels of SHBG are associated with increased fracture risk (total T ≤ 300 ng/dl, total E2 ≤ 16 pg/ml, Bio T < 163.5 ng/dl, Bio E2 < 11.4 pg/ml, SHBG > 59.1Nm).
RESULTS
The baseline characteristics of the three cohorts are shown in Table 1. The rate of incident hip and major osteoporotic fractures was higher in the Swedish compared with the US and Hong Kong cohorts, reflecting established epidemiological patterns. The distribution of sex steroid levels in the MrOS cohorts was broad (Supplementary Table 2) and a substantial proportion of men had low T or E2 levels. For instance, in ~20% of men in the US testosterone levels were ≤ 300 ng/dL. As we have previously reported(24) testosterone levels were higher in Hong Kong than in the US and Sweden, a finding apparently associated with lower BMI in Hong Kong men. In general, sex steroid levels were lower as age increased, while SHBG levels were higher (Supplementary Table 3).
Table 1.
Baseline Characteristics for MrOS analytic cohorts.
| Baseline Characteristics | MrOS Sweden (N = 2542) |
MrOS US (N = 1469) |
MrOS Hong Kong (N = 1476) |
|---|---|---|---|
| Age (years), Mean(SD) | 75 (3) | 74 (6) | 72 (5) |
| Body mass index (kg/m2), Mean (SD) | 26.3 (3.6) | 27.4 (3.7) | 23.3 (3.2) |
| Femoral neck BMD (g/m2), Mean (SD) | 0.83 (0.13)* | 0.78 (0.13) | 0.69 (0.11) |
| Total Testosterone (ng/dl), Mean (SD) | 456.76 (176.35) | 421.48 (161.31) | 548.53 (203.96) |
| Total Testosterone <= 300 ng/dl, N (%) | 437 (17.2) | 313 (21.3) | 112 (7.6) |
| Total Estradiol (pg/ml), Mean (SD) | 21.18 (7.52) | 20.61 (7.86) | 24.67 (14.37) |
| Total Estradiol <= 16 pg/ml, N (%) | 616 (24.2) | 413 (28.1) | 190 (12.9) |
| SHBG (nM), Mean (SD) | 47.87 (22.13) | 49.59 (19.78) | 51.20 (20.37) |
| SHBG > 59.1 nM, N (%) | 569 (22.4) | 372 (25.3) | 407 (27.6) |
| Bioavailable Testosterone (ng/dl), Mean (SD) | 241.29 (86.57) | 213.55 (64.70) | 279.41 (79.19) |
| Bioavailable Testosterone < 163.5 ng/dl, N (%) | 395 (15.5) | 266 (18.1) | 89 (6.0) |
| Bioavailable Estradiol (pg/ml), Mean (SD) | 13.86 (5.04) | 13.10 (4.69) | 15.68 (9.80) |
| Bioavailable Estradiol < 11.4 pg/ml, N (%) | 840 (33.0) | 516 (35.1) | 284 (19.2) |
| FRAX score(10 year fracture probability) with BMD | |||
| Hip fracture, Mean (SD) | 4.73 (4.96)* | 2.43 (3.20) | 3.11 (2.55) |
| Major osteoporotic fracture, Mean (SD) | 9.83 (5.48)* | 7.76 (4.42) | 6.74 (3.17) |
| FRAX score(10 year fracture probability) without BMD | |||
| Hip fracture, Mean (SD) | 5.97 (4.09) | 3.56 (3.64) | 3.48 (2.40) |
| Major osteoporotic fracture, Mean (SD) | 11.07 (4.35) | 9.05 (4.68) | 6.89 (2.80) |
| Incidence of fracture during ~10-year follow-up | |||
| Hip fracture, N (%) | 174 (6.8) | 47 (3.2) | 45 (3.1) |
| Major Osteoporosis fracture, N (%) | 418 (16.4) | 106 (7.2) | 95 (6.4) |
n= 2516
Fracture discrimination
Using ROC analyses, the dichotomous parameters low BioE2 (< 11.4 pg/ml;) and high SHBG (<59.1 nM) slightly increased AUC for major osteoporotic fractures in models including age alone in MrOS Sweden but not in MrOS US or MrOS Hong Kong (Supplementary Table 4). Neither sex steroids nor SHBG provided benefit (p> 0.05) when added to models including FRAX with BMD (Table 2) in discriminating those with incident hip or major osteoporotic fractures. This was true when considering total or bioavailable sex steroid levels, when using continuous or categorical levels, or when these factors were used alone or in combination. Results were similar in all cohorts. The lack of discriminative ability is also illustrated in Figure 1, where ROC curves examining testosterone or estradiol levels in the evaluation of major osteoporotic fractures in the US cohort are shown analyzed alone (a,b) or when included with age, BMI and BMD (d,e).
Table 2.
Discrimination (Area under the ROC curve: AUC) and reclassification for fracture in three MrOS cohort from base model of FRAX (10 year probability of fracture, calculated with knowing BMD)
| Sweden (N = 2516) | US (N = 1469)* | Hong Kong (N = 1476) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Reclassification | Reclassification | Reclassification | ||||||||||
| AUC | Category-free (4-decimal) NRI (95% CI) | AUC | Category-free (4-decimal) NRI (95% CI) | AUC | Category-free (4-decimal) NRI (95% CI) | |||||||
| Combined | Event | Non-event | Combined | Event | Non-event | Combined | Event | Non-event | ||||
| Hip Fracture | N=173 | N=47 | N=45 | |||||||||
|
| ||||||||||||
| Base model | 0.72 | 0.78 | 0.74 | |||||||||
|
| ||||||||||||
| Bio T | 0.71 | 0.05 (−0.10,0.21) | 0.08 | −0.02 | 0.79 | −0.07 (−0.36,0.22) | −0.05 | −0.02 | 0.72 | 0.33 (0.04,0.62) | 0.20 | 0.13 |
|
| ||||||||||||
| Low BioT (<163.5 ng/dl) | 0.72 | 0.03 (−0.12,0.18) | −0.66 | 0.69 | 0.75 | 0.18 (−0.09,0.45) | −0.44 | 0.62 | 0.75 | 0.10 (−0.08,0.29) | −0.78 | 0.88 |
|
| ||||||||||||
| Bio E2 | 0.71 | 0.11 (−0.04,0.26) | 0.11 | 0.00 | 0.78 | −0.03 (−0.34,0.27) | −0.02 | −0.01 | 0.73 | 0.34 (0.05,0.62) | 0.27 | 0.07 |
|
| ||||||||||||
| Low BioE2 (<11.4 pg/ml) | 0.70 | 0.26 (0.10,0.41) | −0.10 | 0.36 | 0.75 | 0.19 (−0.11,0.49) | −0.07 | 0.26 | 0.73 | 0.20 (−0.07,0.47) | −0.42 | 0.62 |
|
| ||||||||||||
| Total T | 0.72 | −0.07 (−0.22,0.08) | 0.02 | −0.09 | 0.78 | −0.07 (−0.35,0.20) | −0.17 | 0.10 | 0.74 | 0.12 (−0.16,0.40) | 0.18 | −0.06 |
|
| ||||||||||||
| Low TotalT (≤300 ng/dl) | 0.72 | 0.02 (−0.14,0.17) | 0.68 | −0.66 | 0.76 | 0.09 (−0.13,0.31) | 0.66 | −0.57 | 0.75 | 0.07 (−0.11,0.26) | −0.78 | 0.85 |
|
| ||||||||||||
| Total E2 | 0.71 | 0.08 (−0.07,0.24) | 0.14 | −0.06 | 0.78 | −0.05 (−0.32,0.23) | −0.13 | 0.08 | 0.73 | 0.08 (−0.21,0.37) | 0.13 | −0.06 |
|
| ||||||||||||
| Low TotalE2 (≤16 pg/ml) | 0.71 | 0.12 (−0.03,0.28) | −0.40 | 0.52 | 0.77 | 0.12 (−0.15,0.40) | −0.32 | 0.44 | 0.74 | 0.28 (0.02,0.55) | −0.47 | 0.75 |
|
| ||||||||||||
| SHBG | 0.71 | 0.12 (−0.04,0.27) | −0.14 | 0.26 | 0.75 | 0.02 (−0.27,0.30) | −0.19 | 0.21 | 0.73 | 0.03 (−0.26,0.32) | −0.20 | 0.23 |
|
| ||||||||||||
| High SHBG (>59.1nM) | 0.70 | 0.14 (−0.01,0.29) | −0.42 | 0.56 | 0.75 | 0.14 (−0.13,0.41) | −0.36 | 0.50 | 0.73 | 0.30 (0.01,0.60) | −0.16 | 0.46 |
|
| ||||||||||||
| Major Osteoporotic Fracture | N=416 | N=106 | N=95 | |||||||||
|
| ||||||||||||
| Base model | 0.65 | 0.65 | 0.69 | |||||||||
|
| ||||||||||||
| Bio T | 0.66 | 0.09 (−0.02,0.19) | 0.12 | −0.03 | 0.65 | 0.14 (−0.06,0.34) | 0.13 | 0.01 | 0.69 | 0.23 (0.03,0.44) | 0.14 | 0.09 |
|
| ||||||||||||
| Low BioT (<163.5 ng/dl) | 0.66 | 0.09 (−0.02,0.19) | −0.62 | 0.71 | 0.65 | 0.19 (0.01,0.37) | −0.44 | 0.63 | 0.70 | 0.14 (−0.01,0.28) | −0.75 | 0.89 |
|
| ||||||||||||
| Bio E2 | 0.66 | 0.10 (−0.01,0.20) | 0.10 | 0.00 | 0.65 | 0.10 (−0.10,0.31) | 0.10 | 0.00 | 0.69 | 0.20 (−0.01,0.41) | 0.16 | 0.04 |
|
| ||||||||||||
| Low BioE2 (<11.4 pg/ml) | 0.65 | 0.21 (0.11,0.32) | −0.16 | 0.37 | 0.65 | 0.25 (0.05,0.45) | −0.02 | 0.27 | 0.68 | 0.20 (−0.01,0.41) | 0.16 | 0.04 |
|
| ||||||||||||
| Total T | 0.65 | −0.01 (−0.12,0.09) | 0.07 | −0.09 | 0.65 | 0.04 (−0.16,0.23) | −0.11 | 0.15 | 0.68 | 0.21 (0.01,0.41) | 0.26 | −0.05 |
|
| ||||||||||||
| Low TotalT (≤300 ng/dl) | 0.66 | 0.02 (−0.09,0.12) | −0.64 | 0.66 | 0.65 | 0.05 (−0.10,0.21) | 0.62 | −0.57 | 0.69 | 0.06 (−0.06,0.19) | −0.79 | 0.85 |
|
| ||||||||||||
| Total E2 | 0.65 | 0.05 (−0.06,0.15) | 0.11 | −0.07 | 0.66 | 0.03 (−0.17,0.22) | −0.07 | 0.09 | 0.69 | 0.11 (−0.10,0.31) | 0.14 | −0.03 |
|
| ||||||||||||
| Low TotalE2 (≤16 pg/ml) | 0.65 | 0.09 (−0.01,0.20) | −0.44 | 0.53 | 0.66 | 0.07 (−0.12,0.25) | −0.38 | 0.44 | 0.69 | 0.22 (0.05,0.39) | −0.54 | 0.76 |
|
| ||||||||||||
| SHBG | 0.66 | 0.17 (0.06,0.27) | −0.11 | 0.27 | 0.67 | 0.21 (0.01,0.41) | −0.06 | 0.26 | 0.69 | 0.01 (−0.19,0.21) | −0.17 | 0.18 |
|
| ||||||||||||
| High SHBG (>59.1nM) | 0.65 | 0.18 (0.07,0.28) | −0.40 | 0.58 | 0.66 | 0.25 (0.06,0.44) | −0.26 | 0.51 | 0.69 | 0.18 (−0.02,0.37) | −0.28 | 0.46 |
Bold AUC or combined NRI indicates significant improvement in prediction, p < 0.05(two-sided);
Base model only contains FRAX with BMD;
Missing measured albumin resulted in missing values for 86 bioavailable testosterone and estradiol values in US data. For bioavailable T and E2 measures: number of hip fracture = 43, AUC of base model = 0.79; number of major osteoporotic fractures = 100, AUC of base model = 0.65
Figure 1.
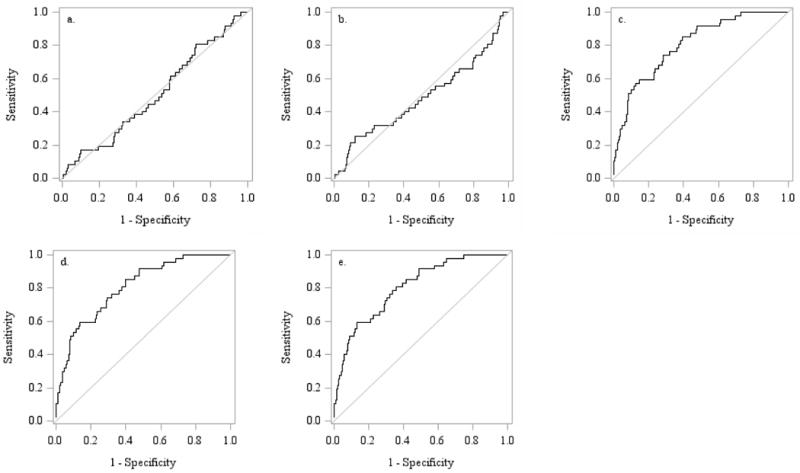
ROC curves showing the discrimination of men with or without incident hip fracture in the US cohort. a. Total testosterone b. Total estradiol c. Age, BMI and femoral neck BMD alone. d. Total testosterone, age, BMI and BMD. e. Total estradiol, age, BMI and BMD
Fracture reclassification
Major osteoporotic fractures: Neither serum T nor BioT improved major osteoporotic fracture reclassification as evaluated by NRI or IDI (Table 2, Supplemental Tables 4–6). Using NRI, statistically significant improvements of FRAX models (with and without BMD) were seen for the dichotomous parameters low BioE2 (< 11.4 pg/ml) and high SHBG (<59.1 nM) in the Swedish and US cohorts, and similar tendencies were observed in the Hong Kong cohort. The improved combined NRI was mainly the result of a correct downward reclassification of those without a fracture (Table 2, Supplemental Table 5). Similarly, IDI was significantly improved by low BioE2 in FRAX models without BMD in all three cohorts (Supplemental Table 6) but this improvement was less for FRAX models with BMD (Supplemental Table 4). However, in all instances these predictive improvements were quantitatively very small and not clinically helpful. Relative IDI figures (Supplemental Tables 4, 6) reflected some influence of estradiol and SHBG levels when added to the models including FRAX, but since base model predicted probabilities differed only slightly between fractured and non-fractured groups the clinical value of adding sex steroids or SHBG to the models was minimal. Results were essentially the same when sex steroids or SHBG were added to a base model that included age alone (Supplemental Tables 7, 8), and were similar when data was examined after 5 years of observation (data not shown). Finally, to assess whether the predictive value of sex steroids or SHBG was more robust in younger men, we examined whether the prediction of major osteoporotic facture was improved in men <75 years of age. In Supplemental Table 9 we show that results in younger men (NRI, IDI) were very similar to those in the entire group.
Hip fractures
Hip fracture reclassification was not significantly improved by T, BioT or SHBG (Table 2, Supplemental Tables 4–6). The lack of improvement by T is illustrated in Figure 2 showing the reclassification data from the US cohort. For hip fracture reclassification a significant improvement of NRI by low BioE2 was observed in the Swedish cohort (Table 2). Evaluation of the improvement using IDI in the Swedish cohort revealed that it was significant in FRAX models without BMD (Supplemental Table 6); the improvement was not significant in FRAX models with BMD (Supplemental Table 4). Again, the magnitude of improvement was minor and results were essentially the same when sex steroids or SHBG were added to a base model that included age alone (Supplemental Tables 7, 8). Results were not substantially different in men <75 years of age (Supplemental Table 9).
Figure 2.
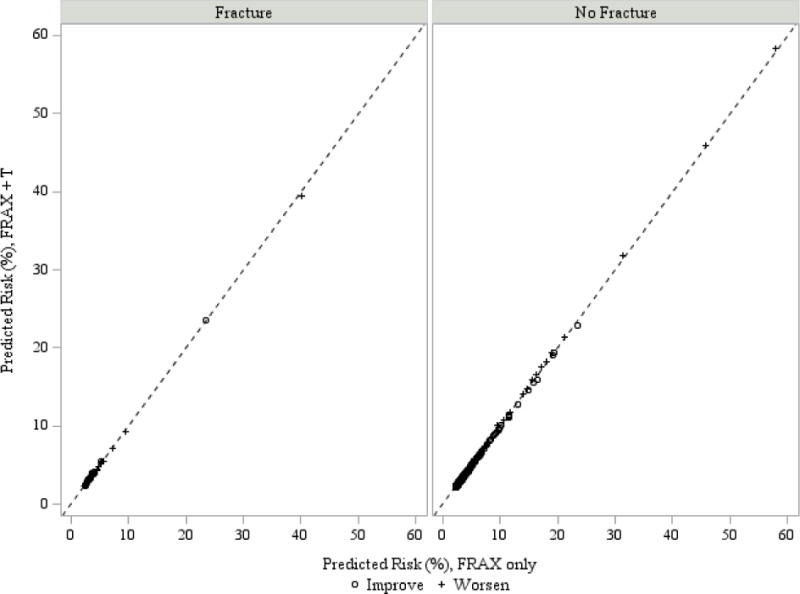
Change in predicted values by the addition of total testosterone to FRAX (with BMD) for hip fracture in the US cohort
In the case of both hip fractures and major osteoporotic fractures there was no interaction between baseline level of BMD or FRAX score and the predictive value of sex steroids, indicating that the limitation of sex steroids in the prediction of fractures appeared across a broad range of underlying risk.
Bone loss
We examined the ability of sex steroids and SHBG to contribute to the prediction of rate of change in BMD in the US cohort. As with fracture outcome, the quantitative improvements in prediction were very small. The predicted values changed <1%, 2% and 4% on average, when adding T, E and SHBG, respectively, to models that included age and BMI.
DISCUSSION
Using three large cohorts of community dwelling older men in the US, Europe and Asia we examined the utility of measures of sex steroid and SHBG for the prediction of fracture risk and bone loss. Consistent with previous analyses we found that the dichotomous parameters low BioE2 and high SHBG contributed statistically to major osteoporotic fracture reclassification while no improvement was seen for T or BioT. However, the magnitudes of the reclassification improvements by BioE2 and SHBG were very small and no clinically meaningful improvements in fracture discrimination using ROC analyses were observed. Similarly, sex steroid measures provided little benefit in predicting the rate of future bone loss. These results suggest that measuring T, E2 or SHBG does not provide important information in the routine evaluation of osteoporosis risk in community dwelling, older men.
In these analyses we consider the clinical value of assessing sex steroid and SHBG measures for the prediction of major osteoporotic and hip fractures. We have also examined similar issues related to vertebral fracture. Vandenput at al.(25) recently reported that higher SHBG levels are associated a greater risk of vertebral fracture in the Swedish and Hong Kong MrOS cohorts, but found that the predictive value of SHBG measures was modest. Cawthon et al. found a similar relationship in men in the US(26). In both analyses, testosterone and estradiol levels were not associated with vertebral fracture risk. These reports reinforce the results we report here.
We and others have reported that sex steroids and SHBG are associated with fracture risk and bone loss in older men.(4,7–9,27) Those reports, in conjunction with an extensive literature that demonstrates the biological importance of sex steroids in bone biology, strongly suggest that they play a role in skeletal homeostasis in men. In fact, our current well-powered 10 year fracture data support these findings; the dichotomous parameters low BioE2 and high SHBG both contributed with statistically significant increments to fracture reclassification for major osteoporotic fractures. Interestingly, the reclassification parameter IDI was moderately but significantly improved by low BioE2 in FRAX models without BMD in all three cohorts but this improvement was attenuated for FRAX models with BMD, suggesting that part of the impact of low BioE2 on major osteoporotic fracture risk is mediated via effects on BMD. However, the statistical significance of these relationships was dependent on the large cohorts and number of events rather than the effect sizes. The key finding in the current analyses is that the reclassification improvements observed were quantitatively marginal, and were not clinically meaningful.
We assessed the clinical usefulness of sex steroid and SHBG measures alone, in combination, and when used in addition to the commonly employed fracture risk prediction algorithm FRAX. In no case was the prediction of fracture risk enhanced. FRAX incorporates a number of variables that might mediate effects of sex steroids or SHBG on bone (e.g., bone mineral density) but does not include indicators of fall risk which might be related to fracture risk and have been associated with lower testosterone levels.(28) That testosterone measures did not enhance the predictive value of FRAX suggests that considering effects of testosterone on fracture risk that are mediated by falls or other mechanisms is not useful when assessing likelihood of incident fracture.
Our findings are important for designing the strategy for the clinical evaluation of osteoporosis risk. The MrOS men are similar to representative cohorts of community dwelling men(29) and our results should be applicable to that broad group. Current guidelines routinely include the measurement of testosterone in the assessment of these men in whom the risk of fractures may be high.(10,11,30) In that context, our results show that it is unlikely that measuring testosterone, estradiol or SHBG is useful in generally healthy, community dwelling older men. Nevertheless, there may be other populations in which these measurements could be appropriate. For instance, younger men with unexplained osteoporosis may have undetected hypogonadism and could benefit from assessments of testosterone levels and therapy with testosterone. In some older men, such as those with clinical evidence of organic causes of hypogonadism, measurement of testosterone would be appropriate, but these men are likely to be uncommon in the general population.(31,32)
Strengths and limitations
Our analysis has substantial strengths. The large size of our populations provided robust power to detect clinically meaningful effect sizes, and the consistency of finding across three independent cohorts reinforces the results. Importantly, the inclusion of cohorts from widely disparate geographical regions suggests that our results have worldwide validity. We used state of the art measures of sex steroid levels. There are also several potential limitations. For instance, the US and Honk Kong cohorts were not randomly selected from their communities. Although their characteristics are similar to representative populations, our finding may not be generalizable to some groups of older men, particularly those with significant co-morbidities that may be under-represented in MrOS. In addition, these results may not be relevant for men with organic causes of severe hypogonadism such as androgen deprivation therapy, pituitary dysfunction or testicular disease.
CONCLUSION
Measurements of sex steroid and SHBG levels in community dwelling older men did not provide clinically useful information for the prediction of the risk of incident fracture or bone loss. This information is essential for designing clinically useful algorithms for the evaluation of osteoporosis in older men.
Supplementary Material
Acknowledgments
The authors acknowledge the invaluable contributions of all the investigators and staff who contributed to the MrOS study, and the study participants who donated their considerable time and energy.
Financial support: The Osteoporotic Fractures in Men (MrOS) Study in the US was supported by the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
MrOS in Sweden is supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Torsten Söderberg’s Foundation, and the Novo Nordisk Foundation
MrOS in Hong Kong was supported by a U.S. National Institute of Health R01 Grant AR049439-01A1, the Research Grants Council Earmarked Grant CUHK 4101/02M, and a direct grant for research of The Chinese University of Hong Kong (No. 2041657)
Footnotes
Supplemental Data: Included in submission
DISCLOSURES/CONFLICTS OF INTEREST: ESO receives research and consulting support from Merck, Lilly and Amgen. JL, PYW, LV, ARH, HF, GAL, MN, OL, AK, ML, MK, DM, SK, AK, TK and CO have nothing to declare.
Author’s roles: EO and CO had full access to data and take responsibility for the integrity of the data and the accuracy of the data analysis. Data analysis: All authors. Study concept and design: EO, JL, CO. Data collection: EO, CO, AK. Drafting of the manuscript: EO, CO, JL. Revising manuscript content: All authors. Statistical analysis: JL, PW, LV, MN. Obtained funding: EO and CO. Administrative, technical, or material support: EO and CO. Study supervision: EO.
Contributor Information
Eric S. Orwoll, Division of Endocrinology, Diabetes, and Clinical Nutrition – Bone and Mineral Unit, School of Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Road CR 113, Portland, OR, USA 97239.
Jodi Lapidus, Email: Lapidusj@ohsu.edu, Dept of Public Health and Preventive Medicine, Division of Biostatistics, Oregon Health & Science University, Portland, OR.
Patty Y. Wang, Email: wangyin@ohsu.edu, Bone and Mineral Unit, Dept of Medicine, Oregon Health & Science University, Portland, OR.
Liesbeth Vandenput, Email: liesbeth.vandenput@medic.gu.se, Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Andrew R. Hoffman, Email: arhoffman@stanford.edu, Dept of Medicine, Stanford University, Stanford, CA.
Howard A. Fink, Email: howard.fink@va.gov, Geriatric Research Education & Clinical Center, VA Medical Center, Minneapolis, MN; Dept of Medicine, University of Minnesota, Minneapolis, MN.
Gail A. Laughlin, Email: glaughlin@ucsd.edu, Division of Epidemiology, Dept of Family Medicine and Public Health, School of Medicine, University of California San Diego, La Jolla, CA.
Maria Nethander, Email: maria.nethander@gu.se, Bioinformatics Core Facility, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Östen Ljunggren, Email: osten.ljunggren@medsci.uu.se, Dept of Medical Sciences, University of Uppsala, Uppsala, Sweden.
Andreas Kindmark, Email: andreas.kindmark@medsci.uu.se, Dept of Medical Sciences, University of Uppsala, Uppsala, Sweden.
Mattias Lorentzon, Email: mattias.lorentzon@medic.gu.se, Centre for Bone and Arthritis Research and Dept of Geriatric Medicine, Inst of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Magnus Karlsson, Email: Magnus.Karlsson@med.lu.se, Clinical and Molecular Osteoporosis Research Unit, Dept of Clinical Sciences, Lund University; Dept of Orthopaedics, Malmö University Hospital, Malmö, Sweden.
Dan Mellström, Email: dan.mellstrom@vgregion.se, Centre for Bone and Arthritis Research and Dept of Geriatric Medicine, Inst of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Anthony Kwok, Email: anthonykwok@cuhk.edu.hk, Department of Orthopaedics and Traumatology, and Jockey Club Centre for Osteoporosis Care and Control, The Chinese University of Hong Kong.
Sundeep Khosla, Email: khosla.sundeep@mayo.edu, Division of Endocrinology, Mayo Clinic, Rochester, MN.
Timothy Kwok, Email: tkwok@cuhk.edu.hk, Jockey Club Centre for Osteoporosis Care and Control, and Department of Medicine and Therapeutics, The Chinese University of Hong Kong.
Claes Ohlsson, Email: claes.ohlsson@medic.gu.se, Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–51. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Laurent MR, Gielen E, Vanderschueren D. Estrogens, the be-all and end-all of male hypogonadal bone loss? Osteoporos Int. 2015;26(1):29–33. doi: 10.1007/s00198-014-2865-4. [DOI] [PubMed] [Google Scholar]
- 7.Cauley JA, Ewing SK, Taylor BC, et al. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density–the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2010;95(9):4314–23. doi: 10.1210/jc.2009-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellstrom D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23(10):1552–60. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94(9):3337–46. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 11.Compston J, Bowring C, Cooper A, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013;75(4):392–6. doi: 10.1016/j.maturitas.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Mellstrom D, Johnell O, Ljunggren O, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21(4):529–35. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 13.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kwok T, Khoo CC, Leung J, et al. Predictive values of calcaneal quantitative ultrasound and dual energy X ray absorptiometry for non-vertebral fracture in older men: results from the MrOS study (Hong Kong) Osteoporos Int. 2012;23(3):1001–6. doi: 10.1007/s00198-011-1634-x. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95(10):E151–60. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 18.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–9. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 19.Woo J, Kwok T, Leung JC, Ohlsson C, Vandenput L, Leung PC. Sex steroids and bone health in older Chinese men. Osteoporos Int. 2012;23(5):1553–62. doi: 10.1007/s00198-011-1552-y. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon PM, Ewing SK, Mackey DC, et al. Change in hip bone mineral density and risk of subsequent fractures in older men. J Bone Miner Res. 2012;27(10):2179–88. doi: 10.1002/jbmr.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 24.Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010 Oct;95(10):E151–60. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenput L, Mellstrom D, Kindmark A, et al. High serum SHBG predicts incident vertebral fractures in elderly men. J Bone Miner Res. 2016 Mar;31(3):683–9. doi: 10.1002/jbmr.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon PM, Schousboe JT, Harrison SL, et al. Sex hormones, sex hormone binding globulin, and vertebral fractures in older men. doi: 10.1016/j.bone.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–60. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006 Oct 23;166(19):2124–31. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 29.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study –a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005 Oct;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358(14):1474–82. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 31.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of A Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. Journal of Clinical Endocrinology & Metabolism. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 32.Rohrmann S, Platz EA, Selvin E, Shiels MS, Joshu CE, Menke A, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clin Endocrinol (Oxf) 2011;75(2):232–9. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


