Abstract
Background
Female sex is conventionally considered a risk factor for coronary artery bypass grafting (CABG) and has been included as a poor prognostic factor in multiple cardiac operative risk evaluation scores. We aimed to investigate the association of sex and the long-term benefit of CABG in patients with ischemic left ventricular (LV) dysfunction enrolled in the prospective Surgical Treatment for Ischemic Heart Failure Study (STICH) trial.
Methods
The STICH trial randomized 1212 patients [148 (12%) women and 1064 (88%) men] with CAD and LV ejection fraction (EF)≤ 35% to CABG + medical therapy (MED) versus MED alone. Long-term (10-year) outcomes with each treatment were compared according to sex.
Results
At baseline, women were older (63.4 vs 59.3, p=0.016) with higher BMI (27.9 vs 26.7, p=0.001). Women had more CAD risk factors (diabetes 55.4% vs 37.2%, hypertension 70.9% vs 58.6%, hyperlipidemia 70.3% vs 58.9%) except for smoking (13.5% vs 21.8%), and had lower rates of prior CABG (0% vs 3.4%, all p<0.05) than men. Moreover, women had higher New York Heart Association (NYHA) class (Class III/IV 66.2% vs 57.0%), lower 6-min walk capacity (300m vs 350m) and lower Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary scores (51 vs 63) (all p<0.05). Over 10-years of follow up, all- cause mortality (49.0% vs 65.8%, adjusted HR 0.67, CI 0.52–0.86, p=0.002) and CV mortality (34.3% vs 52.3%, adjusted HR 0.65, CI 0.48–0.89, p=0.006) were significantly lower in women compared to men. With randomization to CABG + MED vs. MED treatment, there was no significant interaction between sex and treatment group in all-cause mortality, CV mortality, or the composite of all-cause mortality or CV hospitalization (all p>0.05). In addition, surgical deaths were not statistically different (1.5% vs 5.1%, p=0.187) between sexes among patients randomized to CABG per protocol as initial treatment.
Conclusions
Sex is not associated with the effect of CABG + MED vs. MED on all-cause mortality, CV mortality, the composite of death or CV hospitalization, or surgical deaths in patients with ischemic LV dysfunction. Thus, sex should not influence treatment decisions regarding CABG in these patients.
Keywords: heart failure, women, coronary artery bypass grafting
Introduction
Sex-specific differences have been recognized with respect to prevalence, etiology and prognosis of coronary artery disease (CAD) and ischemic heart failure (HF).1–4 Despite having lower burden of obstructive CAD by coronary angiography and better left ventricular ejection fraction (LVEF) compared to men, women with CAD and ischemic HF are usually more symptomatic, have lower functional capacity, worse quality of life, higher rate of ischemia, and possible higher mortality rate post myocardial infarction, all of which could lead to higher health costs associated with frequent office visits and hospitalization.4–12 In fact, CAD is the leading cause of death and HF is the leading cause of hospitalization in women over the age of 65.13, 14 Despite these facts, studies have suggested that physicians are less likely to pursue an aggressive approach to CAD in women than in men.15, 16 In addition, female sex is conventionally considered a risk factor for open-heart surgery, and has been included as a poor prognostic factor in multiple cardiac operative risk scores, e.g. EuroScore II, STS score, modified Parsonnet’s score, New York’s Cardiac Surgery Reporting System score, and Northern New England Cardiovascular Disease Study Group score.17–21
The STICH trial provides a unique opportunity to examine sex differences in the baseline characteristics and clinical outcomes of a high-risk group of patients with severe ischemic LV dysfunction, treated with contemporary guideline directed medical therapy with or without surgical revascularization. Furthermore, the long-term follow-up for mortality in STICHES can provide additional information based on sex.22 Therefore, the objective of this study was to investigate the association of sex on the long-term benefit of CABG in patients enrolled in the prospective STICH trial.
Methods
The STICH/STICHES data, analytic methods, and study results are available for review online.23 The raw datasets and analysis datasets have been deidentified and submitted to NHLBI and will be published at the NHLBI BioLinCC website in the future.24
Study Population
The design of the STICH trial has been described previously.25 In brief, STICH was a prospective, multicenter, randomized controlled trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) that recruited 1212 patients with CAD and LVEF ≤35% from 99 sites in 22 countries between 2002 and 2007. The STICH Hypothesis 1 examined the question whether CABG with optimized medical therapy (MED) improves long-term survival compared with MED alone. The primary results of Hypothesis 1 have been published.26 Detailed inclusion and exclusion criteria have also been previously described. The NHLBI and the ethics committee at each participating institution approved the study protocol. All patients provided written informed consent.
Statistical Analysis
Baseline characteristics for women and men were summarized by the median and interquartile range for continuous variables and by the frequency and percentage for categorical variables. Comparisons of baseline characteristics between men and women were assessed using the Wilcoxon rank-sum test for continuous variables and the Chi-square test or Fisher’s exact test for categorical variables. Cumulative event rates of clinical outcomes (all-cause mortality, cardiovascular mortality, mortality or cardiovascular hospitalization, sudden cardiac death, and heart failure death) were calculated for different patient groups using the method of Kaplan and Meier. The event rates per-person years for each patient group were obtained by dividing the total number of events by the total number of years of follow-up among all patients in the group. For the composite endpoint of mortality or CV hospitalization, the numerator in the event rate per-person year includes only the first event a patient experienced. The effects of treatment as randomized (CABG + MED vs. MED alone) on clinical outcomes were statistically assessed in male and in female patients using the log-rank test and summarized using hazard ratios and 95% confidence intervals generated from the Cox proportional hazards regression model. The Cox model was also used to assess whether the effect of CABG + MED vs. MED was different in women compared to men by examining the interaction between treatment and sex for each clinical endpoint. For comparing men vs. women directly with respect to clinical outcomes, the Cox model was used, adjusting for key prognostic baseline characteristics (including age, race, HF class at baseline, history of MI, previous revascularization, number of diseased vessels, ejection fraction, chronic renal insufficiency, history of atrial flutter/fibrillation, mitral regurgitation, history of stroke, hemoglobin and hyperlipidemia) and randomized treatment (CABG + MED vs. MED). Quality of life measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) Overall Score over the first 36 months of follow-up was compared between men and women using the repeated measures analysis in the PROC MIXED procedure in SAS. The least-square means of KCCQ Overall Scores and their 95% CIs were obtained for men and women at each time point. All calculations were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Demographics and Baseline Parameters
The baseline characteristics of men (n=1064, 88%) and women (n=148, 12%) in the Hypothesis 1 group are listed in Table 1. Women were older than the men (median 63.4 vs. 59.3 years, p=0.016), more likely to be White, and had a higher body mass index (BMI) (median 27.9 vs. 26.7, p=0.001). More risk factors for CAD were reported in women except for smoking (diabetes 55.4% vs. 37.2%, p<0.001, hypertension 70.9% vs. 58.6%, p=0.004, hyperlipidemia, 70.3% vs. 58.9%, p=0.008), while women were less likely to have had prior CABG (0% vs. 3.4%, p=0.017). In addition, women were more likely to report depression and had worse baseline renal function (glomerular filtration median rate 83.8 vs. 91.2 mL/min/1.73m2, p<0.001).
Table 1.
Baseline Characteristics of the Patients
| Women (n=148) | Men (n=1064) | P-value | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, years | 63.4 (54.4,66.4) | 59.3 (53.5, 66.9) | 0.016 |
| White | 71.6% | 67.8% | 0.345 |
|
| |||
| BMI, kg/m2 | 27.9 (24.6, 32.5) | 26.7 (23.9, 29.5) | 0.001 |
| BSA, m2 | 1.79 (1.64, 1.92) | 1.93 (1.79, 2.07) | <0.0001 |
|
| |||
| Medical History | |||
| Previous MI | 75.7% | 77.3% | 0.668 |
| Diabetes | 55.4% | 37.2% | <0.0001 |
| Stroke | 8.1% | 7.5% | 0.800 |
| Hypertension | 70.9% | 58.6% | 0.004 |
| Hyperlipidemia | 70.3% | 58.9% | 0.008 |
| Current Smoker | 13.5% | 21.8% | 0.020 |
| Peripheral Vascular Disease | 14.9% | 15.2% | 0.909 |
| Chronic Renal insufficiency | 7.4% | 7.8% | 0.873 |
| Atrial flutter/fibrillation | 8.8% | 13.2% | 0.133 |
| Depression | 10.8% | 5.6% | 0.015 |
| Performed 6 min walk test | 83.8% | 86.9% | 0.303 |
| Previous CABG | 0% | 3.4% | 0.017 |
| Previous PCI | 16.9% | 12.3% | 0.119 |
| ICD | 1.4% | 2.5% | 0.567 |
| Mitral valve repair or replacement | 0% | 0.4% | 1.000 |
| Pacemaker for heart rate | 1.4% | 1.5% | 1.000 |
| Pacemaker for resynchronization | 0% | 0.7% | 1.000 |
|
| |||
| CCS Angina Class | 0.514 | ||
| No angina | 33.1% | 36.9% | |
| I | 18.2% | 15.0% | |
| II | 42.6% | 43.4% | |
| III | 4.7% | 3.9% | |
| IV | 1.4% | 0.8% | |
|
| |||
| Highest NYHA Heart Failure Class | 0.005 | ||
| I | 5.4% | 5.7% | |
| II | 28.4% | 37.2% | |
| III | 43.9% | 44.6% | |
| IV | 22.3% | 12.4% | |
| Advanced HF (Class III/IV) | 66.2% | 57.0% | 0.034 |
| Distance walked in 6 minute walk test, m | 300 (220, 370) | 350 (270, 410) | <0.0001 |
| KCCQ Overall Summary Score | 51 (33, 69) | 63 (46, 80) | <0.0001 |
|
| |||
| Labs/Biomarkers | |||
| Hemoglobin, g/dL | 12.6 (11.6, 14.0) | 14.0 (12.9, 15.0) | <0.0001 |
| eGFR, mL/min/1.732 | 83.8 (70.0, 89.6) | 91.2 (73.0, 106.4) | <0.0001 |
| BNP, pg/mL | 416 (191, 605) | 310 (180, 559) | 0.163 |
|
| |||
| Medication Use at Baseline | |||
| Beta blocker | 85.8% | 85.4% | 0.905 |
| ACE inhibitor | 73.0% | 83.5% | 0.002 |
| Angiotensin Receptor blocker | 14.9% | 8.7% | 0.017 |
| ACE inhibitor or ARB | 85.1% | 90.1% | 0.063 |
| Statin | 81.8% | 81.0% | 0.829 |
| Antiarrhythmic | 7.4% | 11.0% | 0.186 |
| Amiodarone | 6.8% | 10.2% | 0.192 |
| Digoxin | 13.5% | 21.1% | 0.030 |
| Aspirin | 80.4% | 83.0% | 0.437 |
| Warfarin | 6.8% | 11.0% | 0.115 |
| Clopidogrel | 19.6% | 16.8% | 0.402 |
| Loop diuretic | 68.9% | 64.8% | 0.326 |
| Potassium sparing diuretic | 45.3% | 46.0% | 0.875 |
| Nitrate | 54.1% | 53.2% | 0.853 |
| Insulin | 27.0% | 14.8% | <0.0001 |
| Oral diabetic agent | 29.1% | 22.8% | 0.100 |
Median (twenty-fifth, seventy-fifth percentiles) for continuous variables. BUN, blood urea nitrogen; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate (based on Mayo quadratic formula); ICD indicates Implantable cardioverter defibrillator; MI, myocardial infarct; PCI, percutaneous coronary intervention.
Baseline Left Ventricular Function and Coronary Anatomy
Table 2 details the baseline LV function and the coronary anatomy by sex. Clinical values are the best available data reported by participating sites and/or core labs. The rates of triple vessel disease, left main stenosis ≥ 50% and proximal left anterior descending stenosis ≥ 75% were not statistically different. The median LVEF (30.0% vs 27.0%, p =0.0001) was higher in women.
Table 2.
Baseline Left Ventricular Function and Coronary Anatomy
| Women (n=148) | Men (n=1064) | P-value | |
|---|---|---|---|
|
| |||
| LV Function | |||
| LVEF, % | 30.0 (25.0, 35.6) | 27.0 (22.0, 33.0) | 0.0001 |
| ESVI, mL/m2 | 70.0 (53.0, 89.3) | 80.2 (62.5, 102.6) | <0.0001 |
| EDVI, mL/m2 | 97.4 (79.1, 119.2) | 113.9 (91.1, 141.8) | <0.0001 |
|
| |||
| Anterior akinesis or dyskinesis | 43.0 (29.0, 57.0) | 43.0 (25.0, 57.0) | 0.270 |
|
| |||
| Mitral Regurgitation | 0.026 | ||
| None or trace | 27.7% | 37.1% | |
| Mild | 50.7% | 45.1% | |
| Moderate | 16.9% | 14.7% | |
| Severe | 4.7% | 3.0% | |
|
| |||
| Number of vessels with ≥75% stenosis | 0.617 | ||
| None | 2.7% | 2.0% | |
| 1 | 29.1% | 22.5% | |
| 2 | 29.1% | 39.4% | |
| 3 | 39.2% | 36.1% | |
|
| |||
| Multi-vessel disease | 68.2% | 75.5% | 0.056 |
|
| |||
| Left main stenosis ≥50% | 3.4% | 2.5% | 0.581 |
|
| |||
| Proximal LAD ≥75% | 64.2% | 68.8% | 0.262 |
Median (twenty-fifth, seventy-fifth percentiles) for continuous variables. Clinical values are the best available data reported by participating sites. EDVI, end diastolic volume index; ESVI, end systolic volume index; LAD, left anterior descending artery; LVEF indicates left ventricular ejection fraction.
Symptoms
There was no significant difference in symptoms of angina between sexes by the CCS angina score (Table 1). The percentage of patients with advanced HF class (by highest NYHA class during the 3-month period prior to randomization) was higher in women than men (66.2% vs. 57.0%, p=0.034). In addition, women had a lower functional capacity as noted by the 6 min walk distance (median 300 vs. 350 meters, p<0.0001, Table 1). Health status (HRQoL) measured by Kansas City Cardiomyopathy Questionnaire (KCCQ) Overall Summary Score was lower in women at baseline (51 vs. 63, p<0.0001).
Medical Therapy
More men than women were on ACE inhibitors (83.5% vs. 73.0%, p= 0.002), However, a higher proportion of women were receiving ARBs, thus making the use of ACEI or ARBs relatively similar between sexes (Table 1). Beta-blocker use was also not statistically different between the two groups. While digoxin was more commonly used in men (21.1% vs. 13.5%, p=0.030), more women were on insulin treatment (27.0% vs. 14.8%, p<0.0001).
Clinical Outcomes
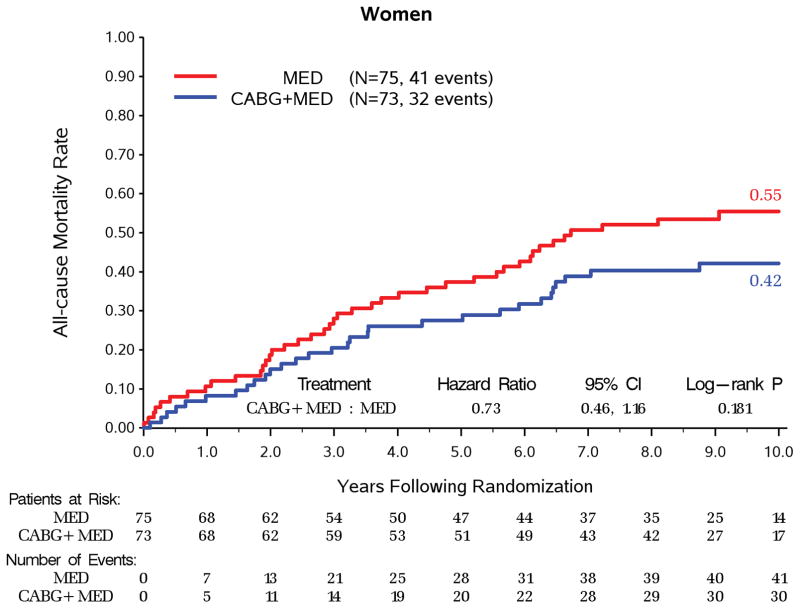
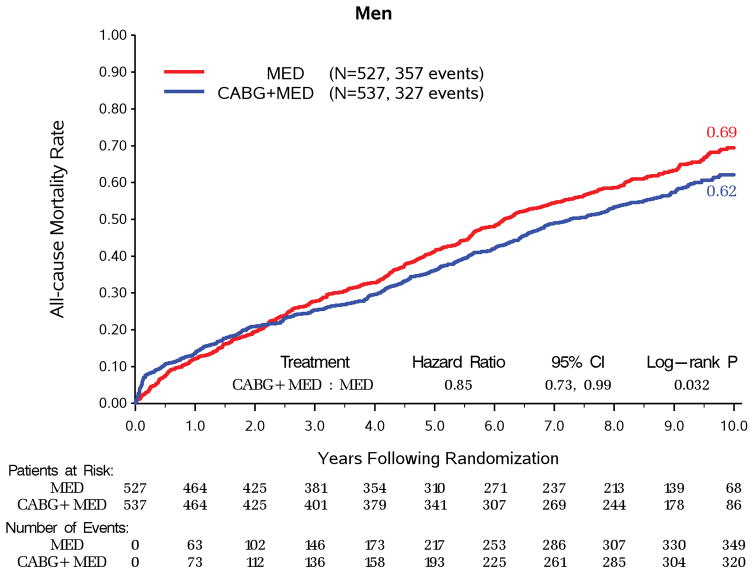
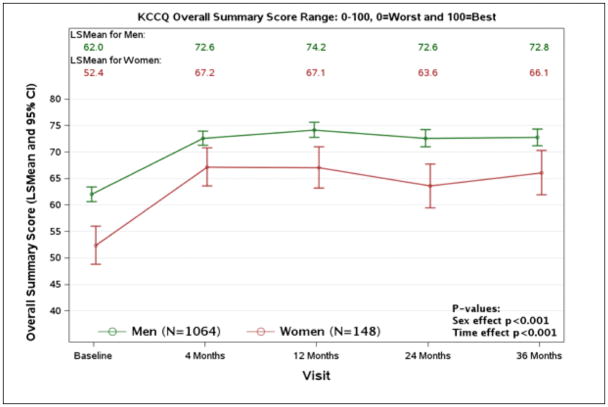
Over a median follow up of 9.8 years, women had significantly lower all-cause mortality compared to men (73/148, 49.3% vs. 684/1064, 64.3%, adjusted HR 0.67, 95% CI 0.52–0.86, p=0.002) and CV mortality (48/148, 32.4% vs. 496/1064, 46.6%, adjusted HR 0.65, 95% CI 0.48–0.89, p=0.006, Table 3). With randomization to CABG vs MED treatment, there was no significant interaction between sex and treatment group in all- cause mortality (p=0.495, Figure 1), CV mortality (p=0.386), mortality or CV hospitalization (p=0.176). For both women and men, CABG + MED patients had lower event rates than MED patients (Table 4) on all-cause mortality (32/73, 43.8% vs. 41/75, 54.7% for women; 327/537, 60.9% vs. 357/527, 67.7% for men), CV mortality (19/73, 26.0% vs. 29/75, 38.7% for women; 228/537, 42.5% vs. 268/527, 50.9% for men), and mortality or CV hospitalization (50/73, 68.5% vs. 62/75, 82.7% for women; 417/537, 77.7% vs 462/527, 87.7% for men). The same pattern of results was also observed on the event rates for sudden cardiac death and heart failure death (Table 3 and Table 4). Moreover, surgical deaths were not statistically different for both sexes among patients randomized to CABG and received CABG per protocol as initial treatment (men 25/488, 5.1% vs. women 1/67, 1.5%, p = 0.187). In addition, both sexes had significant improvements in HRQoL as measured by the KCCQ Overall Score, lasting up to 36 months of follow up (p<0.0001, Figure 2), but remained lower in the women.
Table 3.
Clinical Event Rate and Hazard Ratio for Women versus Men
| Clinical Event | Women (N=148) | Men (N=1064) | Model | Hazard Ratio (95% CI) | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) of Events | 10-Year KM Rate* (%) (95% CI) | Event Rate per person- year | N (%) of Events | 10-Year KM Rate* (%) (95% CI) | Event Rate per person- year | ||||
| All-cause Mortality | 73 (49.3) | 49.0 (40.8, 57.3) | 0.073 | 684 (64.3) | 65.8 (62.7, 68.8) | 0.105 | Unadjusted | 0.70 (0.55, 0.89) | 0.003 |
| Adjusted† | 0.67 (0.52, 0.86) | 0.002 | |||||||
| Cardiovascular Mortality | 48 (32.4) | 34.3 (26.3, 42.3) | 0.048 | 496 (46.6) | 52.3 (48.9, 55.8) | 0.076 | Unadjusted | 0.64 (0.48, 0.86) | 0.003 |
| Adjusted† | 0.65 (0.48, 0.89) | 0.006 | |||||||
| Mortality or Cardiovascular Hospitalization | 112 (75.7) | 76.6 (68.5, 84.6) | 0.111 | 879 (82.6) | 85.2 (82.2, 88.2) | 0.135 | Unadjusted | 0.87 (0.72, 1.06) | 0.180 |
| Adjusted† | 0.86 (0.70, 1.05) | 0.144 | |||||||
| Sudden Cardiac Death | 21 (14.2) | 17.6 (10.7, 24.5) | 0.021 | 249 (23.4) | 30.2 (26.8, 33.7) | 0.038 | Unadjusted | 0.56 (0.36, 0.87) | 0.011 |
| Adjusted† | 0.62 (0.39, 0.97) | 0.038 | |||||||
| Heart Failure Death | 10 (6.8) | 7.5 (2.7, 12.3) | 0.010 | 148 (13.9) | 21.9 (18.5, 25.4) | 0.023 | Unadjusted | 0.44 (0.23, 0.83) | 0.012 |
| Adjusted† | 0.40 (0.21, 0.77) | 0.007 | |||||||
10-year KM Rate=Kaplan-Meier estimates of event rate at 10 years after randomization.
The adjustment variables include treatment, age, race, HF class at baseline, history of MI, previous revascularization, number of diseased vessels, baseline ejection fraction, chronic renal insufficiency, history of atrial flutter/fibrillation, mitral regurgitation, history of stroke, hemoglobin and hyperlipidemia.
Figure 1. Kaplan-Meier Rates of All-cause Mortality by Randomized Treatment for Women and Men.
A. Kaplan-Meier Rates of All-cause Mortality by Randomized Treatment for Women.
B. Kaplan-Meier Rates of All-cause Mortality by Randomized Treatment for Men.
Interaction P-value = 0.495
Table 4.
Clinical Event Rate for Women and Men in Different Treatment Groups
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Clinical Event | CABG + MED (N=73) | MED (N=75) | Total (N=148) | CABG + MED (N=537) | MED (N=527) | Total (N=1064) |
| All-cause Mortality | 32 (43.8) | 41 (54.7) | 73 (49.3) | 327 (60.9) | 357 (67.7) | 684 (64.3) |
| Cardiovascular Mortality | 19 (26.0) | 29 (38.7) | 48 (32.4) | 228 (42.5) | 268 (50.9) | 496 (46.6) |
| Mortality or Cardiovascular Hospitalization | 50 (68.5) | 62 (82.7) | 112 (75.7) | 417 (77.7) | 462 (87.7) | 879 (82.6) |
| Sudden Cardiac Death | 10 (13.7) | 11 (14.7) | 21 (14.2) | 106 (19.7) | 143 (27.1) | 249 (23.4) |
| Heart Failure Death | 3 (4.1) | 7 (9.3) | 10 (6.8) | 63 (11.7) | 85 (16.1) | 148 (13.9) |
Figure 2.
Kansas City Cardiomyopathy Questionnaire Scores by Sex Group
Discussion
The STICH trial is the first and only contemporary randomized clinical trial designed to compare CABG plus intensive HF medical therapy with intensive HF medical therapy only in patients with severe LV dysfunction, in an era with the availability of an evidence-based HF medical regimen. Historically, due to limited numbers of women, data based on predominantly male subjects in cardiovascular clinical trials have been extrapolated to women in practice. This is the first sub-analysis of a contemporary trial to suggest that despite having more cardiovascular comorbidities and worse functional status at baseline, female sex is not associated with the effect of CABG on all-cause mortality, CV mortality or surgical death rates in these patients. Furthermore, women had significantly lower rates of long-term all-cause mortality and CV mortality than men.
Baseline Clinical Characteristics
In light of these findings, a brief review of baseline demographics in comparison to other HF studies is relevant. Baseline characteristics of the STICH Hypothesis 1 cohort are similar to other studies such as CASS, CABG Patch, CHARM and MERIT-HF with a higher prevalence of comorbidities in the women compared to the men.1, 3, 27, 28 In addition, in the STICH trial, women were more likely to experience depression and had lower KCCQ Overall scores. In comparison, the HF-ACTION and BEST trials showed that women were younger and had a lower or same prevalence of hypertension and diabetes.2, 8 Moreover, in HF-ACTION, the scores on the Beck Depression Inventory II (8 vs. 8) and the KCCQ (68 vs. 69) were similar, and the history of depression (21% vs. 22%) was similar as well in both men and women.8 It was speculated that this seeming inconsistency could be due to the recollection of a history of hypertension or diabetes in a younger cohort, who may not have these comorbidities yet manifest.8 In addition, these medical and exercise therapy trials included a large portion of patients with non-ischemic heart failure; in contrast, the STICH trial focused on ischemic heart failure, which may explain the high prevalence of cardiovascular comorbidities in the STICH population.
Ischemic Heart Failure Medical Therapy
The STICH trial is a contemporary trial in which participants were well medicated with evidence based heart failure medical therapy. More than 80% of patients in the STICH trial were treated with beta-blocker, ACE inhibitor or ARB, lipid-reducing therapy and antiplatelet therapy. Additionally, more than 45% of patients received potassium-sparing diuretics. Overall, there was no significant sex difference in the medical therapy for ischemic HF at baseline. Our results showed that a lower proportion of women received ACE inhibitor, while a higher proportion of women received ARB. However, the combined proportion receiving ACE inhibitor or ARB was similar between sexes. This pattern is consistent with observations from prior trials, probably related to the higher prevalence of ACE inhibitor-induced cough in women than in men.1, 8
Clinical Outcome of Coronary Bypass Grafting Surgery
Given the distinct differences in sex hormones and their effects on cardiovascular disease process, women and men may respond to therapies differently, including revascularization.4, 7, 29–31 Some studies have shown higher rates of mortality and complications in women compared to men after coronary revascularization; however, after multivariable adjustment, female sex was often deemed not an independent predictor of poor outcome.27, 32–37
The data on the effect of female sex on CABG outcomes have been controversial in both clinical trials and registries. Women participants were more likely to be older, had significantly greater pre-operative comorbidities (including hypertension, hyperlipidemia, diabetes mellitus, unstable angina, congestive heart failure, and peripheral vascular disease), and were more likely to undergo urgent CABG.27, 28, 32, 36, 38–43 Thus, the association or potential impact of female sex as an independent predictor on the poor outcome of isolated CABG surgery has long been debated. The CASS registry showed that women had worse surgical mortality (4.5% vs 1.9%, p=0.02) and 1-year survival than men despite risk variable adjustment, but there was no significant difference between sexes with regard to long-term 6-year mortality (8.7 vs. 7.9%, p=0.41).27, 44 A meta-analysis of 20 studies reported a higher mortality in women post CABG not only at short term, but also at mid- and long-term follow up.42 Recent studies from the 1990’s and 2000’s attributed this elevated mortality to the higher prevalence of pre-operative risk factors at baseline and later referral bias in women.32, 35, 41, 43, 45 Furthermore, a retrospective analysis of patients undergoing CABG in 1999–2000 suggested that female sex was an independent predictor of increased perioperative mortality, even after adjusting for all comorbidities.39 Despite this seemingly high perioperative mortality, a number of studies suggest that long-term survival (2.6 to 10 years) was reported similar between sexes after risk variable adjustment, but nevertheless women were likely to remain symptomatic from angina and subsequent heart failure.
On the other hand, one subset analysis from the BARI registry (patients enrolled from 1988 to 1991 and majority with preserved EF) reported better outcome in women.38 The in-hospital mortality was similar between sexes, and female sex was an independent predictor of better 5-year survival in both of the CABG and PTCA groups, after adjusting for multiple risk variables.38 Whether an improvement in surgical technique has added to a better survival has not been examined but could account for differences across time.
Data on patients with impaired ventricular function undergoing CABG have been quite limited, as most patients in previous trials or retrospective cohorts had preserved EF. In the CASS trial, only 160 patients (20.5%) had EF 34–50% and of those only 5% were women.46 The CABG Patch trial included 900 patients with an EF≤35%, and of those 15.7% were women. The 2-year all-cause mortality was higher in CABG Patch (22%) than STICH (15.1% in women, 20.9% in men), as well as re-hospitalization rates. This could be a result of better underlying HF medical therapy and possible advances in surgical technique over time. For example, the CABG Patch cohort was sub-optimally medicated for HF with beta-blocker, ACEI and lipid-lowering agents in comparison to STICH. Both of these studies showed that female sex was not associated with increased mortality.
Limitations
This analysis of the STICH Hypothesis 1 study by sex has several limitations including its post hoc nature. Women and men had markedly different baseline characteristics. The sex difference on clinical outcomes was assessed by Cox model after adjusting for key baseline characteristics. Moreover, the number of women represented was small. Some patients may have been excluded or never offered the study due to symptoms or the inherent bias by their providers or from the literature available at the time STICH was initiated. Some of these biases can include older data showing a higher mortality in women after revascularization and concern about the background comorbidities making these women worse candidates as a “fait accompli”. Additionally, there was an exclusion criterion that if angina symptoms were Class III–IV, the clinician could decide against randomization in Hypothesis 1. This inclusion/exclusion may have also precluded more symptomatic women from being enrolled. In STICH, the inclusion and exclusion criteria added to a protocolized application of medical therapy may have made the women more similar to their male counterparts and received better medical therapy when compared to cohort studies without those stipulations. Others have reported a lower use of medical therapy in women with CAD with less CVD risk stratification.47 Nonetheless, the value of the data is the prospective inclusion of patients with a solid protocol and collection of large amounts of baseline characteristics allowing comparisons between sexes. In addition, the proportion of women with CAD and reduced LV function enrolled in more contemporary coronary revascularization trials remained very low 1–2 % (FREEDOM, BEST, EXCEL).48–50 While the number of women enrolled in the STICH trial was low (n=148, 12%), it is comparable to more recent trials and provides probably the largest cohort for analysis.
Conclusions
In summary, this subset analysis of the STICH Hypothesis 1 population suggested that while women appeared to have higher preoperative risk profiles at baseline, when randomized to CABG + MED vs. MED alone treatment, there was no significant interaction between sex and treatment group in all-cause mortality, CV mortality, CV hospitalization or surgical deaths in patients with ischemic LV dysfunction. Mechanisms responsible for this observation are speculative. However, regardless of mechanisms, these findings carry significant implications for clinical practice in the future. Sex should not influence treatment decisions regarding CABG in these patients.
Clinical Perspective.
What is new?
Studies have shown sex-specific differences regarding CAD and heart failure.
Whether these differences affect the benefit of CABG in patients with ischemic LV dysfunction has not been studied prospectively.
Our study examined the association of sex on the long-term benefit of CABG in patients enrolled in the prospective Surgical Treatment for Ischemic Heart Failure Study (STICH) trial.
This is the largest prospectively collected group of women with impaired ventricular function and coronary artery disease enrolled in a protocol-driven trial.
What are the clinical implications?
Sex is not associated with the effect of CABG on all-cause mortality, CV mortality, CV hospitalization or surgical deaths in patients with ischemic LV dysfunction.
When assessing revascularization strategy in a patient with ischemic heart failure with LV dysfunction, although women may appear to have seemingly high preoperative risks, sex should not influence treatment decisions regarding CABG in these patients.
Clinicians should base their decision to recommend CABG to women not based on their baseline risk factors or perceptions of poor outcome, but on the data presented here.
Acknowledgments
Funding:
This work was supported by grants U01-HL69015, U01-HL69013, and R01- HL105853 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Clinical trial registration: ClinicalTrials.gov#NCT00023595; www.stichtrial.org
Abbreviations
- ACEI
angiotensin-converting-enzyme inhibitor
- ARB
angiotensin II receptor blocker
- BARI
bypass angioplasty revascularization investigation
- BEST
beta-blocker evaluation in survival trial
- BNP
brain natriuretic peptide
- BUN
blood urea nitrogen
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CASS
Coronary Artery Surgery Study
- CS
Canadian Cardiovascular Society
- CI
confidence interval
- CHARM
candesartan in heart failure - assessment of mortality and morbidity
- CV
cardiovascular
- EDVI
end diastolic volume index
- EF
ejection fraction
- ESVI
end systolic volume index
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HF-ACTION
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training
- HFrEF
heart failure with reduced ejection fraction
- HRQoL
health status related quality of life
- HR
hazard ratio
- ICD
Implantable cardioverter defibrillator
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LAD
left anterior descending artery
- LV
left ventricle
- MED
medical therapy
- MERIT-HF
metroprolol CR/XL randomized intervention trial in congestive heart failure
- MI
myocardial infarct
- NHLBI
National Heart, Lung, and Blood Institute
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- STICH
Surgical Treatment for Ischemic Heart Failure Trial
Footnotes
Disclosures:
Dr. Velazquez reports PI/Grants with the National Heart, Lung, and Blood Institute, Alnylam Pharmaceuticals, Inc., Amgen, Inc., Pfizer, Novartis; Consulting Fees/Honoraria: Amgen, Inc., Expert Exchange, Merck & Co., New Century Health, Novartis. There are no other relationships with industry and financial associations to disclose.
References
- 1.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA Investigators C. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–20. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 2.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, Young JB, Goldman S, Peberdy MA, Lindenfeld J. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–34. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Ghali JK, Pina IL, Gottlieb SS, Deedwania PC, Wikstrand JC, Group M-HS. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in Metoprolol Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF) Circulation. 2002;105:1585–91. doi: 10.1161/01.cir.0000012546.20194.33. [DOI] [PubMed] [Google Scholar]
- 4.Hsich EM, Pina IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–8. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pina IL, Kokkinos P, Kao A, Bittner V, Saval M, Clare B, Goldberg L, Johnson M, Swank A, Ventura H, Moe G, Fitz-Gerald M, Ellis SJ, Vest M, Cooper L, Whellan D Investigators H-A. Baseline differences in the HF-ACTION trial by sex. American heart journal. 2009;158:S16–23. doi: 10.1016/j.ahj.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED American College of Cardiology-National Cardiovascular Data Registry I. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 10.Nabel EG, Selker HP, Califf RM, Canto JG, Cao JJ, Desvigne-Nikkens P, Goldberg RJ, Finnegan JR, Jr, Vaccarino V, Virmani R National Heart L, Blood I, American College of Cardiology F. Women’s Ischemic Syndrome Evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute workshop: October 2–4, 2002: Section 3: diagnosis and treatment of acute cardiac ischemia: gender issues. Circulation. 2004;109:e50–2. doi: 10.1161/01.CIR.0000116208.41269.75. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G Women’s Ischemia Syndrome Evaluation I. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 12.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PloS one. 2014;9:e93170. doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelling TM, Chen RS, Lubwama RN, L’Italien GJ, Eagle KA. The expanding national burden of heart failure in the United States: the influence of heart failure in women. American heart journal. 2004;147:74–8. doi: 10.1016/j.ahj.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 15.Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, Sollano J, Katz S, Moye L, Basta LL, et al. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. The New England journal of medicine. 1991;325:226–30. doi: 10.1056/NEJM199107253250402. [DOI] [PubMed] [Google Scholar]
- 16.Giles WH, Anda RF, Casper ML, Escobedo LG, Taylor HA. Race and sex differences in rates of invasive cardiac procedures in US hospitals. Data from the National Hospital Discharge Survey. Archives of internal medicine. 1995;155:318–24. [PubMed] [Google Scholar]
- 17.EuroSCORE calculator. Available at: http://www.euroscore.org/calc.html.
- 18.STS Score. Available at: http://riskcalc.sts.org/stswebriskcalc/#/
- 19.Hattler BG, Madia C, Johnson C, Armitage JM, Hardesty RL, Kormos RL, Pham SM, Payne DN, Griffith BP. Risk stratification using the Society of Thoracic Surgeons Program. The Annals of thoracic surgery. 1994;58:1348–52. doi: 10.1016/0003-4975(94)91911-9. [DOI] [PubMed] [Google Scholar]
- 20.Hannan EL, Wu C, Bennett EV, Carlson RE, Culliford AT, Gold JP, Higgins RS, Isom OW, Smith CR, Jones RH. Risk stratification of in-hospital mortality for coronary artery bypass graft surgery. J Am Coll Cardiol. 2006;47:661–8. doi: 10.1016/j.jacc.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, Gott JP, Herrmann HC, Marlow RA, Nugent WC, O’Connor GT, Orszulak TA, Rieselbach RE, Winters WL, Yusuf S, Gibbons RJ, Alpert JS, Eagle KA, Garson A, Jr, Gregoratos G, Russell RO, Smith SC., Jr ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262–347. doi: 10.1016/s0735-1097(99)00389-7. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL Investigators S. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. The New England journal of medicine. 2016;374:1511–20. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://clinicaltrials.gov/ct2/show/study/NCT00023595?term=STICH&cond=Heart+Failure&rank=1.
- 24.https://biolincc.nhlbi.nih.gov/home/
- 25.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH Investigators S. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. The Journal of thoracic and cardiovascular surgery. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL Investigators S. Coronary-artery bypass surgery in patients with left ventricular dysfunction. The New England journal of medicine. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaker ED, Kronmal R, Kennedy JW, Davis K. Comparison of the long-term, postsurgical survival of women and men in the Coronary Artery Surgery Study (CASS) American heart journal. 1989;117:71–81. doi: 10.1016/0002-8703(89)90658-3. [DOI] [PubMed] [Google Scholar]
- 28.Argenziano M, Spotnitz HM, Whang W, Bigger JT, Jr, Parides M, Rose EA. Risk stratification for coronary bypass surgery in patients with left ventricular dysfunction: analysis of the coronary artery bypass grafting patch trial database. Circulation. 1999;100:II119–24. doi: 10.1161/01.cir.100.suppl_2.ii-119. [DOI] [PubMed] [Google Scholar]
- 29.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA National Heart L, Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA : the journal of the American Medical Association. 2002;287:1153–9. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 31.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–6. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 32.Abramov D, Tamariz MG, Sever JY, Christakis GT, Bhatnagar G, Heenan AL, Goldman BS, Fremes SE. The influence of gender on the outcome of coronary artery bypass surgery. The Annals of thoracic surgery. 2000;70:800–5. doi: 10.1016/s0003-4975(00)01563-0. discussion 806. [DOI] [PubMed] [Google Scholar]
- 33.Lansky AJ. Outcomes of percutaneous and surgical revascularization in women. Progress in cardiovascular diseases. 2004;46:305–19. doi: 10.1016/j.pcad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Tillmanns H, Waas W, Voss R, Grempels E, Holschermann H, Haberbosch W, Waldecker B. Gender differences in the outcome of cardiac interventions. Herz. 2005;30:375–89. doi: 10.1007/s00059-005-2716-3. [DOI] [PubMed] [Google Scholar]
- 35.Tamis-Holland JE, Lu J, Korytkowski M, Magee M, Rogers WJ, Lopes N, Mighton L, Jacobs AK, Group BDS. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the BARI 2D Trial (Bypass Angioplasty Revascularization Investigation 2 Diabetes) J Am Coll Cardiol. 2013;61:1767–76. doi: 10.1016/j.jacc.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 36.den Ruijter HM, Haitjema S, van der Meer MG, van der Harst P, Rouleau JL, Asselbergs FW, van Gilst WH Investigators I. Long-term outcome in men and women after CABG; results from the IMAGINE trial. Atherosclerosis. 2015 doi: 10.1016/j.atherosclerosis.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 37.Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ. Sex difference in clinical outcomes after percutaneous coronary intervention in Korean population. American heart journal. 2014;167:743–52. doi: 10.1016/j.ahj.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs AK, Kelsey SF, Brooks MM, Faxon DP, Chaitman BR, Bittner V, Mock MB, Weiner BH, Dean L, Winston C, Drew L, Sopko G. Better outcome for women compared with men undergoing coronary revascularization: a report from the bypass angioplasty revascularization investigation (BARI) Circulation. 1998;98:1279–85. doi: 10.1161/01.cir.98.13.1279. [DOI] [PubMed] [Google Scholar]
- 39.Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou YB, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 Midwestern hospitals. Circulation. 2005;112:I323–7. doi: 10.1161/CIRCULATIONAHA.104.525139. [DOI] [PubMed] [Google Scholar]
- 40.Bukkapatnam RN, Yeo KK, Li Z, Amsterdam EA. Operative mortality in women and men undergoing coronary artery bypass grafting (from the California Coronary Artery Bypass Grafting Outcomes Reporting Program) The American journal of cardiology. 2010;105:339–42. doi: 10.1016/j.amjcard.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Lehmkuhl E, Kendel F, Gelbrich G, Dunkel A, Oertelt-Prigione S, Babitsch B, Knosalla C, Bairey-Merz N, Hetzer R, Regitz-Zagrosek V. Gender-specific predictors of early mortality after coronary artery bypass graft surgery. Clinical research in cardiology : official journal of the German Cardiac Society. 2012;101:745–51. doi: 10.1007/s00392-012-0454-0. [DOI] [PubMed] [Google Scholar]
- 42.Alam M, Bandeali SJ, Kayani WT, Ahmad W, Shahzad SA, Jneid H, Birnbaum Y, Kleiman NS, Coselli JS, Ballantyne CM, Lakkis N, Virani SS. Comparison by meta-analysis of mortality after isolated coronary artery bypass grafting in women versus men. The American journal of cardiology. 2013;112:309–17. doi: 10.1016/j.amjcard.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Loop FD, Golding LR, MacMillan JP, Cosgrove DM, Lytle BW, Sheldon WC. Coronary artery surgery in women compared with men: analyses of risks and long-term results. J Am Coll Cardiol. 1983;1:383–90. doi: 10.1016/s0735-1097(83)80064-3. [DOI] [PubMed] [Google Scholar]
- 44.Myers WO, Blackstone EH, Davis K, Foster ED, Kaiser GC. CASS Registry long term surgical survival. Coronary Artery Surgery Study. J Am Coll Cardiol. 1999;33:488–98. doi: 10.1016/s0735-1097(98)00563-4. [DOI] [PubMed] [Google Scholar]
- 45.Khan SS, Nessim S, Gray R, Czer LS, Chaux A, Matloff J. Increased mortality of women in coronary artery bypass surgery: evidence for referral bias. Annals of internal medicine. 1990;112:561–7. doi: 10.7326/0003-4819-112-8-561. [DOI] [PubMed] [Google Scholar]
- 46.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Survival data. Circulation. 1983;68:939–50. doi: 10.1161/01.cir.68.5.939. [DOI] [PubMed] [Google Scholar]
- 47.Shah T, Palaskas N, Ahmed A. An Update on Gender Disparities in Coronary Heart Disease Care. Current atherosclerosis reports. 2016;18:28. doi: 10.1007/s11883-016-0574-5. [DOI] [PubMed] [Google Scholar]
- 48.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, 3rd, Bertrand M, Fuster V Investigators FT. Strategies for multivessel revascularization in patients with diabetes. The New England journal of medicine. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 49.Park SJ, Ahn JM. Everolimus-Eluting Stents or Bypass Surgery for Coronary Disease. The New England journal of medicine. 2015;373:581–2. doi: 10.1056/NEJMc1506944. [DOI] [PubMed] [Google Scholar]
- 50.Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM, 3rd, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP Investigators ET. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. The New England journal of medicine. 2016;375:2223–2235. doi: 10.1056/NEJMoa1610227. [DOI] [PubMed] [Google Scholar]