Abstract
ATP-binding cassette (ABC) transporters are transmembrane efflux transporters mediating the extrusion of an array of substrates ranging from amino acids and lipids to xenobiotics, and many therapeutic compounds, including anticancer drugs. The ABC transporters are also recognized as important contributors to pharmacokinetics, especially in drug-drug interactions and adverse drug effects. Drugs and xenobiotics, as well as pathologic conditions, can influence the transcription of ABC transporters, or modify their activity or intracellular localization. Kinases can affect the aforementioned processes for ABC transporters as do protein interactions. In this review, we focus on the ABC transporters ABCB1, ABCB11, ABCC1, ABCC4, and ABCG2 and illustrate how kinases and protein-protein interactions affect these transporters. The clinical relevance of these factors is currently unknown; however, these examples suggest that our understanding of drug-drug interactions will benefit from further knowledge of how kinases and protein-protein interactions affect ABC transporters.
Introduction
Mammalian ATP-binding cassette (ABC) transporters are integral membrane proteins that actively extrude various endogenous and exogenous molecules across cell membranes in a unidirectional fashion. While these transporters can be found in many organelles including the mitochondria, lysosome, peroxisome, and endoplasmic reticulum, only those at the plasma membrane are discussed here because they can determine the intracellular concentration of drugs, endogenously synthesized molecules, as well as compounds formed from our microbiome. Drugs may act as substrates and/or inhibitors of these transporters. Such direct interactions [between the so-called victim (the drug) and perpetrator (the inhibitor)] are well known to attenuate or alter transporter function. Less well known, but possibly as important, is the knowledge of how drugs can affect a transporter’s intracellular location (e.g., by phosphorylation or by affecting interactions with partner proteins) or how the transporter is synthesized or degraded. In this review, we will highlight some of these processes and illustrate how this type of regulation of transporter activity could account for important drug-drug interactions or other adverse reactions that are distinct from, but just as important as, the typical victim-perpetrator paradigm. Moreover, we suggest that these types of interactions may be especially important since targeted therapies can interfere with important signaling pathways (e.g., kinase regulated pathways).
Structural studies have indicated that the minimal composition of an ABC transporter includes a minimum of four domains: two membrane-spanning domains (MSDs) and two nucleotide-binding domains (NBDs). The MSD consists of alpha-helices (transmembrane domains) that span the membrane and may determine substrate specificity. The energy for transport is provided by both binding and hydrolysis of ATP by the NBD. The NBD harbors crucial motifs, such as the Walker A (G-X4-GK-)-(T-S), with its conserved lysine contributing to both ATP-binding and hydrolysis, and Walker B (hhhhDE), with a catalytic glutamate that is indispensable for ATP hydrolysis. ABC transporters also contain a characteristic Q-loop that is responsible for communication with the transmembrane domains. Some members of the ABC family, such as ABCG2, are half-transporters that contain only one MSD and NBD; therefore, these half-transporters require either homo- or heterodimerization for their transport function. There are also ABC transporters that have a third MSD, or an N-terminal extension, such as ABCC1.
This review will focus on the transporters ABCB1 [P-glycoprotein (Pgp); multidrug resistance protein (MDR) 1], ABCB11 (bile salt export pump), ABCC1 [multidrug resistance associated protein (MRP) 1], ABCC2 (MRP2), ABCC4 (MRP4), and ABCG2 (breast cancer resistance protein). A brief overview of each of these transporters is given in the subsequent sections.
ABCB1.
The best characterized of the drugs transporting ABC proteins is the 170 kDa P-glycoprotein. Pgp, also referred to as ABCB1 or MDR1, rose to prominence in the mid-1970s due to the challenge to understand how cancer cells acquired resistance to multiple structurally and mechanistically unrelated therapeutic cytotoxins; as a mechanism, Pgp’s poly-substrate specificity was especially attractive. Subsequently, through the efforts of many laboratories, Pgp, from multiple species, was cloned and characterized (Juliano and Ling, 1976; Gottesman and Ling, 2006). Today, overwhelming evidence supports the idea that Pgp expression is important in a subset of human tumors; in particular, acute myeloid leukemia and breast cancer. The demonstration that Pgp was expressed at the plasma membrane in barrier organs (i.e., those separating the blood from the organ) such as the intestines, the liver, the testis, and cerebral vasculature (Thiebaut et al., 1987; Beaulieu et al., 1997) coupled with no obvious endogenous substrate led to the hypothesis that Pgp was a general protector against xenobiotics; in vivo proof of this proposition did not emerge until the development of Abcb1-null mice, and the serendipitous discovery of Pgp’s function at the blood-brain barrier by the Borst Laboratory (Schinkel et al., 1994, 1995, 1996). ABCB1 has been implicated in many drug-drug interactions because of its high expression in tissues relevant for drug absorption, disposition, excretion, and substrate overlap with CYP3A inducers and substrates (Schuetz et al., 1996a,b; Aszalos, 2007). As such, the Food and Drug Administration and European Medicines Agency suggest that all new drug candidates be screened for in vitro ABCB1 substrate and inhibition potential. Known substrates used for clinical studies are digoxin, dabigatran, and fexofenadine, while known inhibitors include verapamil and carvedilol.
ABCB11.
ABCB11, bile salt export pump, or the sister of Pgp belongs to the same subfamily as ABCB1; thus, these two proteins share high sequence and structural homology. Because ABCB1 is not highly expressed in liver, a prominent excretory organ with canalicular membranes displaying ATP-dependent transport of bile acids (Nishida et al., 1991), the search for a bile acid ABC transporter began. The sister of Pgp was identified by a low-stringency hybridization screen of a liver cDNA library, using as a probe a cDNA fragment from ABCB1; ABCB11 was almost exclusively expressed in hepatocytes (Childs et al., 1995), although low levels of mRNA and protein expression were detected in testes and adrenal glands (Langmann et al., 2003; Uhlen et al., 2010). Intriguingly, ABCB11 is present in subapical vesicles under basal conditions, which can then be mobilized to the plasma membrane at the canalicular (apical) membrane following stimulus by cAMP or taurocholate (Kipp et al., 2001). Unlike ABCB1, ABCB11 has a more restricted range of substrates, which are mostly monovalent taurine- and glycine-conjugated bile salts (Byrne et al., 2002). However, some non-bile acid substrates have been reported such as pravastatin and fexofenadine (Hirano et al., 2005; Matsushima et al., 2008), as well as the fluorescent dye, calcein-acetoxymethyl ester (Lecureur et al., 2000), a substrate thought to be primarily an ABCB1 substrate.
Thus, ABCB11’s main physiologic role is in the export of bile salts during enterohepatic circulation. The essential role for ABCB11 in bile salt secretion was revealed by studies of inherited cholestatic diseases; gene mutations in ABCB11 accounted for the autosomal recessive disease known as progressive familial intrahepatic cholestasis type 2. Importantly drug-induced ABCB11 inhibition, possibly coupled with functionally impaired ABCB11, might predispose one to intrahepatic cholestasis of pregnancy, a condition that can lead to neonatal death due to bile acid accumulation in the lungs (Zhang et al., 2015). Both progressive familial intrahepatic cholestasis type 2 and intrahepatic cholestasis of pregnancy involve liver damage, which appears to be due to bile acid disruption of mitochondrial function rather than bile acids damaging the liver (Zhang et al., 2012).
ABCC1.
ABCC1, is a 190 kDa protein that was discovered through efforts to understand drug resistance beyond the classical resistance provided by ABCB1. It is also known as MRP1, is the founding member of the C subfamily, and contains two NBDs and three MSDs. ABCC1 is expressed in many normal tissues, including lung, skin, small intestine, colon, kidney, and placenta, and localizes to the basolateral membrane in polarized cells; its role in the disposition of drugs used in treating nonmalignant disease is not well-defined. Nonetheless, ABCC1 appears to protect certain normal tissues, since Abcc1−/− mice show hypersensitivities to various xenobiotics including etoposide and vincristine (Wijnholds et al., 1997). Additionally, ABCC1 transports endogenous molecules including the inflammatory molecule leukotriene C4 and the pro-oxidant glutathione disulfide (Leier et al., 1994, 1996; Loe et al., 1996; Wijnholds et al., 1997). Finally, ABCC1 exports many glutathione, glucuronide, and sulfate conjugates that are synthesized during phase II metabolism (Müller et al., 1994; Jedlitschky et al., 1996).
ABCC2.
ABCC2, previously known as a canalicular multispecific organic anion transporter, has broad substrate specificity that includes both endogenous compounds, such as bilirubin and epinephrine metabolites, and exogenous compounds, such as irinotecan and methotrexate (Nies and Keppler, 2007). ABCC2 likely has multiple substrate binding sites (Borst et al., 2006) since transport activity can occur in a cooperative manner and substrate transport is stimulated by the presence of other substrates. For example, indomethacin and sulfinpyrazone can stimulate glutathione efflux in MDCKII cells at low concentrations (Evers et al., 2000). In contrast to ABCC1, ABCC2 is expressed on apical membranes (Paulusma et al., 1997; Schaub et al., 1997; Mottino et al., 2001). Genetic mutations in ABCC2 lead to Dubin-Johnson syndrome (Kartenbeck et al., 1996; Paulusma et al., 1997; Kajihara et al., 1998; Wada et al., 1998; Toh et al., 1999; Mor-Cohen et al., 2001; Pacifico et al., 2010), a condition characterized by conjugated hyperbilirubinemia and a dark pigmented liver (Dubin and Johnson, 1954), caused by the build-up of epinephrine metabolites (Kitamura et al., 1992). The connection between ABCC2 and Dubin-Johnson syndrome was illustrated by the transport-deficient rat strain, which was used to model the Dubin-Johnson syndrome (Paulusma et al., 1996). Using a fragment of rat MRP1 as a probe, a cDNA of 1541 amino acids was isolated that had high expression in the liver of Wistar rats, but had reduced expression in the transport-deficient rat liver. The causative mutation was found to be a deletion of amino acid 393, which caused a frameshift and introduction of an early stop codon that decreased expression of the protein. The link between ABCC2 and Dubin-Johnson has been further confirmed in another rat strain, the Esai hyperbilirubinemic rat (Ito et al., 1997), Abcc2−/− mice (Chu et al., 2006), and human patients (Kartenbeck et al., 1996; Paulusma et al., 1997; Kajihara et al., 1998; Wada et al., 1998; Toh et al., 1999; Mor-Cohen et al., 2001; Pacifico et al., 2010).
ABCC4.
ABCC4 is the shortest member of the ABCC subfamily, and like ABCB1 it has a typical ABC transporter structure with four domains: two NBDs and two MSDs. Originally, it was identified as the first mammalian ABC transporter capable of exporting nucleotide analogs (Schuetz et al., 1999; Adachi et al., 2002; Wielinga et al., 2002), a phenomenon that had been initially described in the 1960s, but had not been accounted for mechanistically. ABCC4 also contains a C-terminus postsynaptic density protein 95, Drosophila disc large tumor suppressor 1, and zonula occludens-1 protein (PDZ) interaction motif (Li et al., 2007). ABCC4 can be found in many tissues, including the blood-brain and blood-cerebrospinal fluid barriers, liver, kidneys, Leydig cells, and platelets. ABCC4 is unique in that it can be found on either the basal or apical membrane in polarized cell types. For example, in hepatocytes and the choroid plexus, ABCC4 is found on the basolateral membrane, while in the renal proximal tubule and blood-brain barrier ABCC4 is located on the apical membrane (Leggas et al., 2004). The mechanisms accounting for the basolateral versus apical localization of ABCC4 are unknown. ABCC4 has been shown to transport many drugs including nucleoside analogs (Ray et al., 2006; Imaoka et al., 2007), loop and thiazide diuretics (Hasegawa et al., 2007), and cephalosporins (Ci et al., 2007) with sulfate conjugated drugs favored over glucuronyl conjugates. The preference for sulfate conjugates is notable because it is coordinately upregulated with Sult2a by the nuclear constitutive androstane receptor Nr1i3 (Assem et al., 2004). ABCC4 is also capable of exporting endogenous substrates including cAMP (Chen et al., 2001), urate (Van Aubel et al., 2005), and prostaglandin E2 (Reid et al., 2003). Transport of such signaling molecules suggests ABCC4 may play a key regulatory role in cell signaling, in addition to drug transport.
ABCG2.
ABCG2 (breast cancer resistance protein) is a half-transporter that homodimerizes for transport function (Kage et al., 2002), although some evidence suggests it is found in cells as an oligomer (Litman et al., 2002; Xu et al., 2004; Bhatia et al., 2005; McDevitt et al., 2006; Dezi et al., 2010; Ni et al., 2010) and cross-linking studies indicate that the predominant functional form is as a dimer (Bhatia et al., 2005). ABCG2 is ubiquitously expressed, but is most abundant in the placenta (for this reason, it was once referred to as ABCP) (Allikmets et al., 1998), small intestine, liver, and mammary tissue (Maliepaard et al., 2001). Like ABCB1, ABCG2’s apical localization and ubiquitous expression suggests that it plays a role in the protection of tissues from environmental insults; however, it is also highly upregulated in many cancers, both solid and hematologic (Fukuda et al., 2017; Wijaya et al., 2017), leading to multidrug resistance. Substrates of ABCG2 include the topoisomerase inhibitor mitoxantrone, tyrosine kinase inhibitors such as sorafenib (Agarwal et al., 2011; Shukla et al., 2012), the antimetabolite methotrexate (Chen et al., 2003), and the endogenous substrate protoporphyrin IX. Besides being isolated as a new drug resistance gene (Doyle et al., 1998), ABCG2 was also established as a key protein that was both a marker of stem cells (the so-called side population, a transport phenotype typified by a characteristic export signature of Hoechst 3382 dye) and had an intrinsic role in protection of hematopoietic stem cells (Kim et al., 2002; Scharenberg et al., 2002). During hematopoiesis, ABCG2 is highly expressed in primitive bone marrow stem cells and is reduced dramatically following differentiation (Zhou et al., 2001; Bunting, 2002; Scharenberg et al., 2002), providing further support that ABCG2 both protects and helps to maintain hematopoietic stem cells. This role is further supported by studies in Abcg2−/− mice. Hematopoietic cells reside in a hypoxic niche and Abcg2−/− hematopoietic stem cells have impaired survival under hypoxic conditions; moreover, ABCG2 is upregulated by the hypoxia responsive Hif1-α transcription factor (Krishnamurthy et al., 2004). ABCG2 may play a similar role in cancer stem cells in a variety of solid tumors (Alvi et al., 2003; Hirschmann-Jax et al., 2004; Raaijmakers et al., 2005; Seigel et al., 2005; Chen et al., 2006; Haraguchi et al., 2006; Mohan et al., 2006; Ho et al., 2007; Olempska et al., 2007; Wang et al., 2007).
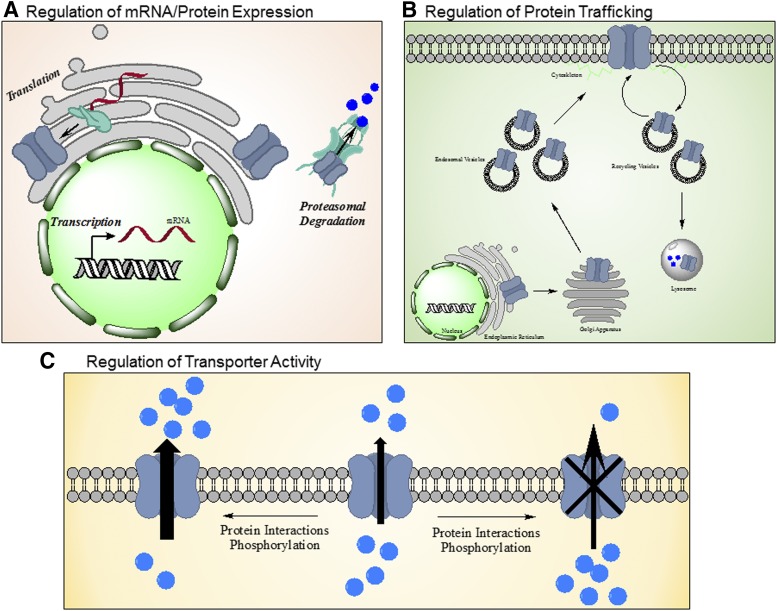
Due to the role these transporters play in regulating the cellular concentrations of endogenous and exogenous molecules, as well as being located in the barrier tissues of drug distribution and excretion, the opportunity for drug-drug interactions is quite high. Screening of new drugs (e.g., kinase inhibitors) (Table 1) for direct interactions with ABC transporters is performed to minimize potential drug-drug interactions. However, interference with ABC transporter function may be more complex than the simple victim and perpetrator competitive interactions. Review of the literature reveals three key additional mechanisms of drug-transporter interaction, beyond direct interaction with the transporter (Fig. 1): 1) regulation of protein expression via changes in transcription, translation, or degradation; 2) interruption of trafficking to or from the membrane; and 3) modulation of activity of the transporter via phosphorylation. Each of these potential mechanisms is explored with examples within this review.
TABLE 1.
Food and Drug Administration–approved tyrosine kinase inhibitors and known interactions with ABC transporters
All information is from labeling information that has been submitted to the Food and Drug Administration by the manufacturer.
| Generic Name | Brand Name | Kinase Target | Use | In vitro Transporter Interaction |
|---|---|---|---|---|
| Afatinib | Gilotrif | EGFT, HER2, HER4 | NSCLC | ABCB1 substrate and inhibitor |
| Alectinib | Alecensa | ALK, RET | ALK-positive, metastatic NSCLC | May inhibit ABCB1 and ABCG2 |
| Axitinib | Inlyta | VEGFR 1-3 | Renal Cell Cancer, advanced | ABCB1 inhibitor, but not at therapeutic levels |
| Bosutinib | Bosulif | BCR-ABL, Src, Lyn, Hck | CML, resistant | |
| Cabozantinib | Cometriq, Cabometyx | RET, MET, VEGFR 1-3, KIT, TRKB, FLT-3, AXL, ROS1, TYRO3, MER, and TIE2 | Metastatic medullary thyroid cancer, Renal cell cancer | MRP2 Substrate |
| Ceritinib | Zykadia | ALK, IGF-1R, InsR, and ROS1 | ALK-positive, metastatic NSCLC | ABCB1 substrate |
| Cobimetinib | Cotellic | MEK1 and MEK2 | Melanoma with BRAF mutations V600E or V600K | ABCB1 substrate |
| Crizotinib | Xalkori | ALK, HGFR, c-Met, c-Ros, RON | Metastatic NSCLC | ABCB1 inhibitor |
| Dabrafenib | Tafinlar | BRAF, CRAF, SIK1, NEK11, LIMK1 | Melanoma or NSCLC with BRAF mutation | ABCB1, ABCG2 substrate, ABCG2 inhibitor |
| Dasatinib | Sprycel | BCR-ABL, Src, LCK, YES, FYN, c- KIT, EPHA2, and PDGFRβ | Philadelphia chromosome positive CML and ALL | ABCB1 substrate |
| Erlotinib | Tarceva | EGFR | Metastatic NSCLC, Pancreatic Cancer | |
| Gefitinib | Iressa | EGFR, IGF, PDGF | Metastitic NSCLC with EGFR mutation | ABCB1 substrate |
| Ibrutinib | Imbruvica | BTK | Mantle Cell Lymphoma, CLL, SLL, Waldenström’s macroglobulinemia, marginal zone lymphoma, chronic Graft vs. host disease | May inhibit ABCB1 and ABCG2 |
| Idelalisib | Zydelig | PI3Kδ | Relapsed CLL, Follicular B-cell non- Hodgkin lymphoma, SLL | ABCB1, ABCG2 substrate, ABCB1 inhibitor |
| Imatinib | Gleevec | BCR-ABL, c-Kit, PDGF, SCF | Philadelphia chromosome positive CML and ALL, myelodysplastic/myeloproliferative diseases associated with PDGFR gene rearrangements, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, GIST | |
| Lapatinib | Tykerb | EGFR, HER2 | Breast Cancer, Her2 positive | ABCB1 and ABCG2 inhibitor |
| Lenvatinib | Lenvima | VEGFR 1-3, FGFR 1-4, PDGFR, c- Kit, RET | Differentiated Thyroid Cancer, Renal Cell Cancer | ABCB1 and ABCG2 substrate, ABCB11 inhibitor |
| Nilotinib | Tasigna | BCR-ABL, PDGFR, c-KIT, CSF-1R,and DDR1 | Philadelphia chromosome positive CML | ABCB1 inhibitor |
| Niraparib | Zejula | PARP1 and -2 | Ovarian, fallopian tube, or primary peritoneal cancer | Weak ABCG2 inhibitor; ABCB1 and ABCG2 substrate |
| Olaparib | Lynparza | PARP 1-3 | Recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, advanced ovarian cancer | ABCG2 and ABCB1 inhibitor, ABCB1 substrate |
| Osimertinib | Tagrisso | EGFR, HER 2-4, ACK1, and BLK | Metastatic, EGFR mutated, NSCLC | ABCG2 and ABCB1 substrate, ABCG2 inhibitor |
| Palbociclib | Ibrance | CDK4/6 | Breast Cancer, Hormone Receptor positive, Her2 negative | Weak ABCB1 and ABCG2 inhibitors |
| Pazopanib | Votrient | VEGFR 1-3, FGFR 1,3, Kit, Itk, Lck, c-Fms, PDGFRβ | Renal Cell Cancer, Soft tissue sarcoma | ABCB1 and ABCG2 substrate |
| Ponatinib | Iclusig | ABL, BCR-ABL, VEGFR, PDGFR, FGFR, SRC, KIT, RET, TIE2, and FLT3 | CML, ALL | ABCB1, ABCG2, and ABCB11 inhibitor |
| Regorafenib | Stivarga | RET, VEGFR 1-3, KIT, PDGFR, PDGFRβ, FGFR1-2, TIE2, DDR2, TRKA, Eph1A, RAF-1, BRAF, SAPK2, PTK5, Abl, CSF1R | Colorectal Cancer, GIST, hepatocellular carcinoma | ABCG2 inhibitor |
| Ribociclib | Kisqali | CDK4/6 | Breast Cancer, Hormone Receptor positive, Her2 negative | ABCG2, ABCB11 inhibitor |
| Rucaparib | Rubraca | PARP 1-3 | Ovarian Cancer, Advanced | ABCB1, ABCG2 substrate and inhibitor, ABCC4 inhibitor at ultra- therapeutic concentrations |
| Ruxolitinib | Jakafi | JAK1 and 2 | myelofibrosis, polycythemia vera | |
| Sorafenib | Nexavar | c-CRAF, BRAF, KIT, FLT-3, RET, RET/PTC, VEGFR 1-3, PDGFRβ | Renal Cell carinoma, hepatocellular carcinoma, refractory thyroid carcinoma | ABCB1 inhibitor |
| Sunitinib | Sutent | PDGFR and β, c-Ki, FLT3, CSF- 1R, RET, VEGFR2 | GIST, renal cell carcinoma, pancreatic neuroendocrine tumors | |
| Tofacitinib | Xeljanz | JAK1-3, | Rheumatoid arthritis | |
| Trametinib | Mekinist | MEK1-2 | Malignant Melanoma or NSCLC with BRAF mutation | ABCB1, ABCB11 substrate |
| Vandetanib | Caprelsa | EGFR, VEGFR, RET, BRK, TIE2, EPHR, Src | Medullary thyroid cancer | ABCB1 inhibitor |
| Vemurafenib | Zelboraf | BRAF, CRAF, ARAF, SRMS, ACK1, MAP4K5, and FGR | Melanoma or Erheim-Chester Disease with BRAF V600 mutation | ABCB1 and ABCG2 substrate and inhibitor |
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; EGFR, EGR receptor; GIST, gastrointestinal stromal tumor; NSCLC, non-small cell lung cancer; SLL, small lymphocytic leukemia; VEGFR VEGF receptor.
Fig. 1.
Indirect mechanisms of drug interactions with ABC transporters. Drugs influencing the activity of ABC transporters by factors distinct from direct binding to the transporter. These mechanisms include regulation of mRNA and protein expression through influences on transcription, translation, and/or proteasomal degradation (A); regulation of protein trafficking leading to changes in stabilization of the transporter on the membrane and/or internalization (B); or regulation of transporter activity through protein interactions or phosphorylation by kinases (C).
Regulation of ABC Transporter Expression by Growth Factor Receptor Kinases
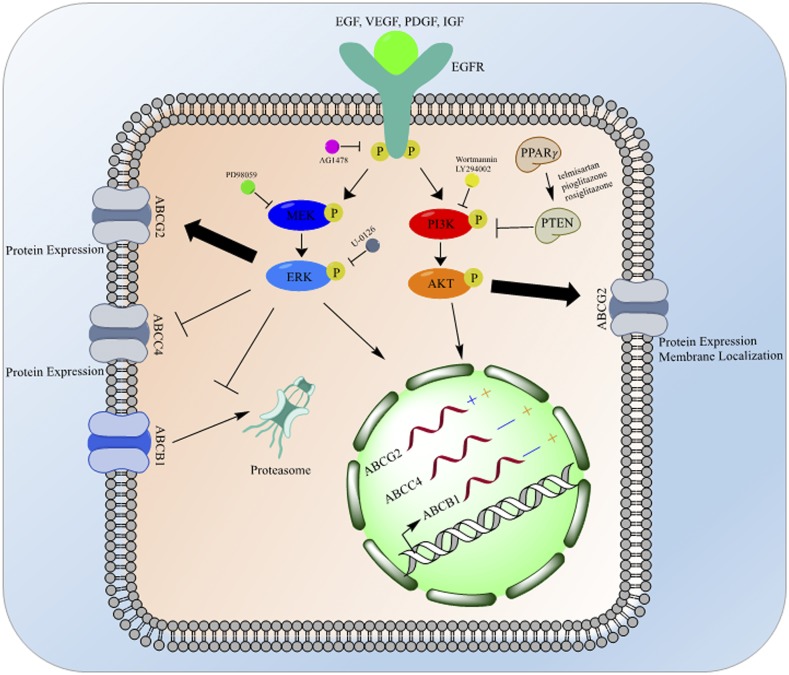
The epidermal growth factor (EGF) is a growth factor that when bound to its EGF receptor (EGFR), a tyrosine kinase receptor, stimulates a signaling cascade through two main pathways: the mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) pathway and the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway. Each of these pathways has been shown to regulate the expression of ABC transporters (Fig. 2).
Fig. 2.
EGF receptor (EGFR) effects on ABC transporters. The EGFR signals through both MAPK and PI3K pathways to affect ABC transporters, differentially, at the transcriptional, translational, and post-translational levels. Following ligand binding, the EGFR dimerizes and signals through its tyrosine kinase domain to lead to the phosphorylation and activation of MEK, which in turn phosphorylates and activates ERK. ERK signaling increases the mRNA and protein expression of ABCG2, decreases the mRNA and protein expression of ABCC4, and decreases the mRNA expression of ABCB1 while preventing the degradation of ABCB1 protein by the proteasome. PI3K can also be activated downstream from EGFR tyrosine kinase activity leading to the activation of Akt. Akt signaling then increases the mRNA expression of ABCG2, ABCB1, and ABCC4, and also increases the protein expression and membrane localization of ABCG2. PI3K signaling can be inhibited by PTEN; PTEN is activated by PPARγ signaling. Pharmacological inhibitors used to elucidate this pathway have been noted at the level of their inhibition; mRNA changes induced by the ERK pathway are noted in blue, while changes induced by the Akt pathway are noted in orange.
Induction of ABCG2 expression following EGF treatment has been demonstrated in several cell lines and under various conditions. Treatment of cultured BeWo (human trophoblasts) and MCF-7 (human breast adenocarcinoma) cells led to increased ABCG2 mRNA and protein expression (Meyer zu Schwabedissen et al., 2006). This induction was not due to a global increase in transporters since there was no change in ABCC2 expression following EGF treatment. The increase in expression was paralleled by increased drug resistance to mitoxantrone and topotecan, known ABCG2 substrates, but not to doxorubicin, which is not an ABCG2 substrate, except when it acquires a mutation producing an amino acid substitution at arginine 482 (Allen et al., 2002). Pharmacological inhibition of the EGFR signaling cascade with a tyrosine kinase inhibitor (AG1478) or a MEK inhibitor (PD98059) abrogated EGF increase in ABCG2 mRNA and protein expression in resensitized cells to mitoxantrone, as expected. The ability of the MEK inhibitor to block the increase in ABCG2 expression does suggest that the MAPK cascade regulates ABCG2 expression at the mRNA level following EGF activation of its receptor.
Similar results were found in kidney and liver cells, although these studies also suggest a role for the Akt pathway downstream from the EGFR in regulation of ABCG2 expression. Treatment of conditionally immortalized human proximal tubule epithelial cells with EGF increased the expression of ABCG2 (Caetano-Pinto et al., 2017). Treatment of these cells with either an Akt inhibitor (LY294002) or ERK inhibitor (U-0126) led to a reduction in ABCG2 expression, although it is unclear whether these inhibitors suppressed basal ABCG2 expression or blocked EGF induction of ABCG2. These data suggest regulation of ABCG2 expression is through both Akt and MAPK pathways in response to EGF stimulation. An in vivo extension, hepatocyte-specific knockout of EGFR (Egfrhep) reduced ABCG2 protein in both total lysate and membrane-enriched fractions (Traxl et al., 2017). Monitoring the clearance of the substrate [11C] erlotinib via positron emission tomography in Egfrhep mice demonstrated a reduction in hepatic clearance and shift to renal excretion. These data suggest the shift in clearance of [11C] erlotinib is due to the loss of ABCG2 protein in hepatocytes caused by the knockout of Egfr, whereas Egfr and ABCG2 expression are unchanged in the kidney, leading to renal excretion. Overall, it is clear that activation of the EGF pathway enhances ABCG2 expression at the mRNA and protein levels in many relevant tissues. However, further data are needed to determine the mechanism by which this occurs.
Data suggest that EGF signaling can also regulate the expression of ABCB1 and ABCC4, but in more complex ways. Treatment of conditionally immortalized human proximal tubule epithelial cells with EGF decreases the mRNA level of both ABCB1 and ABCC4 (Caetano-Pinto et al., 2017). Interestingly, pharmacological blockade of ERK increased ABCB1 and ABCC4. Paradoxically, Akt inhibition decreased expression of these two transporters at the mRNA level. Thus, unlike ABCG2, the MAPK and Akt pathways seem to have opposing roles in the control of the expression of ABCB1 and ABCC4. In the Egfrhep mice mentioned previously, ABCB1 protein expression increased, in addition to the changes in ABCG2 (Traxl et al., 2017). Finally, treatment of human colorectal cells, HCT-15 and SW620-14, or breast cancer cells, MCF-7/MDR and MDA-MB-231/MDR, with EGF or basic fibroblast growth factor enhanced ABCB1 expression at the protein level with no effect on mRNA expression (Katayama et al., 2007). Both siRNA-mediated knockdown and pharmacological inhibition of ERK decreased ABCB1 protein expression (Fig. 2). Further pulse-chase experiments determined that ERK inhibition promoted degradation of ABCB1 with no effect on its biosynthesis. Thus, these data suggest that the MAPK pathway (downstream from growth factor signaling) increases ABCB1 by preventing its proteasomal degradation.
Overall, these data illustrate how disruption of growth factor signaling by kinase inhibitors (Table 1) can produce unanticipated alterations in transporter protein expression; such changes can be due to alterations in transcription or protein stability. More broadly, these changes could impact drug disposition and clearance, consequently increasing adverse effects, drug-drug interactions, and nonoptimal plasma concentrations of drug.
Kinase Modifications of ABC Transporters
Phosphorylation sites have been identified in many ABC transporters (Stolarczyk et al., 2011) (see the PHOSIDA searchable database; http://141.61.102.18/phosida/index.aspx). Phosphorylation of proteins changes their conformation, and is reversible; however, unlike our knowledge of the impact on transcription factors (e.g., TP53), the evidence that specific phosphorylation changes ABC transporter activity is still nascent. Furthermore, the relationship between phosphorylation sites and the kinases mediating the modifications is virtually unknown. Figure 3 illustrates the predicted phosphorylation sites for ABCB1, C1, C4, and G2 discussed in this review, and Table 2 lists experimentally identified phosphorylation sites for some of these proteins and the putative effect of phosphorylation at these sites. In addition to direct phosphorylation of ABC transporters, kinases may contribute to signaling cascades that ultimately affect the trafficking and/or activity of transporters. Given that kinase inhibitors may ultimately be deployed in a variety of therapeutic settings, and also given the well-known, off-target effects of kinase inhibitors (Anastassiadis et al., 2011), it is likely that alterations in ABC transporter function may occur secondary to altered transporter phosphorylation. The importance and identity of kinases affecting ABC transporter activity is emerging; some examples are discussed subsequently.
Fig. 3.
Predicted phosphorylation sites on ABCB1, ABCC1, ABCG2, and ABCC4. Potential post-translational modifications of the indicated proteins were performed using the default parameters at the following website: www.phosphosite.org.
TABLE 2.
Experimentally identified phosphorylation sites on ABCB1, ABCG2, and ABCC1
| Gene | Species | Modification | Effect of Modification | Reference |
|---|---|---|---|---|
| ABCB1 | Homo sapiens | S661 | PKC regulation of transport activity | Chambers et al. (1993) |
| ABCB1 | Homo sapiens | S667 | PKA and PKC regulation of transport activity | Chambers et al. (1994) |
| ABCB1 | Homo sapiens | S667 | PKA and PKC regulation of transport activity | Chambers et al. (1993) |
| ABCB1 | Homo sapiens | S671 | PKA and PKC regulation of transport activity | Chambers et al. (1994) |
| ABCB1 | Homo sapiens | S671 | PKA and PKC regulation of transport activity | Chambers et al. (1993) |
| ABCB1 | Homo sapiens | S683 | PKA regulation of transport activity | Chambers et al. (1994) |
| ABCB1 | Homo sapiens | S683 | PKA regulation of transport activity | Chambers et al. (1993) |
| ABCB1 | Homo sapiens | NI | Pim-1 regulation of membrane localization | Xie et al. (2010) |
| ABCG2 | Homo sapiens | T362 | Pim-1 modulation of dimerization | Xie et al. (2008) |
| ABCC1 | Homo sapiens | T249 | CK2α negative regulation of transport activity | Stolarczyk et al. (2012) |
NI, not identified; PKA, protein kinase A.
Kinases and Trafficking
In addition to the transcriptional changes associated with EGF treatment discussed previously, there is evidence that EGF downstream signaling may also regulate the trafficking of ABCG2. Side population cells, first identified in the laboratory of Richard Mulligan (Goodell et al., 1996), express high levels of ABCG2 and are enriched in hematopoietic stem cell activity. Brief treatment of side population cells with the PI3K inhibitor LY294002 led to the translocation of ABCG2 to the cytosol (Fig. 2), as visualized via immunofluorescence detection of endogenous protein (Mogi et al., 2003). There was no change in total fluorescence intensity, suggesting no change in the total protein expression, only localization. This phenomenon was independent of ABCG2 transcription because LLC-Pk1 cells that heterologously express ABCG2 when treated with EGF display a 2-fold increase in cell surface expression of ABCG2 with no change in total protein expression (Takada et al., 2005). Accordingly, treatment with PI3K inhibitors to disrupt this pathway showed ∼50% decreased surface expression as measured by surface biotinylation and confocal microscopy. Finally, PPARγ agonists, which activate PTEN, a negative regulator of PI3K activity, also reduced surface expression and transport activity of ABCG2 with no change in mRNA or protein expression in MCF-7 FLV1000 cells (To and Tomlinson, 2013). Thus, while EGF affects ABCG2 transcription (Fig. 2), evidence indicates EGF, through the PI3K/Akt pathway, stabilizes ABCG2 at the membrane of cells. It is unknown if this pathway produces specific phosphorylation of ABCG2 to facilitate its membrane localization or retention. Although speculative, one could imagine phosphorylation altering ABCG2 membrane recycling or its interaction with the actin network.
The PI3K/Akt pathway and protein kinase C (PKC) have been implicated in the retrieval of ABCC2 and ABCB11 from the membrane during cholestasis induced by estradiol 17β-D-glucoronide (E2-17G)–induced cholestasis (Crocenzi et al., 2008; Boaglio et al., 2010), an endogenous metabolite of estrogen. The endocytic retrieval of both ABCC2 and ABCB11 from the membrane occurs following treatment with E2-17G (Meyers et al., 1980; Mottino et al., 2002; Crocenzi et al., 2003). Pharmacological inhibition of the conventional PKC isoform with Gö6976, partially rescued the E2-17G-induced reduction in biliary secretory function and ABCC2 and ABCB11 transport activity and was able to maintain membrane expression of both transporters (Crocenzi et al., 2008). However, since conventional PKC activity only partially prevents the effects of E2-17G, further work was required. Inhibition of PI3K with wortmannin or LY294002 also significantly rescued the activity and membrane of ABCC2 and ABCB11 following E2-17G treatment (Boaglio et al., 2010). Downstream from PI3K, Akt inhibition also partially rescued ABCC2 and ABCB11 activity in response to E2-17G treatment; however, this was not to the same magnitude as PI3K inhibition, suggesting there are other PI3K downstream pathways that are involved in transporter trafficking. The PI3K and conventional PKC signaling was shown to be additive, suggesting complementary rather than overlapping pathways. Thus, both PI3K and PKCs contribute to maintaining plasma membrane ABCC2 and ABCB11 expression in hepatocytes.
Preliminary evidence suggests that the Src kinase pathway may regulate transporter protein trafficking downstream from the vascular endothelial growth factor (VEGF) (Hawkins et al., 2010). VEGF reversibly decreases ABCB1 transport activity with no changes in protein expression in isolated brain capillaries. In fact, the effect of VEGF could be reversed within 30 minutes of washout. Both Flk1 and Src activity are needed for this signaling pathway since pretreatment with inhibitors abolished VEGF-induced reduction in transport activity. This effect was specific for Src downstream from Flk1 since treatment with both PI3K and PKC inhibitors had no effect. Intracerebroventricular injection of VEGF increased the brain distribution of ABCB1 substrates morphine and verapamil to the ipsilateral cerebral hemisphere with no change in sucrose distribution, indicating no general change in blood-brain barrier permeability. This increase in brain distribution of verapamil could be abrogated by intraperitoneal injection of a Src kinase inhibitor. Finally, to determine the mechanism by which VEGF affects transporter activity, nocodazole, a microtubule polymerization inhibitor, blocked the VEGF-induced loss of ABCB1 activity in isolated brain capillaries but the proteasome inhibitor lactacystin did not. These data together suggest a change in ABCB1 trafficking, but whether it is related to altered endosomal trafficking or loss of membrane retention remains the subject of future investigation. In another report, ABCB1 and Src were found to coimmunoprecipitate from MCF-7 drug resistant cells (Zhang et al., 2014), further supporting a role for Src signaling in ABCB1 membrane localization. However, these results are preliminary and further work is needed to verify and determine the clinical relevance, if any, of this interaction.
Pim-1 kinase, an oncogenic serine/threonine kinase, is overexpressed in many cancers and has diverse substrates that include proapoptotic proteins (Aho et al., 2004), cell cycle regulatory proteins (Mochizuki et al., 1999; Bachmann et al., 2006; Zhang et al., 2007; Morishita et al., 2008), and transcription factors (Chen et al., 2002; Peltola et al., 2004; Glazova et al., 2005; Aho et al., 2006; Kim et al., 2008; Zhang et al., 2008). ABCB1 and ABCG2 have been identified as putative substrates for Pim-1 kinase, where phosphorylation is necessary for the plasma membrane localization of both transporters (Xie et al., 2008, 2010; Natarajan et al., 2013; Darby et al., 2015).
Pim-1 directly phosphorylates ABCG2, and the 44 kDa isoform of Pim-1 coimmunoprecipitates ABCG2 when exogenously expressed in HEK293T cells or endogenously expressed in CWR-R1 prostate cancer cells (Xie et al., 2008). This interaction requires the Pim-1 kinase domain and its N-terminus proline-rich domain, which is present only in the 44 kDa isoform. The siRNA-mediated knockdown of Pim-1 in CWR-R1 cells reduced mitoxantrone or docetaxel resistance, while overexpression of Pim-1 in cells that express very little ABCG2 (LNCaP cells) increased drug resistance. ABCG2 has a Pim-1 substrate consensus sequence located in the cytoplasmic linker region. To determine if ABCG2 was a substrate of Pim-1, they were coexpressed in HEK293T cells, and ABCG2 was phosphorylated at T362. This phosphorylation could be abrogated by Pim-1 knockdown. Overexpression of a T362A ABCG2 mutant, which blocks phosphorylation at this site, in LNCaP cells did not change drug sensitivity, unlike wild-type ABCG2, which strongly enhanced drug resistance. Accordingly, a phospho-mimicking T362D mutant increased drug resistance even when Pim-1 had been knocked down, suggesting this specific phosphorylation event is important for ABCG2 transport activity. Finally, the wild-type or T362D ABCG2 in LNCaP cells could be observed on the plasma membrane of cells by immunofluorescence and cell fractionation, but the T362A mutant ABCG2 could only be observed in the cytosol, suggesting the phosphorylation site is required for membrane localization. This was further validated by either T362A mutant overexpression or Pim-1 knockdown, each of which exhibited a decrease in oligomerization of ABCG2. These data suggest phosphorylation by Pim-1 at T362 is necessary for proper localization and function of ABCG2.
Pharmacological inhibition of Pim kinase with the inhibitor SGI-1776 decreased the surface expression of ABCG2 and increased the uptake of substrate drugs in cells that expressed high levels of ABCG2 (Natarajan et al., 2013). Additionally, use of novel Pim kinase inhibitors in MCF-7 drug-resistanct cells overexpressing ABCG2 increased the potency of flavopiridol, mitoxantrone, topotecan, and doxorubicin, while also decreasing the protein expression of ABCG2 (Darby et al., 2015). While preliminary, these results suggest that Pim kinase inhibition could regulate ABCG2 activity, localization, and expression.
Pim-1 phosphorylation is also required for ABCB1 membrane expression, likely due to stabilization of immature ABCB1 to allow glycosylation to occur (Xie et al., 2010). Total and plasma membrane expression of ABCB1 is decreased when Pim-1 is knocked down via shRNA or siRNA. This decrease in protein expression is probably due to an increase in degradations; cyclohexamide treatment in cells with Pim-1 knockdown decreased the half-life of ABCB1 in comparison with cells with Pim-1 present. A consensus Pim-1 sequence exists in ABCB1 between NBD1 and MSD1, and an in vitro kinase assay demonstrates ABCB1 phosphorylation following incubation with Pim-1. Therefore, the stabilization of ABCB1 appears to be through its phosphorylation. Pharmacological inhibition of Pim-1 with SGI-1776 demonstrated similar effects as genetic inhibition, namely, a decrease in mature ABCB1 and a decrease in ABCB1 half-life. Cotreatment of cells with SGI-1776 and MG132, a proteasome inhibitor, maintained ABCB1 protein stability, suggesting that phosphorylation of ABCB1 prevents its proteasomal degradation. Finally, it was determined that this stabilization appears to be required for effective glycosylation of immature ABCB1. Inhibition of glycosylation by 2-deoxyglucose led to accumulation of 150 kDa ABCB1 protein, while cotreatment with SGI-1776 decreased this accumulation. Thus, these data suggest that Pim-1 phosphorylation of ABCB1 enhances its maturation and membrane trafficking. However, another report suggests that treatment of NCIADR/Res cells, which overexpress ABCB1, with novel Pim kinase inhibitors does not affect sensitivity to doxorubicin, a well-known ABCB1 substrate (Darby et al., 2015). That these reports do not agree suggests further work is needed to fully determine what, if any, effect Pim-1 kinase activity may have on ABCB1 trafficking.
Kinases and Transporter Activity
ABCC1 can be regulated by several kinases including glycogen synthase kinase 3 (GSK3) and casein kinase 2 (CK2). GSK3 is a serine/threonine kinase that has two isoforms (GSK3α and β), which have been shown to modulate the PI3K-Akt pathway. The GSK3 isoforms have a diverse array of substrates that do not depend on mitogenic signaling. Phosphorylation of a serine (Ser9 for GSK3α, or Ser21 for GSK3β) within the basic pocket near the catalytic site leads to autoinhibition of kinase activity (Hermida et al., 2017).
Changes in ABCC1 expression in response to cadmium exposure are complex, but GSK3 inhibitors provide insight. ABCC1 is both induced and redistributed from the nuclear-rich membrane fraction to the cytosolic fraction following cadmium exposure, which can be suppressed by the GSK3 inhibitor (Kim et al., 2015). GSK3 knockout decreased ABCC1 mRNA and protein expression in response to cadmium. Notably, ABCC1 and p-Ser GSK3 can be coimmunoprecipitated, indicating a physical interaction. While it is clear that GSK3 regulates ABCC1 expression following cadmium treatment, the consequence (or role) of GSK3 in normal regulation of ABCC1 is unknown.
CK2, a ubiquitous and constitutively active serine/threonine kinase, also modulates ABCC1. In yeast, the homolog of CK2 phosphorylates Ser251 in the intracellular loop that connects MSD0 to MSD1 producing an increase in activity (Paumi et al., 2008). This CK2 consensus site is conserved in human ABCC1 as Thr249. In a variety of cancer cells, treatment with CK2 inhibitors decreases ABCC1-dependent efflux of doxorubicin, thereby increasing doxorubicin cytotoxicity (Stolarczyk et al., 2012). How this specific phosphorylation site changes ABCC1 activity is unknown; one could speculate that this occurs by altering degradation, localization, or protein-protein interactions. Further work is needed to determine the consequences of ABCC1 phosphorylation by either GSK3 or CK2.
ABCB1 activity can be acutely modulated at the blood-brain barrier through PKC signaling. Freshly isolated brain capillaries, exposed to subnanomolar-to-nanomolar concentrations of endothelin-1 (ET1), reduced ABCB1 transport activity within 15 minutes (Hartz et al., 2004), with ET1 washout returning ABCB1 activity to baseline within 60 minutes. An inhibitor of the ETB receptor prevented the effects of ET1 on ABCB1 transport activity, suggesting signaling occurs through this receptor. Signaling through inducible nitric oxide synthase (iNOS) and PKC are required for the effect of ET1 on ABCB1; blocking these downstream targets, iNOS with NG-Monomethyl-L-arginine or PKC with bisindolylmaleimide prevents ET1’s suppression of ABCB1 function. Furthermore, increasing nitric oxide with the donor sodium nitroprusside mimics ET1’s reduction of ABCB1, and bisindolylmaleimide also blocks this activation, suggesting PKC is downstream of iNOS. Further work showed that upstream of ET1, tumor necrosis factor α (TNFα) exposure also reduces ABCB1 activity, by signaling the release of ET1. This effect is both acute, reduction of activity is seen within 30 minutes, and reversible, washout of TNFα returns activity to baseline within 150 minutes, suggesting it is not a change in protein stability (Hartz et al., 2006). Reduction of ABCB1 activity could also be replicated by treatment with lipopolysaccharide, a component of gram negative bacterial cell walls that activates an inflammatory response, which includes release of TNFα. Intraperitoneal injection of lipopolysaccharide in mice increased brain uptake of [3H]-verapamil 18 hours after injection, indicative of decreased ABCB1 activity (Salkeni et al., 2009). Increased brain uptake was evident until 36 hours after injection and occurred despite an increase in ABCB1 protein expression. In mice, inhibition of nitric oxide signaling did not restore ABCB1 transport activity, suggesting there may be factors present in a live animal that are not in isolated brain capillaries and are a part of this signaling cascade.
TNFα signaling stimulates sphingosine-1-phosphate (S1P) from sphingosine by activating sphingosine kinase 1 (Xia et al., 1999; De Palma et al., 2006). S1P signals through its receptor S1P receptor (S1PR) 1. Treatment of brain capillaries with sphingosine kinase 1 or S1PR1 antagonists blocks the TNFα- and PKC agonist-mediated reduction in ABCB1 transport activity; suggesting sphingosine kinase 1 signaling is downstream from TNFα and PKC (Cannon et al., 2012). An approved small molecule used for the treatment of multiple sclerosis, FTY720, is a nonselective S1PR agonist. Treatment of capillaries with this S1PR agonist rapidly reduces ABCB1 activity. Brain perfusion with FTY720 increased accumulation of ABCB1 substrates of verapamil by ∼4-fold and loperamide and paclitaxel by ∼5-fold, which is indicative of decreased ABCB1 activity. Downstream from S1P, actin filament-associated protein 1 is required for TNFα-induced reduction of ABCB1 activity, since siRNA-mediated knockdown of actin filament-associated protein 1 maintains ABCB1 activity following treatment with TNFα, lipopolysaccharide, and S1P (Hoshi et al., 2017). Interestingly, long-term (3–6 hours) treatment with ET1 or TNFα produces a 2-fold increase in protein in isolated rat brain capillaries, which correlates with an increase in ABCB1 activity through a NF-κB-mediated cascade (Bauer et al., 2007). These data illustrate that a signaling cascade, mediated by multiple phosphorylation events, can profoundly affect ABCB1 activity, suggesting a potential mechanism for ABCB1-mediated drug-drug interactions with drugs that effect ET1, iNOS, PKC, inflammatory, or sphingosine signaling.
PKC also regulates ABCB1 activity in other contexts (Table 2). Phosphorylation of the linker region of ABCB1 by PKC has been well documented at Ser661, Ser 671, and one or more of Ser667, Ser675, and Ser683 (Chambers et al., 1990, 1993, 1994; Orr et al., 1993), and this phosphorylation increases the activity of ABCB1 (Chambers et al., 1990, 1992; Sachs et al., 1996; Szabó et al., 1997; Idriss et al., 2000; Masanek et al., 2002). This phosphorylation site is similar to the location of phosphorylation sites within the cystic fibrosis transmembrane-conductance regulator (CFTR), a member of the ABC transporter C family. While the fact that ABCB1 is phosphorylated by PKC is well established, the mechanism by which this phosphorylation event increases transporter activity is less obvious. Coexpression of PKCα with ABCB1 increased the ATPase activity in insect and ovarian cells (Idriss et al., 2000), while PKC inhibitor treatment did not alter ATPase activity in MCF-7 cells (Sachs et al., 1996). Perhaps this difference is due to the expression systems or differences between mammals and insects, but it may also be an isoform-specific effect since coexpression of PKCε with ABCB1 in insect ovarian cells had no effect on ATPase activity (Idriss et al., 2000). Mutation of Ser661, Ser667, and Ser671 from serine to alanine creates a protein that is not phosphorylated and required an increased substrate concentration to achieve half-maximal activity (Szabó et al., 1997). This suggested a change in substrate binding. However, this effect was only true for verapamil, vinblastine, and rhodamine 123, but not valinomycin or calcein acetoxymethylester. These data suggest phosphorylation changes the substrate binding site selectively. However, it is important to note that there is some discrepancy in the role PKC plays in ABCB1 activity because inhibition of PKC failed to reverse drug resistance in some human cancer cells (Scala et al., 1995). Notably, in fish proximal tubule cells activation of PKC decreased ABCB1 transport activity (Miller et al., 1998). Similarly, activation of the PKCβ1 isoform at the blood-brain barrier in rats led to the rapid decrease in ABCB1 transport activity (Rigor et al., 2010). Thus, the effects of PKC on ABCB1 function vary, and may depend on the phosphorylation sites as other inputs from upstream stimuli, or perhaps the species and/or cell-type. It is clear that phosphorylation is an attractive mechanism to control ABC transporter activity, and may contribute to drug-drug interactions or adverse events by changing ABC transporter protein activity, although the clinical consequences remain unexplored.
Protein-Protein Interactions
Direct interactions between proteins may be another source of regulation since these interactions might contribute to the proper localization and stabilization of transporters at the plasma membrane. To determine the interactome of nonmitochondrial ABC transporters, Snider et al. (2013) used the unbiased membrane yeast two-hybrid method, which allows interrogation of protein-protein interactions of membrane proteins in their native state. The membrane yeast two-hybrid system is more effective for determination of protein interactions with membrane-bound proteins than traditional yeast two-hybrid methodologies. It is based on the split-ubiquitin protein complementation assay and allows for detection of novel protein-protein interactions (Lentze and Auerbach, 2008). When combined with the BioGRID database (see https://thebiogrid.org), 537 unique binary interactions across 366 proteins involving the 19 nonmitochondrial ABC transporters encoded by the yeast genome were identified. Interactions were classified using gene ontology and included interactions between transporters and proteins involved with metabolism, cell cycle, growth, and division; cytoskeleton, DNA replication, maintenance, and repair; nuclear function, protein degradation, folding, and modification; protein synthesis and ribosome, RNA processing, and regulation; stress response; and transport, trafficking, and secretion. Additionally, the transporters were found to interact with one another, not only known half-transporter interactions, but also interactions between full transporters (e.g., Pdr5p and Snq2p, i.e., members of the pleiotropic drug resistance family, which has homology to the ABCG family in mammals). Thus, there are many diverse protein-protein interactions that if disrupted may have an effect on transporter activity. Specific examples and the consequences of the protein interactions between ABC proteins and ezrin-radixin-moesin (ERM) or PDZ proteins are discussed in more detail subsequently as examples of protein-protein interactions that modulate ABC protein activity.
ERM Proteins
The ERM family of proteins are scaffolding proteins that crosslink actin filaments and integral membrane proteins (McClatchey, 2014). ERM proteins may be important for ABCB1 stabilization at the membrane; ABCB1 has been shown to interact with each of the ERM proteins. In HepG2 cells with radixin (Rdx) knocked out there is decreased ABCB1 at the membrane and decreased transport activity (Kano et al., 2011). This interaction has also been found in Rdx−/− mouse small intestines, where the amount of ABCB1 at the membrane was reduced compared with wild-type mice (Yano et al., 2013). Ezrin coimmunoprecipitates with ABCB1 and is required for optimal ABCB1 membrane localization and transporter activity (Brambilla et al., 2012). ABCB1 and ezrin also co-localize with the lipid raft marker GM1, and ezrin is required for ABCB1 localization to lipid rafts. In both cells and mice, the importance of an interaction between ABCB1 and ERM proteins has been shown to be important for stabilization at the membrane. However, the nature of this interaction and the domains mediating it remain to be defined.
That the proper subcellular localization of ABCC2 (which modulates bile acid–independent bile flow) in the bile canalicular membranes (BCMs), required an interaction with the major liver ERM, Rdx, was unknown, until the Rdx−/− mouse was developed (Kikuchi et al., 2002). The Rdx−/− mouse appeared normal at birth; however, by age 4 weeks, concentrations of conjugated bilirubin began to rise in the serum, and reached 15-fold higher levels than in wild-type mice by 16 weeks. This correlated with histologic evidence for mild liver injury and increased serum levels of alkaline phosphatase and aspartate aminotransferase. This is similar to Dubin-Johnson syndrome, a human genetic disease, producing hyperbilirubinemia associated with ABCC2 loss of function. The expression of ABCC2 in the Rdx−/− mice was determined in the BCM by immunofluorescence and western blot; a decrease in Abcc2 relative to other BCM-resident proteins was evident in Rdx−/− mice compared with wild-type mice. In the bile canaliculi, co-localization of Abcc2 and Rdx was histologically demonstrated in liver frozen sections, suggesting a direct interaction that was then confirmed by coimmunoprecipitation from wild-type liver. In MDCK cells transiently expressing FLAG-ABCC2, ezrin, the dominant isoform of ERM proteins in these cells, was coimmunoprecipitated, and this interaction was prevented by the removal of the C-terminus of ABCC2. To map the location of ABCC2’s interaction with Rdx, in vitro studies were conducted whereby a purified fusion protein composed of the C-terminus of ABCC2 fused to glutathione-S-transferase was incubated with the recombinant N-terminus half of Rdx. The complex between these two proteins suggested that the N-terminus of Rdx interacted with the C-terminus of ABCC2. However, ABCC2 lacks the prototypical PDZ motif at its C-terminus, thus the exact nature of this interaction is unknown. In combination, the studies from the MDCK cells and Rdx−/− mice suggest that the ERM proteins are required for the expression of ABCC2 at the membrane, and that in BCMs, radixin interacts with ABCC2. At this point, it is unknown if ERM proteins like radixin interact due to their affinity or abundance.
PDZ Domain Proteins: Postsynaptic Density Protein 95, Drosophila Disc Large Tumor Suppressor 1, and Zonula Occludens-1 Protein
PDZ domains are the most common protein-protein interaction domains in humans with over 250 PDZ domains in 150 unique proteins (Ponting et al., 1997; Feng and Zhang, 2009; Walsh et al., 2015). An individual protein may contain from one to multiple PDZ domains that facilitate the formation of multiprotein complexes. The canonical PDZ domain is composed of a stretch of 80–90 amino acid residues that are arranged as six β-strands and two α-helices in a globular structure (Karthikeyan et al., 2001). Although some PDZ motifs can be found throughout the target protein, PDZ motifs are typically C-terminal sequences and are generally three to four residues long. These protein-protein interactions are mediated through binding of the PDZ motif to a hydrophobic binding pocket in the PDZ-domain protein that is formed by a β-strand and α-helix, and the loop that connects these two (Walsh et al., 2015).
PDZ motifs are found in ABCC2 (-STKF) and ABCC4 (-ETAL). ABCC2 interacts with the PDZ domain containing proteins NHERF1, NHERF3 (also known as PDZK1), and NHERF4 (Kocher et al., 1999; Hegedüs et al., 2003). Interestingly, the serine present in the PDZ motif of ABCC2 is also a PKC consensus phosphorylation site. In Sf9 cells, phospho-mimicking mutants of this serine (S1542E) interacted with NHERF1 and -4 more strongly than wild-type MRP2, while a dephosphorylated mimicking mutant (S1542A) had no effect on the interaction with NHERF4 but decreased the interaction with NHERF1 (Hegedüs et al., 2003). These data suggest that the phosphorylation of the serine in the PDZ domain of MRP2 may regulate its protein-protein interactions. PDZ protein interactions appear to be important for ABCC2 protein localization. In Nherf1-knockout mouse liver, Abcc2 mRNA expression was unchanged, but the total lysate and membrane fraction protein expression of Abcc2 was significantly decreased compared with wild-type mice (Li et al., 2010). Abcc2 was still localized to the apical membrane, detected by immunofluorescence, suggesting that trafficking to the membrane was unchanged when Nherf1 was absent. Functional outputs of Abcc2, glutathione, and glutathione-methylfluorescein excretion were also decreased in Nherf1-knockout mice, further supporting a decrease in Abcc2 function. Using immunofluorescence, the requirement for NHERF1 was further confirmed and refined in WIF-B cells. Mutation of the first PDZ domain or the radixin-binding domain of NHERF1 decreased ABCC2 membrane localization and function (Karvar et al., 2014). These data suggest that the interaction between ABCC2 and NHERF1 is important for the proper membrane localization of ABCC2 in hepatocytes.
The interaction between ABCC2 and NHERF3 was the first to be discovered, using a yeast two-hybrid system (Kocher et al., 1999), and was further confirmed in later studies (Hegedüs et al., 2003; Emi et al., 2011). However, in Nherf3-knockout mouse kidney proximal tubule cells, there was no difference in the expression or localization of Abcc2 when compared with wild-type cells (Kocher et al., 2003). In contrast, in HepG2 cells, overexpression of the PDZ domain of NHERF3 can destabilize ABCC2’s membrane localization (Emi et al., 2011). Thus, the importance of NHERF3 on ABCC2 localization may be dependent on cell type and other PDZ-containing proteins present.
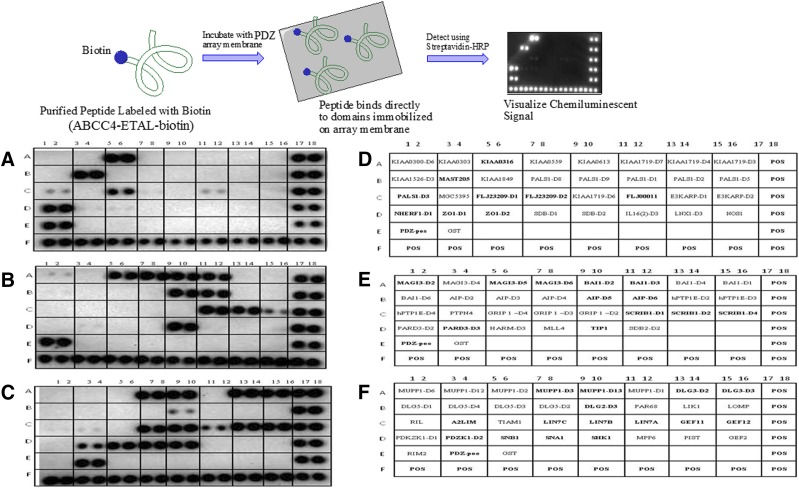
The C-terminal PDZ motif of ABCC4 (-ETAL) is highly conserved from fish to humans (Pitre et al., 2017). In an attempt to catalog the extent of PDZ domain proteins capable of interacting with this ABCC4 motif, we used an in vitro strategy and interrogated the Panomics PDZ Domain Arrays (Fig. 4) (Fremont, CA). These commercial arrays include a total of 123 different human PDZ domains representing recombinant conserved binding sites from the individual PDZ domain proteins that have been fused with glutathione-S-transferase. These proteins were affinity-purified, immobilized onto a membrane, spotted in duplicate, and then probed with a biotinylated peptide harboring the ABCC4 PDZ motif (see the Fig. 4 legend for the description). We discovered almost 30 PDZ domain–containing proteins that interact with the ABCC4 PDZ motif. Among these, some were already known, such as PDZK1 and NHERF1, but many have never been reported before.
Fig. 4.
ABCC4 PDZ motif binds to a variety of PDZ-domain containing proteins. (Upper panel) Overview of the workflow for PDZ arrays. (A–C) The purified PDZ domains from the indicated PDZ-domain proteins were immobilized, in duplicate, on the array membrane (Panomics). Biotin-containing ABCC4 purified peptide harboring the ABCC4 PDZ motif was incubated with the array membrane. Unbound ABCC4 was then washed away and bound ABCC4 detected using streptavidin-horseradish peroxidase. The ABCC4 was peptide: biotin-KSGSG-STLTIFETALCOOH (blue = linker, orange = spacer; green = PDZ motif). (Lower panel) Results of three different PDZ arrays. (D–F) Proteins that have been immobilized on the membrane are noted, with positive binding indicated by the bold font.
As mentioned previously, several PDZ-domain proteins have been reported to interact with ABCC4’s PDZ motif. Initial studies evaluated several PDZ-domain proteins, and pull-down assays demonstrated that ABCC4 binds with the highest affinity to PDZK1 (also known as NHERF3) (Li et al., 2007). PDZK1, a protein with four PDZ domains, mediated a functional coupling between ABCC4 and another ABC transporter protein in the C subfamily, the CFTR, a cAMP-activated (via protein kinase A–mediated phosphorylation of CFTR) Cl− channel. Genetic deletion and pharmacological inhibition of ABCC4, a cAMP exporter (Li et al., 2007), potentiated CFTR Cl− currents, likely due to an accumulation of cAMP near CFTR, resulting in increased transport activity of CFTR. In gut epithelia, CFTR, ABCC4, and PDZK1 were coimmunoprecipitated, suggesting that formation of this macromolecular complex, facilitated by a PDZ protein, functionally couples CFTR and ABCC4. Disruption of this macromolecular complex with competitive ABCC4 PDZ peptides attenuated the potentiation of CFTR conductance, suggesting that the extent of complex formation can regulate CFTR activity. Unanswered questions include: what signals promote the formation or disassociation of this macromolecular complex?
PDZK1 is reportedly required for ABCC4 transport activity in kidney, presumably through this PDZ interaction (Park et al., 2014); however, given that ABCC4 only interacts with one of the four PDZ domains, other interacting proteins could modulate the activity. Nonetheless, when coexpressed in HEK293 cells, PDZK1 increased ABCC4 expression, membrane stability, and drug transport activity, and reduced the internalization of the transporter and lysosome-mediated degradation. These results were extended to in vivo studies in the kidney of Pdzk1-knockout mice, which had decreased Abcc4 expression, membrane localization, and plasma clearance of [3H]-adefovir, a measurement of Abcc4 function in the kidney. However, given that PDZK1 has the potential to bind more than one PDZ motif–containing protein, it remains to be determined if another PDZ-motif protein interacts with ABCC4 and PDZK1 within this context.
An interaction between ABCC4 and NHERF1 has also been defined (Li et al., 2007; Hoque and Cole, 2008; Hoque et al., 2009), although the consequence of this interaction is unclear. In HeLa cells, NHERF1 knockdown by siRNA increased ABCC4 total and membrane protein abundance and decreased cellular accumulation of ABCC4 substrates [3H]-bis(POM)PMEA and [14C]6-mercaptopurine, indicative of increased transport activity (Hoque and Cole, 2008). It has been proposed that NHERF1 promotes ABCC4 internalization since NHERF1 siRNA-mediated knockdown reduced ABCC4 internalization. The role of NHERF1 is likely cell context dependent and dictated by the proteins occupying its two PDZ domains because, unexpectedly, NHERF1 was also found to be required for membrane localization of ABCC4 (Hoque et al., 2009). ABCC4 is unique in that it can locate to both basolateral and apical membranes in polarized cell types and it has been proposed that NHERF1 might contribute to polarized cellular localization of ABCC4. In MDCKI cells, ABCC4 is expressed on the basolateral membranes. In contrast, ABCC4 is found predominantly on the apical membrane of LLC-PK1 cells. Interestingly, NHERF1 protein could be detected only in LLC-PK1 cells, suggesting NHERF1 contributes to the membrane localization of ABCC4. High ectopic expression of NHERF1 in MDCKI cells appeared to redirect ABCC4 away from the basolateral toward the apical membrane. Thus, the localization of ABCC4, by interactions with NHERF1, seems to depend on the cell type and/or a cell’s ability to polarize. These data suggest that in nonpolarized cells, NHERF1 promotes transporter internalization, but in polarized cells, NHERF1 facilitates ABCC4 trafficking to the apical membrane. However, what happens to ABCC4 localization when NHERF1 is knocked down in polarized cells and where it is expressed is currently unknown. Moreover, given that NHERF1 has more than one PDZ, and the ABCC4 PDZ motif binds to a single PDZ domain, it would be important to know the other proteins in the NHERF1, ABCC4 complex. These might influence ABCC4 function or activity.
Another PDZ adaptor protein, SNX27, was also identified in a pull-down assay to bind to ABCC4 (Hayashi et al., 2012). Knockdown of SNX27 in HEK293 cells led to increased plasma membrane expression and transport activity of ABCC4. SNX27 siRNA-mediated knockdown leads to a decrease in ABCC4 internalization; this combined with SNX27’s localization to endosomes suggests that SNX27 promotes ABCC4 internalization from the membrane.
To determine if single-domain PDZ-domain proteins impacted ABCC4 function in relation to disease (in this case, acute myeloid leukemia), we elucidated a new functional relationship between ABCC4 and MPP1, a gene associated with poor survival in acute myeloid leukemia; this interaction enhanced both leukemogenesis and ABCC4 chemoresistance (Pitre et al., 2017). ABCC4 and MPP1 reciprocally immunoprecipitated one another in myeloid leukemia cell lines, and removal of the C-terminal PDZ motif from ABCC4 prevented this interaction. In vitro mapping studies indicated ABCC4 interaction only required the MPP1 PDZ domain. This interaction stabilized ABCC4 at the plasma membrane since degradation of surface ABCC4 was reduced when MPP1 was coexpressed and transport activity increased as measured by cell sensitivity to ABCC4 substrates 6-mercaptopurine and cytosine arabinoside. Loss of MPP1 via CRISPR-Cas9 genetic ablation decreased 6-mercaptopurine resistance and ABCC4 surface expression, further validating that MPP1 is crucial for endogenous ABCC4 plasma-membrane localization and function in myeloid leukemia. Subsequently, a small molecule (time-resolved fluorescence resonance energy transfer) screen was developed to identify drugs capable of disrupting this interaction; 11,297 chemicals were tested and 144 unique disrupting compounds were identified. Further refinement led to the characterization of 17 compounds that displayed dose-response activity with antimycin A being the most potent. Antimycin A treatment recapitulated the effects of Mpp1 gene deletion or competition with ABCC4-PDZ peptides, i.e., antimycin A sensitized cells to the ABCC4 substrate 6-mercaptopurine and blocked growth of leukemic progenitors. These data demonstrate that drugs disrupting protein-protein interactions can alter membrane localization and function of ABCC4 and suggest that other reported inhibitors might act through a similar mechanism.
Perspective
ABC transporters impact the cellular concentrations of drugs, xenobiotics, and endogenous compounds. Direct interactions between drugs, acting as substrates and/or inhibitors, and ABC transporters, often referred to as victim and perpetrator interactions, are well known to affect therapeutic outcomes, generally in a negative manner. In this review, we have tried to briefly provide an overview that illustrates how biologic factors alter the function of transporters; these pathways regulating transporter expression, trafficking, and activity are less well understood. By focusing on kinases, we have illustrated how drug-induced alterations in receptor kinase activity can have myriad effects, from altering transcription (and possibly translation) to altering the phosphorylation of the transporters themselves. The effects of phosphorylation on a transporter are idiosyncratic to the transporter; some transporters have increased expression and function, while others are diminished in both. These pathways by and large have not been previously elucidated or have been understudied. Furthermore, we have shown how protein-protein interactions via specific domains can alter the function of ABC transporters. Post-translational regulation of ABC transporters requires further study since relatively little is known about how many of these clinically relevant proteins are regulated and/or trafficked. By extension, we propose that the clinical implications of regulation of transporter expression, degradation, trafficking, phosphorylation, and protein-protein interaction need to be considered in drug development.
Acknowledgments
The authors thank all of the members of the Schuetz laboratory for the suggestions to improve this manuscript.
Abbreviations
- ABC
ATP-binding cassette
- Akt
protein kinase B
- BCM
bile canalicular membrane
- CFTR
cystic fibrosis transmembrane-conductance regulator
- CK2
casein kinase 2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- E2-17G
estradiol 17β-D-glucoronide
- ERK
extracellular signal regulated kinase
- ERM
ezrin-radixin-moesin
- ET1
endothelin-1
- GSK3
glycogen synthase kinase 3
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- MDR
multidrug resistance protein
- MRP
multidrug resistance associated protein
- MSD
membrane-spanning domain
- NBD
nucleotide-binding domains
- PDZ
postsynaptic density protein 95, Drosophila disc large tumor suppressor 1, and zonula occludens-1 protein
- Pgp
P-glycoprotein
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- Rdx
radixin
- S1P
sphingosine-1-phosphate
- S1PR
sphingosine-1-phosphate receptor
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
Authorship Contributions
Participated in research design: J.D. Schuetz.
Conducted experiments: Potukuchi, E.G. Schuetz, J.D. Schuetz.
Performed data analysis: J.D. Schuetz.
Wrote or contributed to the writing of the manuscript: Crawford, J.D. Schuetz.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01CA194057, P30 CA21745, CA21865, CA194057, and CA096832] and the American Lebanese Syrian Associated Charities.
References
- Adachi M, Sampath J, Lan LB, Sun D, Hargrove P, Flatley R, Tatum A, Edwards MZ, Wezeman M, Matherly L, et al. (2002) Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem 277:38998–39004. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF. (2011) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho TL, Sandholm J, Peltola KJ, Ito Y, Koskinen PJ. (2006) Pim-1 kinase phosphorylates RUNX family transcription factors and enhances their activity. BMC Cell Biol 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. (2004) Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett 571:43–49. [DOI] [PubMed] [Google Scholar]
- Allen JD, Jackson SC, Schinkel AH. (2002) A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for doxorubicin resistance. Cancer Res 62:2294–2299. [PubMed] [Google Scholar]
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. (1998) A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58:5337–5339. [PubMed] [Google Scholar]
- Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco Md, Dale TC, Smalley MJ. (2003) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res 5:R1–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol 29:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279:22250–22257. [DOI] [PubMed] [Google Scholar]
- Aszalos A. (2007) Drug–drug interactions affected by the transporter protein, P-glycoprotein (ABCB1, MDR1): II. Clinical aspects. Drug Discov Today 12:838–843. [DOI] [PubMed] [Google Scholar]
- Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Möröy T. (2006) The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol 38:430–443. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS. (2007) Tumor necrosis factor α and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. (1997) P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J 326:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A, Schäfer HJ, Hrycyna CA. (2005) Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry 44:10893–10904. [DOI] [PubMed] [Google Scholar]
- Boaglio AC, Zucchetti AE, Sánchez Pozzi EJ, Pellegrino JM, Ochoa JE, Mottino AD, Vore M, Crocenzi FA, Roma MG. (2010) Phosphoinositide 3-kinase/protein kinase B signaling pathway is involved in estradiol 17β-D-glucuronide-induced cholestasis: complementarity with classical protein kinase C. Hepatology 52:1465–1476. [DOI] [PubMed] [Google Scholar]
- Borst P, Zelcer N, van de Wetering K, Poolman B. (2006) On the putative co-transport of drugs by multidrug resistance proteins. FEBS Lett 580:1085–1093. [DOI] [PubMed] [Google Scholar]
- Brambilla D, Zamboni S, Federici C, Lugini L, Lozupone F, De Milito A, Cecchetti S, Cianfriglia M, Fais S. (2012) P-glycoprotein binds to ezrin at amino acid residues 149-242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int J Cancer 130:2824–2834. [DOI] [PubMed] [Google Scholar]
- Bunting KD. (2002) ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20:11–20. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. (2002) The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology 123:1649–1658. [DOI] [PubMed] [Google Scholar]
- Caetano-Pinto P, Jamalpoor A, Ham J, Goumenou A, Mommersteeg M, Pijnenburg D, Ruijtenbeek R, Sanchez-Romero N, van Zelst B, Heil SG, et al. (2017) Cetuximab prevents methotrexate-induced cytotoxicity in vitro through epidermal growth factor dependent regulation of renal drug transporters. Mol Pharm 14:2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. (2012) Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 109:15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TC, McAvoy EM, Jacobs JW, Eilon G. (1990) Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem 265:7679–7686. [PubMed] [Google Scholar]
- Chambers TC, Pohl J, Glass DB, Kuo JF. (1994) Phosphorylation by protein kinase C and cyclic AMP-dependent protein kinase of synthetic peptides derived from the linker region of human P-glycoprotein. Biochem J 299:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TC, Pohl J, Raynor RL, Kuo JF. (1993) Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. J Biol Chem 268:4592–4595. [PubMed] [Google Scholar]
- Chambers TC, Zheng B, Kuo JF. (1992) Regulation by phorbol ester and protein kinase C inhibitors, and by a protein phosphatase inhibitor (okadaic acid), of P-glycoprotein phosphorylation and relationship to drug accumulation in multidrug-resistant human KB cells. Mol Pharmacol 41:1008–1015. [PubMed] [Google Scholar]
- Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. (2006) EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope 116:401–406. [DOI] [PubMed] [Google Scholar]
- Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P. (2002) Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci USA 99:2175–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Kruh GD. (2001) Transport of cyclic nucleotides and estradiol 17-β-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem 276:33747–33754. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. (2003) Transport of methotrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 63:4048–4054. [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. (1995) Identification of a sister gene to P-glycoprotein. Cancer Res 55:2029–2034. [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, et al. (2006) Characterization of mice lacking the multidrug resistance protein Mrp2 (Abcc2). J Pharmacol Exp Ther 317:579–589. [DOI] [PubMed] [Google Scholar]
- Ci L, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. (2007) Involvement of MRP4 (ABCC4) in the luminal efflux of ceftizoxime and cefazolin in the kidney. Mol Pharmacol 71:1591–1597. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Mottino AD, Cao J, Veggi LM, Pozzi EJ, Vore M, Coleman R, Roma MG. (2003) Estradiol-17β-D-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol 285:G449–G459. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Sánchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, Vore M. (2008) Ca2+-dependent protein kinase C isoforms are critical to estradiol 17β-D-glucuronide-induced cholestasis in the rat. Hepatology 48:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RA, Unsworth A, Knapp S, Kerr ID, Callaghan R. (2015) Overcoming ABCG2-mediated drug resistance with imidazo-[1,2-b]-pyridazine-based Pim1 kinase inhibitors. Cancer Chemother Pharmacol 76:853–864. [DOI] [PubMed] [Google Scholar]
- De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. (2006) Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol 26:99–105. [DOI] [PubMed] [Google Scholar]
- Dezi M, Fribourg PF, Di Cicco A, Arnaud O, Marco S, Falson P, Di Pietro A, Lévy D. (2010) The multidrug resistance half-transporter ABCG2 is purified as a tetramer upon selective extraction from membranes. Biochim Biophys Acta 1798:2094–2101. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95:15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin IN, Johnson FB. (1954) Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore) 33:155–197. [DOI] [PubMed] [Google Scholar]
- Emi Y, Nomura S, Yokota H, Sakaguchi M. (2011) ATP-binding cassette transporter isoform C2 localizes to the apical plasma membrane via interactions with scaffolding protein. J Biochem 149:177–189. [DOI] [PubMed] [Google Scholar]
- Evers R, de Haas M, Sparidans R, Beijnen J, Wielinga PR, Lankelma J, Borst P. (2000) Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br J Cancer 83:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Zhang M. (2009) Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci 10:87–99. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Wang Y, Lian S, Lynch J, Nagai S, Fanshawe B, Kandilci A, Janke LJ, Neale G, Fan Y, et al. (2017) Upregulated heme biosynthesis, an exploitable vulnerability in MYCN-driven leukemogenesis. JCI Insight 2:e92409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazova M, Aho TL, Palmetshofer A, Murashov A, Scheinin M, Koskinen PJ. (2005) Pim-1 kinase enhances NFATc activity and neuroendocrine functions in PC12 cells. Brain Res Mol Brain Res 138:116–123. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Ling V. (2006) The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 580:998–1009. [DOI] [PubMed] [Google Scholar]
- Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. (2006) Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 24:506–513. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 66:387–394. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. (2006) Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-α and lipopolysaccharide. Mol Pharmacol 69:462–470. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. (2007) Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol 18:37–45. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. (2010) Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci 30:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Naoi S, Nakagawa T, Nishikawa T, Fukuda H, Imajoh-Ohmi S, Kondo A, Kubo K, Yabuki T, Hattori A, et al. (2012) Sorting nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J Biol Chem 287:15054–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedüs T, Sessler T, Scott R, Thelin W, Bakos E, Váradi A, Szabó K, Homolya L, Milgram SL, Sarkadi B. (2003) C-terminal phosphorylation of MRP2 modulates its interaction with PDZ proteins. Biochem Biophys Res Commun 302:454–461. [DOI] [PubMed] [Google Scholar]
- Hermida MA, Dinesh Kumar J, Leslie NR. (2017) GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul 65:5–15. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. (2005) Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther 314:876–882. [DOI] [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 101:14228–14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MM, Ng AV, Lam S, Hung JY. (2007) Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 67:4827–4833. [DOI] [PubMed] [Google Scholar]
- Hoque MT, Cole SP. (2008) Down-regulation of Na+/H+ exchanger regulatory factor 1 increases expression and function of multidrug resistance protein 4. Cancer Res 68:4802–4809. [DOI] [PubMed] [Google Scholar]
- Hoque MT, Conseil G, Cole SP. (2009) Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem Biophys Res Commun 379:60–64. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Uchida Y, Tachikawa M, Ohtsuki S, Terasaki T. (2017) Actin filament-associated protein 1 (AFAP-1) is a key mediator in inflammatory signaling-induced rapid attenuation of intrinsic P-gp function in human brain capillary endothelial cells. J Neurochem 141:247–262. [DOI] [PubMed] [Google Scholar]
- Idriss H, Urquidi V, Basavappa S. (2000) Selective modulation of P-glycoprotein’s ATPase and anion efflux regulation activities with PKC alpha and PKC epsilon in Sf9 cells. Cancer Chemother Pharmacol 46:287–292. [DOI] [PubMed] [Google Scholar]
- Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. (2007) Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol 71:619–627. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. (1997) Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am J Physiol 272:G16–G22. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. (1996) Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res 56:988–994. [PubMed] [Google Scholar]
- Juliano RL, Ling V. (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162. [DOI] [PubMed] [Google Scholar]
- Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. (2002) Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer 97:626–630. [DOI] [PubMed] [Google Scholar]
- Kajihara S, Hisatomi A, Mizuta T, Hara T, Ozaki I, Wada I, Yamamoto K. (1998) A splice mutation in the human canalicular multispecific organic anion transporter gene causes Dubin-Johnson syndrome. Biochem Biophys Res Commun 253:454–457. [DOI] [PubMed] [Google Scholar]
- Kano T, Wada S, Morimoto K, Kato Y, Ogihara T. (2011) Effect of knockdown of ezrin, radixin, and moesin on P-glycoprotein function in HepG2 cells. J Pharm Sci 100:5308–5314. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J, Leuschner U, Mayer R, Keppler D. (1996) Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology 23:1061–1066. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S, Leung T, Birrane G, Webster G, Ladias JA. (2001) Crystal structure of the PDZ1 domain of human Na+/H+ exchanger regulatory factor provides insights into the mechanism of carboxyl-terminal leucine recognition by class I PDZ domains. J Mol Biol 308:963–973. [DOI] [PubMed] [Google Scholar]
- Karvar S, Suda J, Zhu L, Rockey DC. (2014) Distribution dynamics and functional importance of NHERF1 in regulation of Mrp-2 trafficking in hepatocytes. Am J Physiol Cell Physiol 307:C727–C737. [DOI] [PubMed] [Google Scholar]
- Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. (2007) Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther 6:2092–2102. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S, et al. (2002) Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 31:320–325. [DOI] [PubMed] [Google Scholar]
- Kim HR, Lee KY, Ahn SG, Lee BH, Jung KT, Yoon JH, Yoon HE, Oh SH. (2015) Transcriptional regulation, stabilization, and subcellular redistribution of multidrug resistance-associated protein 1 (MRP1) by glycogen synthase kinase 3αβ: novel insights on modes of cadmium-induced cell death stimulated by MRP1 [published correction appears in Arch Toxicol (2015) 89:1285]. Arch Toxicol 89:1271–1284. [DOI] [PubMed] [Google Scholar]
- Kim HR, Oh BC, Choi JK, Bae SC. (2008) Pim-1 kinase phosphorylates and stabilizes RUNX3 and alters its subcellular localization. J Cell Biochem 105:1048–1058. [DOI] [PubMed] [Google Scholar]
- Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. (2002) The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 8:22–28. [PubMed] [Google Scholar]
- Kipp H, Pichetshote N, Arias IM. (2001) Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem 276:7218–7224. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Alroy J, Gatmaitan Z, Inoue M, Mikami T, Jansen P, Arias IM. (1992) Defective biliary excretion of epinephrine metabolites in mutant (TR−) rats: relation to the pathogenesis of black liver in the Dubin-Johnson syndrome and Corriedale sheep with an analogous excretory defect. Hepatology 15:1154–1159. [DOI] [PubMed] [Google Scholar]
- Kocher O, Comella N, Gilchrist A, Pal R, Tognazzi K, Brown LF, Knoll JH. (1999) PDZK1, a novel PDZ domain-containing protein up-regulated in carcinomas and mapped to chromosome 1q21, interacts with cMOAT (MRP2), the multidrug resistance-associated protein. Lab Invest 79:1161–1170. [PubMed] [Google Scholar]
- Kocher O, Pal R, Roberts M, Cirovic C, Gilchrist A. (2003) Targeted disruption of the PDZK1 gene by homologous recombination. Mol Cell Biol 23:1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. (2004) The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 279:24218–24225. [DOI] [PubMed] [Google Scholar]
- Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, Schmitz G. (2003) Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem 49:230–238. [DOI] [PubMed] [Google Scholar]
- Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. (2000) Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol 57:24–35. [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, et al. (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24:7612–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Center M, Cole SP, Deeley RG, Keppler D. (1996) ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J 314:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. (1994) The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem 269:27807–27810. [PubMed] [Google Scholar]
- Lentze N, Auerbach D. (2008) Membrane-based yeast two-hybrid system to detect protein interactions, in Current Protocols in Protein Science (Coligan JE, Dunn BM, Speicher DW, and Wingfield PT eds) pp 19.17.1–19.17.28, John Wiley and Sons Inc., Hoboken. [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, et al. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131:940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, Boyer JL. (2010) NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem 285:19299–19307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, Abati A, Hansen PR, Horn T, Skovsgaard T, et al. (2002) Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta 1565:6–16. [DOI] [PubMed] [Google Scholar]
- Loe DW, Almquist KC, Deeley RG, Cole SP. (1996) Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem 271:9675–9682. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61:3458–3464. [PubMed] [Google Scholar]
- Masanek U, Stammler G, Volm M. (2002) Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol 2:37–41. [DOI] [PubMed] [Google Scholar]
- Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. (2008) Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol 73:1474–1483. [DOI] [PubMed] [Google Scholar]
- McClatchey AI. (2014) ERM proteins at a glance. J Cell Sci 127:3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. (2006) Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 14:1623–1632. [DOI] [PubMed] [Google Scholar]
- Meyers M, Slikker W, Pascoe G, Vore M. (1980) Characterization of cholestasis induced by estradiol-17 beta-D-glucuronide in the rat. J Pharmacol Exp Ther 214:87–93. [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Völker U, Kroemer HK. (2006) Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos 34:524–533. [DOI] [PubMed] [Google Scholar]
- Miller DS, Sussman CR, Renfro JL. (1998) Protein kinase C regulation of p-glycoprotein-mediated xenobiotic secretion in renal proximal tubule. Am J Physiol 275:F785–F795. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. (1999) Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem 274:18659–18666. [DOI] [PubMed] [Google Scholar]
- Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, Summer R, Fine A, Quesenberry PJ, Walsh K. (2003) Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem 278:39068–39075. [DOI] [PubMed] [Google Scholar]
- Mohan A, Kandalam M, Ramkumar HL, Gopal L, Krishnakumar S. (2006) Stem cell markers: ABCG2 and MCM2 expression in retinoblastoma. Br J Ophthalmol 90:889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor-Cohen R, Zivelin A, Rosenberg N, Shani M, Muallem S, Seligsohn U. (2001) Identification and functional analysis of two novel mutations in the multidrug resistance protein 2 gene in Israeli patients with Dubin-Johnson syndrome. J Biol Chem 276:36923–36930. [DOI] [PubMed] [Google Scholar]
- Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. (2008) Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res 68:5076–5085. [DOI] [PubMed] [Google Scholar]
- Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. (2002) Altered localization and activity of canalicular Mrp2 in estradiol-17β-D-glucuronide-induced cholestasis. Hepatology 35:1409–1419. [DOI] [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Jennes L, Cao J, Vore M. (2001) Expression of multidrug resistance-associated protein 2 in small intestine from pregnant and postpartum rats. Am J Physiol Gastrointest Liver Physiol 280:G1261–G1273. [DOI] [PubMed] [Google Scholar]
- Müller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL. (1994) Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci USA 91:13033–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Bhullar J, Shukla S, Burcu M, Chen ZS, Ambudkar SV, Baer MR. (2013) The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms. Biochem Pharmacol 85:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Mark ME, Cai X, Mao Q. (2010) Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int J Biochem Mol Biol 1:1–11. [PMC free article] [PubMed] [Google Scholar]
- Nies AT, Keppler D. (2007) The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch 453:643–659. [DOI] [PubMed] [Google Scholar]
- Nishida T, Gatmaitan Z, Che M, Arias IM. (1991) Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci USA 88:6590–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. (2007) Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int 6:92–97. [PubMed] [Google Scholar]
- Orr GA, Han EK, Browne PC, Nieves E, O’Connor BM, Yang CP, Horwitz SB. (1993) Identification of the major phosphorylation domain of murine mdr1b P-glycoprotein. Analysis of the protein kinase A and protein kinase C phosphorylation sites. J Biol Chem 268:25054–25062. [PubMed] [Google Scholar]
- Pacifico L, Carducci C, Poggiogalle E, Caravona F, Antonozzi I, Chiesa C, Maggiore G. (2010) Mutational analysis of ABCC2 gene in two siblings with neonatal-onset Dubin Johnson syndrome. Clin Genet 78:598–600. [DOI] [PubMed] [Google Scholar]
- Park J, Kwak JO, Riederer B, Seidler U, Cole SP, Lee HJ, Lee MG. (2014) Na+/H+ exchanger regulatory factor 3 is critical for multidrug resistance protein 4-mediated drug efflux in the kidney. J Am Soc Nephrol 25:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. (1997) A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology 25:1539–1542. [DOI] [PubMed] [Google Scholar]
- Paumi CM, Chuk M, Chevelev I, Stagljar I, Michaelis S. (2008) Negative regulation of the yeast ABC transporter Ycf1p by phosphorylation within its N-terminal extension. J Biol Chem 283:27079–27088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola KJ, Paukku K, Aho TL, Ruuska M, Silvennoinen O, Koskinen PJ. (2004) Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood 103:3744–3750. [DOI] [PubMed] [Google Scholar]
- Pitre A, Ge Y, Lin W, Wang Y, Fukuda Y, Temirov J, Phillips AH, Peters JL, Fan Y, Ma J, et al. (2017) An unexpected protein interaction promotes drug resistance in leukemia. Nat Commun 8:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Phillips C, Davies KE, Blake DJ. (1997) PDZ domains: targeting signalling molecules to sub-membranous sites. BioEssays 19:469–479. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheper RJ, Scheffer GL, de Witte TJ, Raymakers RA. (2005) Breast cancer resistance protein in drug resistance of primitive CD34+38− cells in acute myeloid leukemia. Clin Cancer Res 11:2436–2444. [DOI] [PubMed] [Google Scholar]
- Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. (2006) Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50:3297–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. (2003) The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA 100:9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS. (2010) Activation of PKC isoform βI at the blood–brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab 30:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs CW, Ballas LM, Mascarella SW, Safa AR, Lewin AH, Loomis C, Carroll FI, Bell RM, Fine RL. (1996) Effects of sphingosine stereoisomers on P-glycoprotein phosphorylation and vinblastine accumulation in multidrug-resistant MCF-7 cells. Biochem Pharmacol 52:603–612. [DOI] [PubMed] [Google Scholar]
- Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. (2009) Lipopolysaccharide impairs blood–brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J Neuroimmune Pharmacol 4:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S, Dickstein B, Regis J, Szallasi Z, Blumberg PM, Bates SE. (1995) Bryostatin 1 affects P-glycoprotein phosphorylation but not function in multidrug-resistant human breast cancer cells. Clin Cancer Res 1:1581–1587. [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, Torok-Storb B. (2002) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99:507–512. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, König J, Vogel O, Witzgall R, Kriz W, Keppler D. (1997) Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol 8:1213–1221. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. (1996) P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. (1995) Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Beck WT, Schuetz JD. (1996a) Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol 49:311–318. [PubMed] [Google Scholar]
- Schuetz EG, Schinkel AH, Relling MV, Schuetz JD. (1996b) P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc Natl Acad Sci USA 93:4001–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. (1999) MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med 5:1048–1051. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. (2005) Cancer stem cell characteristics in retinoblastoma. Mol Vis 11:729–737. [PubMed] [Google Scholar]
- Shukla S, Chen ZS, Ambudkar SV. (2012) Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat 15:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J, Hanif A, Lee ME, Jin K, Yu AR, Graham C, Chuk M, Damjanovic D, Wierzbicka M, Tang P, et al. (2013) Mapping the functional yeast ABC transporter interactome. Nat Chem Biol 9:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarczyk EI, Reiling CJ, Paumi CM. (2011) Regulation of ABC transporter function via phosphorylation by protein kinases. Curr Pharm Biotechnol 12:621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarczyk EI, Reiling CJ, Pickin KA, Coppage R, Knecht MR, Paumi CM. (2012) Casein kinase 2α regulates multidrug resistance-associated protein 1 function via phosphorylation of Thr249. Mol Pharmacol 82:488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó K, Bakos E, Welker E, Müller M, Goodfellow HR, Higgins CF, Váradi A, Sarkadi B. (1997) Phosphorylation site mutations in the human multidrug transporter modulate its drug-stimulated ATPase activity. J Biol Chem 272:23165–23171. [DOI] [PubMed] [Google Scholar]
- Takada T, Suzuki H, Gotoh Y, Sugiyama Y. (2005) Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos 33:905–909. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A 84:7735–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Tomlinson B. (2013) Targeting the ABCG2-overexpressing multidrug resistant (MDR) cancer cells by PPARγ agonists. Br J Pharmacol 170:1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, Adachi Y, Sakisaka S, Kuwano M. (1999) Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Am J Hum Genet 64:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxl A, Komposch K, Glitzner E, Wanek T, Mairinger S, Langer O, Sibilia M. (2017) Hepatocyte-specific deletion of EGFR in mice reduces hepatic Abcg2 transport activity measured by [11C]erlotinib and positron emission tomography. Drug Metab Dispos 45:1093–1100. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. (2010) Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28:1248–1250. [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. (2005) Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol 288:F327–F333. [DOI] [PubMed] [Google Scholar]
- Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, Yoshida I, Kimura A, Sakisaka S, Adachi Y, et al. (1998) Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet 7:203–207. [DOI] [PubMed] [Google Scholar]
- Walsh DR, Nolin TD, Friedman PA. (2015) Drug transporters and Na+/H+ exchange regulatory factor PSD-95/drosophila discs large/ZO-1 proteins. Pharmacol Rev 67:656–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. (2007) Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res 67:3716–3724. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Reid G, Challa EE, van der Heijden I, van Deemter L, de Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, et al. (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol 62:1321–1331. [DOI] [PubMed] [Google Scholar]
- Wijaya J, Fukuda Y, Schuetz JD. (2017) Obstacles to brain tumor therapy: key ABC transporters. Int J Mol Sci 18:2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. (1997) Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med 3:1275–1279. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Gamble JR, Vadas MA. (1999) Activation of sphingosine kinase by tumor necrosis factor-α inhibits apoptosis in human endothelial cells. J Biol Chem 274:34499–34505. [DOI] [PubMed] [Google Scholar]
- Xie Y, Burcu M, Linn DE, Qiu Y, Baer MR. (2010) Pim-1 kinase protects P-glycoprotein from degradation and enables its glycosylation and cell surface expression. Mol Pharmacol 78:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, Nakanishi T, Ross DD, Chen H, Fazli L, et al. (2008) The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem 283:3349–3356. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. (2004) Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem 279:19781–19789. [DOI] [PubMed] [Google Scholar]
- Yano K, Tomono T, Sakai R, Kano T, Morimoto K, Kato Y, Ogihara T. (2013) Contribution of radixin to P-glycoprotein expression and transport activity in mouse small intestine in vivo. J Pharm Sci 102:2875–2881. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhang H, Wang Z, Yu M, Tian R, Ji W, Yang Y, Niu R. (2014) P-glycoprotein associates with Anxa2 and promotes invasion in multidrug resistant breast cancer cells. Biochem Pharmacol 87:292–302. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li F, Patterson AD, Wang Y, Krausz KW, Neale G, Thomas S, Nachagari D, Vogel P, Vore M, et al. (2012) Abcb11 deficiency induces cholestasis coupled to impaired β-fatty acid oxidation in mice. J Biol Chem 287:24784–24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li F, Wang Y, Pitre A, Fang ZZ, Frank MW, Calabrese C, Krausz KW, Neale G, Frase S, et al. (2015) Maternal bile acid transporter deficiency promotes neonatal demise. Nat Commun 6:8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Li X, Magnuson NS. (2008) Pim kinase-dependent inhibition of c-Myc degradation. Oncogene 27:4809–4819. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Magnuson NS. (2007) Pim-1 kinase-dependent phosphorylation of p21Cip1/WAF1 regulates its stability and cellular localization in H1299 cells. Mol Cancer Res 5:909–922. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034. [DOI] [PubMed] [Google Scholar]