Abstract
Purpose: Drug delivery has a critical role in the treatment of cancer, in particular, carbon nanotubes for their potential use in various biomedical devices and therapies. From many other materials which could be more biocompatible and biodegradable and which could form single-walled nanotubes, silicon carbide was selected.
Methods: To compare two drug delivery systems based on single-walled nanotubes, molecular dynamic simulations were applied and encapsulation behavior of the drug carboplatin was investigated inside the silicon carbide nanotube and the carbon nanotube.
Results: Localization of the carboplatin inside the nanotubes indicated that the carboplatin moves throughout the tubes and possesses a greater probability of finding the drug molecule along the nanotubes in the first quarter of the tubes. The energy analysis exhibited the lowest free energy of binding belongs to the encapsulation of the drug carboplatin in the silicon carbide nanotube, about -145 Kcal/mol.
Conclusion: The results confirmed that the silicon carbide nanotube is a more suitable model than the carbon nanotube for drug delivery system based on nanotubes as a carrier of platinum-based anticancer drugs.
Keywords: Molecular Dynamic Simulation, Carbon nanotube, Silicon Carbide nanotube, Platinum-based anticancer drug
Introduction
Carbon nanotubes (CNTs), due to their unique atomic configuration, mechanical, optical and electronic properties, have been envisioned in designing biomedical devices and therapies on novel delivery platforms.1-5 The physicochemical versatility of carbon nanotubes is related to their high surface-area-to-volume ratios and facile functionalization along the nanotube axis and a great inner content that can be filled with the desired drug molecules.6-8 Additionally, the ability of single-walled carbon nanotubes (SWCNT) to incorporate inside cells has been proven, independent of cell type and functional groups linked to the nanotubes.9,10 In vivo assays have demonstrated that SWCNTs as carriers have no obvious toxicity,11 and in animal models have demonstrated the efficacy of drug-loaded CNTs through targeting tumors.6,12 Whereas water is the main component in biological systems and CNTs are hydrophobic, heterogeneous CNTs could be considered including silicon carbide nanotube (SiCNT) in aqueous media.13 In previous studies, several advantages have been shown for SiCNTs compared to CNTs. First, there is the relative stability increase from the CNTs to SiCNTs when the ratio of Si over C is 50:50, due to alternative sp2 and sp3 hybridization bond structures that are more stable than a smooth-walled tube.14 Additionally, the external surface of SiCNTs has higher reactivity than that of CNTs to facilitate aimed side wall functionalization.15,16 Furthermore, the experimental results prove the biocompatibility of silicon nanotubes and hence an alternative option for applications in nanomedicine.17,18 Platinum-based anticancer drugs are used to treat many types of solid tumors via binding to DNA and inducing cellular apoptosis, despite their adverse side effects,19,20 so that many of these side effects for healthy cells can be greatly reduced by nanoscale drug delivery. A fast and reliable tool to evaluate theoretically such systems is molecular modeling, which could interpret the details of the interaction between the drug and both the DNA and the nanostructures.21 On the other hand, our previous study allowed the assessment of a drug delivery system caused by another nanotube apart from CNTs, as carrier on theoretical level for the first time.22 Therefore, in the present study, molecular dynamics (MD) simulation was employed to investigate another so-named nanotube SiCNT as delivery system compared to CNT. Carboplatin (diammineplatinum(II) cyclobutane-1,1-dicarboxylate) was selected as a drug model because it encompasses fewer adverse effects and has greater water solubility than other platinum agents.23-25 Our simulations have been performed to assess the encapsulation behavior and localization of the anticancer drug carboplatin inside pristine CNT and SiCNT. For this purpose, the placement of the drug inside nanotubes and the free energy calculations were implemented to determine the preference between the two predicted drug delivery systems.
Materials and Methods
Systems preparation
In the present work, the zigzag open-ended single-walled nanotubes with 14 Å diameter and 40 Å length were considered, so (18, 0) carbon nanotube and (14, 0) silicon carbide nanotube were applied. Molecular dynamic simulations were applied for two systems; both of them containing a carboplatin molecule at a position along the CNT and SiCNT z-axis, i.e., drug_CNT and drug_SiCNT. Two systems were solvated in an aqueous solution. The parameters of CNT were modeled using the AMBER99SB force field.26,27 All the Lennard-Jones parameters and partial charge values for the carbon and silicon atoms for SiCNT were obtained from density functional theory calculations, summarized in Table 1.14,28 Other parameters were taken from the DREIDING force field and used in MD simulations. The structure of carboplatin was obtained from our previous works,22,29 and the parameters were identified from the literature and the GAFF;30,31 additionally, the carboplatins’ partial charges using the RESP module of AMBER 12 were calculated. The desired structures were immersed in a periodic water box of octagonal shape with a minimal distance of 12 Å from the system surface. Two systems were solvated with a TIP3P solvation model.32
Table 1. Lennard-Jones parameters and partial charges for SiCNT atoms .
| Atom | ε (kcal/mol) | σ (Å) | Atomic Charge (e) |
| C | 0.086 | 3.4 | 0.45 |
| Si | 0.469 | 3.7364 | -0.45 |
Molecular dynamics simulations
Molecular dynamics simulations were performed by employing the AMBER 12 simulation package.33 All simulations were conducted under the isothermal-isobaric ensemble at 1 atm and 300 K using the SANDER module in the AMBER 12 program. Constant temperature and pressure were maintained using Langevin dynamics.34 Bond lengths containing hydrogen atoms were constrained using the SHAKE algorithm.35 Periodic boundary conditions were applied and the long-range electrostatic interactions were handled with the particle mesh Ewald method; the nonbonded interactions were treated with cutoff at 12Å.36 Each simulation in energy minimization stage included 5000 steps for solvent and 5000 steps for solute relaxation. All systems were then heated from 0K to 300K for 120 ps and equilibrated at 300K for 200 ps. The production stages were run for 10 ns with a time step of 2 fs, and the trajectories for structural coordinates were saved every 1 ps for data analysis. Configuration analysis of the trajectories was performed with the ptraj module included in AmberTools14. Additionally, binding free energies were evaluated using the MM/PBSA (molecular mechanics/Poisson-Boltzmann surface area) method by previous experiment.29,37 The analysis of the interaction energies between the nanotubes and the drug was accomplished with AmberTools14 and implementation of MMPBSA.py script.
Results
The results from MD simulations of the two systems revealed that throughout the simulation time, both encapsulated carboplatin molecules resided inside the CNT and SiCNT cavity. Time-averaged radial distribution functions (RDF) were calculated to assess the localization of carboplatin inside the nanotubes (Figure 1), also RMSD plots were illustrated the drug movement inside the carbon and silicon carbide nanotubes (Figure 2). Moreover, MM/PBSA analysis of the trajectories was performed to estimate the binding free energy of the drug_CNT and the drug_SiCNT. Binding and absolute free energies of molecules are evaluated using this attractive method:
Figure 1.
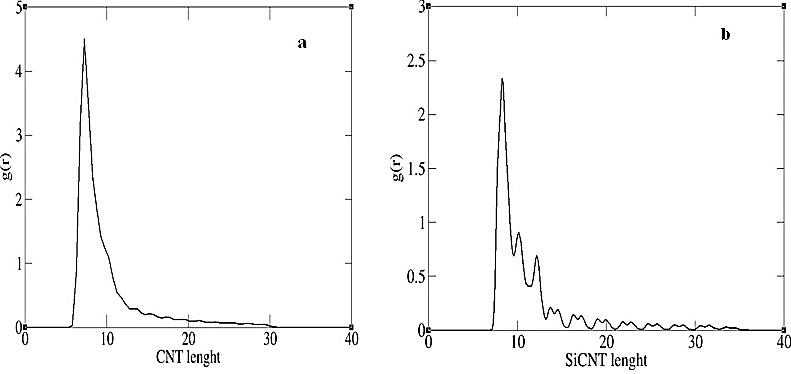
The RDF plot between the carboplatin center of mass and (a) the carbon atoms of the CNT, (b) the carbon and silicon atoms of the SiCNT
Figure 2.
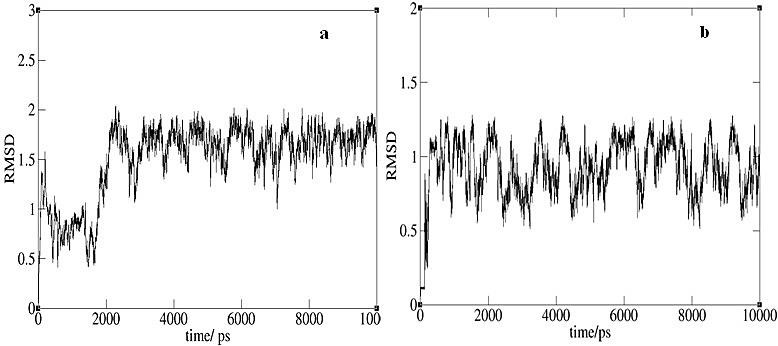
RMSD plots of encapsulation of the carboplatin inside the (a) CNT, (b) SiCNT.
Additionally, separation of the total free energy of binding into the van der Waals, electrostatic, and solute-solvent interactions, and discussion of each of the terms can be allowed and is calculated from:
where the molecular mechanics energy of the molecule (∆EMM) includes the van der Waals (EvdW), internal energy (bonds, angles and dihedrals) (Eint) and the electrostatic energy (Eele) terms. The solvation energy (Gsolv) containing the polar and nonpolar parts is obtained from an implicit solvent description. Table 2 summarizes all energy terms for the two simulated systems in order to evaluate binding free energy using the MM/PBSA method.
Table 2. Energy values for encapsulation of drug inside the nanotubes .
| Complex | drug_CNT | drug_SiCNT |
| EvdW | -50.7566 | -147.1443 |
| Eele | -0.9297 | -2.9051 |
| Gsolvele | 7.0830 | 6.0232 |
| Gsolvnonpolar | -1.7366 | -1.0369 |
| EMM | -51.6863 | -150.0495 |
| GsolvPBSA | 5.3464 | 4.9863 |
| ∆Gbinding | -46.3399 | -145.0631 |
All values in this table are in kcal/mol.
Discussion
Localization of carboplatin inside the CNT and SiCNT
To assess the localization of carboplatin inside the nanotubes, time-averaged radial distribution functions (RDF) were calculated. Figure 1a illustrates the RDF plot between the carboplatin center of mass and the carbon atoms of the CNT. In addition, Figure 1b illustrates the RDF plot between the carboplatin center of mass and the carbon and silicon atoms of the SiCNT. Both plots indicate that the drug moves throughout the tubes and there is a greater probability of finding the drug along the CNT and SiCNT in the first quarter of the tubes. Since drug exposure at the end of the nanotube is an important option for the drug molecules to release,38 the encapsulation and the preferential position of the carboplatin inside the tubes have a significant contribution in drug delivery.
Energy analysis
With regard to RMSD plots of drug movement inside the carbon and silicon carbide nanotubes (Figure 2), it is obvious that the two systems have stable equilibration, so it is possible to assess the thermodynamic properties of systems by sampling in simulation time and achieving the localization and the encapsulation behavior of the carboplatin inside the CNT and SiCNT. From the results of energy terms in Table 2, the total binding free energy in the drug_CNT system with an average of about −46 kcal mol−1 and the drug_SiCNT with −145 kcal mol−1 remains constant. It is derived that the dominant contribution of interactions is related to nonbonded van der Waals energy; evidently indicating that encapsulation of carboplatin inside the silicon carbide nanotube has stronger vdW interactions. The solvation free energies (GsolvPBSA) have a positive and small contribution of the binding free energy for both systems (Table 2), due to the hydrophobic structures of nanotubes; however, this is slightly reduced in the drug_SiCNT system. Whereas the partial charge on carbon atoms of CNT is zero, this reduction of solvation free energies can be related to partial charges on carbon and silicon atoms of the silicon carbide nanotube’s structure. Regard these results, the larger van der Waals value belongs to the drug_SiCNT, so the carboplatin drug has stronger interaction inside the SiCNT than it does inside the carbon nanotube. Comparison of these energy profiles reveals that the carboplatin molecule prefers to spend more time inside the SiCNT until it reaches the target cell. Consequently, using SiCNT nanotube as a platinum drug carrier can be the more suitable option for drug delivery with this type of nanostructure.
Conclusion
Molecular dynamic simulations to investigate the encapsulation behavior of the drug carboplatin inside the silicon carbide nanotube were applied as a comparison with the carbon nanotube as an anticancer drug delivery system based on single-walled nanotubes. The RDF plots show the localization of carboplatin inside the nanotubes, indicating that the drug moves throughout the tubes and has a greater probability of finding the carboplatin along the CNT and SiCNT in the first quarter of the tubes. Additionally, the binding free energy profiles in the encapsulation of drug inside both systems were investigated. The results confirmed that the appropriate drug delivery system for platinum drug is the use of SiCNTs to CNTs. Since the free energy of binding in the silicon carbide nanotube is about three times that of the carbon nanotube, the length of time remaining for the drug in this system will be greater, and the probability of releasing the drug will be less than with carbon nanotube, before reaching the target cells.
Acknowledgments
The authors give special thanks to S. Skies for editing this manuscript.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes--the route toward applications. Science. 2002;297(5582):787–92. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Jain N, Sareen R. Nanocarriers for diagnosis and targeting of breast cancer. BioMed Res Int. 2013;2013:960821. doi: 10.1155/2013/960821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CH, Vijayaraghavan V. Compressive characteristics of single walled carbon nanotube with water interactions investigated by using molecular dynamics simulation. Phys Lett A. 2014;378(5-6):570–6. doi: 10.1016/j.physleta.2013.12.026. [DOI] [Google Scholar]
- 4.Adeli M, Hakimpoor F, Ashiri M, Kabiri R, Bavadi M. Anticancer drug delivery systems based on noncovalent interactions between carbon nanotubes and linear-dendritic copolymers. Soft Matter. 2011;7(8):4062–70. doi: 10.1039/c0sm01550d. [DOI] [Google Scholar]
- 5. Madani SY, Mandel A, Seifalian AM. A concise review of carbon nanotube's toxicology. Nano Reviews 2013;4. [DOI] [PMC free article] [PubMed]
- 6.McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C. et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48(7):1180–9. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X. et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68(16):6652–60. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castle AB, Gracia-Espino E, Nieto-Delgado C, Terrones H, Terrones M, Hussain S. Hydroxyl-functionalized and N-doped multiwalled carbon nanotubes decorated with silver nanoparticles preserve cellular function. ACS Nano. 2011;5(4):2458–66. doi: 10.1021/nn200178c. [DOI] [PubMed] [Google Scholar]
- 9.Kang B, Chang S, Dai Y, Yu D, Chen D. Cell response to carbon nanotubes: Size-dependent intracellular uptake mechanism and subcellular fate. Small. 2010;6(21):2362–6. doi: 10.1002/smll.201001260. [DOI] [PubMed] [Google Scholar]
- 10.Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, Luangsivilay J. et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2(2):108–13. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 11.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z. et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3(4):216–21. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Drelich J, Yap Y. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir. 2009;25(9):4853–60. doi: 10.1021/la900511z. [DOI] [PubMed] [Google Scholar]
- 14.Mavrandonakis A, Froudakis GE, Schnell M, Mühlhäuser M. From pure carbon to silicon−carbon nanotubes: An Ab-initio study. Nano Lett. 2003;3(11):1481–4. doi: 10.1021/nl0343250. [DOI] [Google Scholar]
- 15.Wu IJ, Guo GY. Optical properties of SiC nanotubes: An ab initio study. Phys Rev B. 2007;76(3):035343. [Google Scholar]
- 16.Miyamoto Y, Yu BD. Computational designing of graphitic silicon carbide and its tubular forms. Appl Phys Lett. 2002;80(4):586–8. doi: 10.1063/1.1445474. [DOI] [Google Scholar]
- 17.Mu C, Zhao Q, Xu D, Zhuang Q, Shao Y. Silicon nanotube array/gold electrode for direct electrochemistry of cytochrome c. J Phys Chem B. 2007;111(6):1491–5. doi: 10.1021/jp0657944. [DOI] [PubMed] [Google Scholar]
- 18.Sahu T, Ghosh B, Pradhan SK, Ganguly T. Diverse role of silicon carbide in the domain of nanomaterials. Int J Electrochem. 2012;2012:271285. doi: 10.1155/2012/271285. [DOI] [Google Scholar]
- 19.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 20.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8(1):10–6. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargolzaei M, Nikoofard H, Afshar M. DNA binding mode and affinity of antitumor drugs of 2-aroylbenzofuran-3-ols: Molecular dynamics simulation study. Pharm Chem J. 2016;50(3):137–42. doi: 10.1007/s11094-016-1411-4. [DOI] [Google Scholar]
- 22.Khatti Z, Hashemianzadeh SM. Boron nitride nanotube as a delivery system for platinum drugs: Drug encapsulation and diffusion coefficient prediction. Eur J Pharm Sci. 2016;88:291–7. doi: 10.1016/j.ejps.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 24.Arlt M, Haase D, Hampel S, Oswald S, Bachmatiuk A, Klingeler R. et al. Delivery of carboplatin by carbon-based nanocontainers mediates increased cancer cell death. Nanotechnology. 2010;21(33):335101. doi: 10.1088/0957-4484/21/33/335101. [DOI] [PubMed] [Google Scholar]
- 25.Negureanu L, Salsbury FR Jr. Non-specificity and synergy at the binding site of the carboplatin-induced DNA adduct via molecular dynamics simulations of the mutsα-DNA recognition complex. J Biomol Struct Dyn. 2014;32(6):969–92. doi: 10.1080/07391102.2013.799437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM. et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117(19):5179–97. doi: 10.1021/ja00124a002. [DOI] [Google Scholar]
- 27.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414(6860):188–90. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 28.Taghavi F, Javadian S, Hashemianzadeh SM. Molecular dynamics simulation of single-walled silicon carbide nanotubes immersed in water. J Mol Graph Model. 2013;44:33–43. doi: 10.1016/j.jmgm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Khatti Z, Hashemianzadeh SM. Investigation of thermodynamic and structural properties of drug delivery system based on carbon nanotubes as a carboplatin drug carrier by molecular dynamics simulations. J Incl Phenom Macrocycl Chem. 2015;83(1-2):131–40. doi: 10.1007/s10847-015-0549-0. [DOI] [Google Scholar]
- 30.Yao S, Plastaras JP, Marzilli LG. A molecular mechanics amber-type force field for modeling platinum complexes of guanine derivatives. Inorg Chem. 1994;33(26):6061–77. doi: 10.1021/ic00104a015. [DOI] [Google Scholar]
- 31.Cundari TR, Fu W, Moody EW, Slavin LL, Snyder LA, Sommerer SO. et al. Molecular mechanics force field for platinum coordination complexes. J Phys Chem. 1996;100(46):18057–64. doi: 10.1021/jp961240x. [DOI] [Google Scholar]
- 32.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79(2):926–35. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 33.Case D, Darden T, Cheatham III T, Simmerling C, Wang J, Duke R, Amber 12. San Francisco: University of California; 2012. [Google Scholar]
- 34.Cerutti DS, Duke R, Freddolino PL, Fan H, Lybrand TP. Vulnerability in popular molecular dynamics packages concerning langevin and andersen dynamics. J Chem Theory Comput. 2008;4(10):1669–80. doi: 10.1021/ct8002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto S, Kollman PA. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem. 1992;13(8):952–62. doi: 10.1002/jcc.540130805. [DOI] [Google Scholar]
- 36.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A smooth particle mesh ewald method. J Chem Phys. 1995;103(19):8577–93. doi: 10.1063/1.470117. [DOI] [Google Scholar]
- 37.Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L. et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc Chem Res. 2000;33(12):889–97. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 38.Hampel S, Kunze D, Haase D, Kramer K, Rauschenbach M, Ritschel M. et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine (Lond) 2008;3(2):175–82. doi: 10.2217/17435889.3.2.175. [DOI] [PubMed] [Google Scholar]


