Abstract
Purpose: The worldwide prevalence of metabolic disorders such as diabetes is increasing rapidly. Currently, the complications of diabetes are the major health concern. The aim of this study was to investigate the effect of high performance (HP) inulin supplementation on glucose homeostasis via KLF5 mRNA expression in adults with type 2 diabetes.
Methods: In the present clinical trial conducted for a duration of 6 weeks, 46 volunteers diabetic patients referring to diabetes clinic in Tabriz, Iran, were randomly assigned into intervention (n= 23, consuming 10 gr/d HP inulin) and control groups (n= 23, consuming 10 gr/ d starch). We assessed glycemic and anthropometric indices, blood lipids and plasmatic level of miR-375 as well as KLF5 mRNA expression before and after the intervention.
Results: Findings indicated that inulin supplementation significantly decreased fasting plasma glucose (FPG) in comparison to the placebo group (P<0.001). Also Intra-group and between group results showed that inulin supplementation resulted in significant decrease in KLF5 mRNA expression in peripheral blood mononuclear cells (PBMCs) (Fold change: 0.61± 0.11; P-value= 0.001) and significant increase in plasmatic level of miR-375 (Fold change: 3.75± 0.70; P-value=0.004).
Conclusion: Considering the improvements of FPG level in diabetic patients, it seems that HP inulin supplementation may be beneficial in controlling diabetes via the expression of some genes. However, further studies are needed to achieve concise conclusions.
Keywords: Diabetes, Inulin, KLF5, miR-375, Fasting plasma glucose
Introduction
Diabetes mellitus (DM) as a complex metabolic disorder influenced by various environmental and genetic factors has become a common health problem in the entire world.1 According to recent reports it is estimated that by the year 2030, at least 366 million people will suffer from diabetes.2 Recent scientific advances point to manipulation of gut microbiota as contributing factors for preventing or controlling diabetes.2,3
The intestinal microbiota is a vital organ with trillions of commensally microorganisms which is involved in host metabolism. Nowadays, dietary components, particularly prebiotics are considered as functional foods that provide beneficial health effects on the intestinal tract. Prebiotics are defined as “ non-viable food components that confer health benefits on the host in association with modulation of the microbiota”.4
Inulin- type fructans are a kind of prebiotic fibers that have received much attention in the last decade. Inulin (a mixture of fructo oligo- and polysaccharides) is a very interesting functional ingredient, present as storage carbohydrate in more than 30,000 vegetables and fruits such as garlic, chicory root, wheat and banana.5,6 High performance (HP) inulin, the highly refined kind of inulin, is the average rate of polymerization which consists of 25 monosaccharide units. This form of inulin has many advantages with minimum gastrointestinal side effects such as abdominal tension.7,8 The beneficial health effects of inulin-type fructans have been previously studied. It has been showed that inulin may modulate glucose homeostasis by direct and indirect mechanisms. Alteration in gene expression and its effect on the gut microbiota at different taxonomic levels are few of the beneficial effects of inulin.9,10
Recent evidences showed an elaborate network of certain transcription factors coordinate with the expression of hundreds of genes which are responsible for the beneficial effects of inulin.11,12 The Kruppel Like Factor (KLF) is a kind of zinc finger transcriptional factor which encodes proteins that bind directly to a specific recognition motif in the promoters of target genes. KLF5, a member of the KLF family known as Gut-Enriched Kruppel-Like Factor (GKLF) has been characterized as a transcription factor which is expressed in high amounts in the cells of the intestinal epithelium.13 Previous studies reported an intense association between KLF5 overexpression and some metabolic disorders such as cardiovascular disease and diabetes.14 It has been proved that the translation of KLF5 is controlled by microRNAs (miRNAs).15
MiRNAs are small (~ 22 nucleotides), single strand, noncoding RNAs which are important regulators of gene expression via base pairing with 3´- untranslated regions of messenger RNA (mRNA).16 In fact, MiRNAs could possibly lead to degradation of mRNA or protein translation inhibition.17 Pioneering studies showed that the expression of miR-375 is able to prohibit the translation of KLF5.18 As the miRNA expression is very high in the human intestine,19 we supposed that mediation of the gut microbiota with inulin supplementation may promote glucose homeostasis via overexpression of miR-375 in adult diabetic patients. Thus, the objective of this study was to evaluate the effect of HP inulin supplementation on glucose homeostasis via KLF5 mRNA expression in type II diabetic patients in the form of a randomized, double-blind, placebo-controlled clinical trial.
Material and Methods
Participants
In the current randomized, double-blind, placebo-controlled trial, 46 volunteer diabetic adult patients referring to diabetes clinics in East Azerbaijan, Iran, during September 2016 and November 2016, were recruited. With Confidence Interval: 95% & Power: 90%, calculation of the sample size was accomplished based on the fasting insulin parameter.20 The formula: n = [(Z1 – α/2 + Z1 – β) 2 (SD12 +S D22)]/Δ2 was used to estimate the 23 samples allocated for each group while considering 6 patients for withdrawal. The inclusion criteria were having diabetes mellitus for more than 6 months; aged 30 to 50 years; body mass index) BMI) greater than 25 and less than 35 kg/m2. Exclusion criteria were having kidney disease; liver failure; heart failure; rheumatic diseases inflammatory diseases of the gastrointestinal tract; lactose intolerance; insulin injection and consuming drugs such as: estrogen, progesterone, corticosteroids; smoking; breast feeding and pregnancy; vitamin, mineral, omega-3 and antibiotic supplementation for three weeks before the beginning of the study. These subjects (46 patients) were randomly allocated using randomized block procedure, to one of the 2 treatment groups (A, or B) by computer-generated allocation schedule (Random Allocation Software) in which A was inulin group, B was placebo. Participants were also matched by type of consumed drugs (glucose lowering and anti- hyperlipidemia drugs) and disease duration in this trial.
Study design
After stratifying patients based on gender and age, subjects were randomly allocated into HP inulin (n=23) and placebo groups (n=23). The randomization process was not disclosed to the researchers and diabetic patients until the main analyses were completed. Also, during the study, none of the researchers and patients was aware of the drug randomization procedure. A study technician accomplished the randomization allocation procedure and allocated the participants into two groups. The HP inulin group received 10 g per day HP inulin powder (Sensus, Borchwef 3, 4704 RG Roosendaal the Netherlands) and the placebo group obtained 10g starch powder as placebo for 6 consecutive weeks. The components used for supplementation were sequenced into equal doses (5 grams) which were prescribed to consume before breakfast and dinner for 6 weeks. HP inulin powder and placebo (starch powder) were manufactured by Sensus Company, Netherlands. The HP inulin powder and placebo were in the same appearance such as colour, shape and packaging, which were coded by the producer to guarantee blinding. The participants were encouraged to avoid changing the dose and drugs consuming in order to prevent potential effects on the results of the study. Compliance to the HP inulin was assessed via asking participants to return the medication packages. Subjects were controlled weekly for possible side effects.
Physical activity and dietary intake assessments
For evaluating physical activity level before and after the intervention The International Physical Activity Questionnaire (IPAQ) was used for assessing physical activity level at baseline and end of intervention. According to the categorical scoring guidelines of the concluded form of IPAQ, participants were classified as highly, moderate and/or low physical activity level. we grouped our participants into high, moderate or low physical activity levels.21 Dietary intake was determined using a 24-hour food recall method for 2 average working days and 1 weekend day a week before and at the end of intervention. The dietary recalls were analyzed using the Nutritionist IV software (First Databank, San Bruno, CA, USA) adjusted for Iranian foods. Subjects were informed to continue their usual intake and physical activity until the end of the trial.
RNA extraction and quantitative real-time PCR for gene and miRNA
Peripheral blood mononuclear cells (PBMCs) separation was accomplished by Ficoll-Histopaque solution gradient (ficoll- paque, GmbH) centrifugation. Total RNA was extracted from PBMCs using ambion Trizol LS reagent. Thermo Fisher scientific revertaid first strand cDNA synthesis kit was used for the synthesis of cDNA. The level of KLF5 mRNA were evaluated by SYBR Green Master mix (Thermo Fisher Scientific, USA). The primer sequences were designed using PrimerBank. The amount of the mRNA normalized against the β-actin mRNA and -2ΔΔCT method22 was used for relative mRNA abundance. For miR-375 expression evaluation, complementary DNA was synthesized from isolated total RNA obtained from 200-µL plasma using the Universal cDNA synthesis kit (EXIQON, Denmark). Quantitative reverse transcription PCR (qRT-PCR ) was prepared using ExiLENT SYBER Green Master mix (Exiqon) against LNA based primer sets (Exiqon) and comparative -2ΔΔCT method was used to prove the relative quantitative level of miRNAs using Endogenous Control Primer miR-191 (Exiqon) for miRNA normalization.23,24 All samples were run in duplicate. Fold change of the parameters was computed as relative expression post intervention/control. The primers sequences of KLF5, β-actin, miR- 375 and miR-191 are illustrated as following: KLF5: Forward TCATCTTTCTGTCCCTACCC, Reverse TCCATTGCTGCTGTCTGA, Forward GGTGAAGGTGACAGCAGT, Reverse TGGGGTGGCTTTTAGGAT Hsa-miR-375 (5'-3') UUUGUUCGUUCGGCUCGCGUGA, Hsa-miR-191-5p (5'-3') CAACGGAAUCCCAAAAGCAGCUG.
Anthropometric measurements
At the onset and end of the trial, anthropometric indices such as body weight (BW), height, waist and hip circumferences (WC and HC respectively), waist to hip ratio (WHR) and body mass index (BMI) were recorded. Weight was measured via a calibrated scale (Itin Scale Co., Inc. Germany) with least clothing and 0.1 kg accuracy. Also, height was measured in a standing position next to ruler attached to the wall. For calculating BMI, the following equation was used: weight (kg) / height2 (m). Waist circumference (WC) was obtained by measuring the smallest area below the rib cage and above the umbilicus. Standing HC was measured at the inter trochantric level.25 Waist to hip ratio (WHR) was measured by dividing mean WC to mean HC.
Biochemical Assessment
For biochemical assessment, 7 ml blood sample was collected at baseline and at the end of the trial after 12h overnight fasting for measuring fasting plasma glucose (FPG), fasting insulin, glycated hemoglobin (HbA1c), total Cholestrol (TC), Triglycerides (TG), High density lipoprotein cholesterol (HDL-C) and Low density lipoprotein cholesterol (LDL-C). Auto-analyzer (Mindray Auto Hematology Analyzer, China) was used for biochemical analysis as well as platinum enzyme-linked immunosorbent assay (ELISA) kit (Monobind, Iran) for measuring fasting insulin. Also, NycoCard kit (NycoCard, Norway) was used for measuring HbA1c. Based on the Homeostatic model assessment of insulin resistance (HOMA-IR method, insulin resistance was measured: Fasting Glucose (mg/dL) × fasting insulin (mU/L)/450.
Statistical analysis
The Kolmogorov-Smirnov test was used for testing the normal distribution all of variables. Numerical variables were compared by Student t test and reported as mean ± standard deviation (SD). The Pearson chi-square test was used for comparing categorical variables and reported as number (%). Within group comparisons were determined by paired-sample t test. After adjusting for baseline values, Analysis of Covariance (ANCOVA) was applied to identify any differences between two groups at the end of the study. Statistically significant variables had p-value less than, 0.05 and were analyzed using the SPSS software version 23 (SPSS Inc., Chicago, Illinois, USA).
Results
The study flowchart is shown in Figure 1. A total of 46 diabetic patients completed the study and were interred in the final analyses. No adverse side-effects were reported by diabetic patients following the HP inulin or placebo supplementation. General characteristics (demographic variables and baseline values of anthropometric indices) of the participants are presented in Table 1. None of the demographic and anthropometric variables were different between the study groups, at baseline (P>0.05). As specified in Table 2, no differences were seen in the percent changes of few of the variables such as HbA1c, Weight, BMI, anthropometric indices (WC, HC and WHR) and blood lipids (TC, TG, HDL-C, LDL-C) after Inulin supplementation compared to the placebo group (P>0.05). However, Inulin supplementation significantly decreased FPG in comparison to the placebo group (P<0.001). Also, a statistically insignificant increase was observed in fasting insulin level after inulin supplementation. Intra-group statistical analysis indicated that Inulin supplementation significantly reduced FPG, WC and HC (P<0.05). Inulin supplementation significantly decreased WC from 97.47± 8.41 to 96.28±8.03 (p=0.009).
Figure 1.
Trial flow diagram
Table 1. Baseline characteristics of the study subjects .
| Variable |
HP inulin group
(n=23) |
Placebo group
(n=23) |
P-value b |
| Male/Female | 10/13 | 10/13 | 1.00 |
| Age(year) | 41.50±6.27 | 42.73±5.95 | 0.509 |
| Weight(kg) | 81.87± 11.46 | 79.91± 14.60 | 0.624 |
| Height(cm) | 168.68± 8.83 | 164.09± 10.10 | 0.116 |
| BMI(kg/m2) | 27.71± 4.60 | 28.79±4.77 | 0.444 |
| WC(cm) | 97.47± 8.41 | 93.84± 11.16 | 0.467 |
| HC(cm) | 108.04± 7.39 | 104.50± 10.70 | 0.455 |
| WHR | 0.90±0.08 | 0.88±0.07 | 0.455 |
| Diabetes duration(year) | 8.78± 4.67 | 9.86± 4.95 | 0.546 |
| Physical activity level n(%) | 0.459 | ||
| Low | 14(60.87) | 15(65.23) | |
| Moderate | 7(30.44) | 7(30.43) | |
| High | 2(8.69) | 1(4.34) |
Abbreviations: BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist To hip ratio. aVariables are expressed as mean ± SD. And number (percentage). bp-values resulted from independent t tests for quantitative and Chi-square for qualitative variables between the two groups.
Table 2. The effect of HP inulin supplementation on anthropometric indices and biochemical in patient with diabetes.
| Variable | HP inulin group(n=23) | placebo group(n=23) | P-value a | ||
| mean±sd | Change | mean±sd | Change | ||
| Weight (kg) | -0.61 | -0.47 | 0.317 | ||
| Baseline | 81.87± 11.46 | 79.91± 14.60 | |||
| End of trial | 81.46± 11.39 | 79.40± 13.91 | |||
| P-valueb | 0.362 | 0.259 | |||
| BMI(kg/m 2 ) | -0.710 | -0.62 | 0.792 | ||
| Baseline | 30.37±2.47 | 30.86±2.41 | |||
| End of trial | 30.15±2.73 | 30.64±2.24 | |||
| P-valueb | 0.104 | 0.084 | |||
| WC(cm) | -1.17 | -0.003 | 0.952 | ||
| Baseline | 97.47±8.41 | 93.84±11.16 | |||
| End of trial | 96.28±8.03 | 93.68±9.98 | |||
| P-valueb | 0.009 | 0.846 | |||
| HC(cm) | -1.93 | -1.17 | 0.687 | ||
| Baseline | 108.04±7.39 | 104.50±10.70 | |||
| End of trial | 106.01±8.23 | 103.22±10.47 | |||
| P-valueb | 0.004 | 0.061 | |||
| WHR(cm) | -0.21 | -1.28 | 0.192 | ||
| Baseline | 0.90±0.08 | 0.88±0.07 | |||
| End of trial | 0.90±0.08 | 0.87±0.05 | |||
| P-valueb | 0.744 | 0.135 | |||
| Insulin(µU/ml) | 7.54 | -0.57 | 0.817 | ||
| Baseline | 4.99±1.41 | 5.42±1.56 | |||
| End of trial | 5.20±1.75 | 5.38±1.56 | |||
| P-valueb | 0.546 | 0.598 | |||
| FPG(mg/dl) | -7.46 | 1.93 | <0.001 | ||
| Baseline | 130±36.25 | 127.64±24.19 | |||
| End of trial | 119.28±30.75 | 130.36±27.85 | |||
| P-valueb | 0.001 | 0.216 | |||
| HOMA-IR | 1.05 | 1.47 | 0.815 | ||
| Baseline | 1.53±0.53 | 1.71± 0.60 | |||
| End of trial | 1.55±0.69 | 1.72± 0.61 | |||
| P-valueb | 0.974 | 0.568 | |||
| HbA1c (%) | -0.69 | -2.52 | 0.128 | ||
| Baseline | 8.04±2.45 | 7.02±1.60 | |||
| End of trial | 7.62±1.85 | 7.79± 1.29 | |||
| P-valueb | 0.389 | 0.156 | |||
| TG(mg/dl) | -0.80 | -0.40 | 0.136 | ||
| Baseline | 177.16±72.02 | 186.73± 74.64 | |||
| End of trial | 168.58±53.50 | 185.45± 7.74 | |||
| P-valueb | 0.299 | 0.402 | |||
| TC(mg/dl) | -7.77 | 2.97 | 0.819 | ||
| Baseline | 201.38±73.43 | 174.27± 42.35 | |||
| End of trial | 178.24±55.01 | 176.18±31.02 | |||
| P-valueb | 0.122 | 0.742 | |||
| HDL-C(mg/dl) | 4.79 | 1.49 | 0.388 | ||
| Baseline | 41.18±9.23 | 41.64±8.75 | |||
| End of trial | 42.95±9.19 | 42.09± 9.42 | |||
| P-valueb | 0.091 | 0.681 | |||
| LDL-C(mg/dl) | -8.32 | 6.52 | 0.070 | ||
| Baseline | 111.81±48.54 | 107.20±30.96 | |||
| End of trial | 97.57±44.05 | 112.32±32.60 | |||
| P-valueb | 0.183 | 0.169 | |||
Abbreviations: ANCOVA, analysis of co-variance; BMI, body mass index; HDL-C, High density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance;a obtained from ANCOVA adjusted for baseline value. bobtained from paired T test.
Dietary intake of study participants is shown in Table 3 demonstrates subject’s dietary intake. Within group ad between group differences for dietary intake of energy and macro-nutrients such as carbohydrates, proteins, fats and dietary fiber was not statistically significant. There were no within-or between-group differences observed for dietary intake of total energy, carbohydrates, proteins, fats and dietary fiber.
Table 3. Dietary intake of study participants at Baseline and End of triala .
| Variable | HP inulin group (n=23) | Placebo group(n=23) | P-value b |
| Energy(Kcal/day) | |||
| Baseline | 1680.24±387.81 | 1739.57±421.05 | 0.603 |
| End of trial | 1786.84±495.04 | 1666.15±458.37 | 0.370 |
| P-valuec | 0.213 | 0.505 | |
| Carbohydrate(g/day) | |||
| Baseline | 242.92±62.84 | 255.25±77.19 | 0.536 |
| End of trial | 259.25±80.20 | 242.38±60.06 | 0.398 |
| P-valuec | 0.18 | 0.58 | |
| Protein(g/day) | |||
| Baseline | 66.95±19.01 | 70.73±19.83 | 0.491 |
| End of trial | 74.35±24.41 | 67.69±19.14 | 0.283 |
| P-valuec | 0.13 | 0.45 | |
| Fat(g/day) | |||
| Baseline | 50.73±15.90 | 51.05±16.50 | 0.944 |
| End of trial | 50.55±13.38 | 47.56± 18.79 | 0.518 |
| P-valuec | 0.96 | 0.37 | |
| Dietary fiber(g/day) | |||
| Baseline | 18.35± 6.62 | 17.60± 4.60 | 0.519 |
| End of trial | 17.92± 3.95 | 16.95±4.30 | 0.109 |
| P-valuec | 0.231 | 0.401 |
aVariables are expressed as mean ± SD,). bp-values resulted from independent T tests.cobtained from paired T test.
Intra-group and between-group statistical analysis revealed that Inulin supplementation resulted in significant decrease in KLF5 mRNA expression of KLF5 (Fold change: 0.61± 0.11; P-value= 0.001) (Figure 2). Additionally, Inulin supplementation significantly increased miR-375 level (Fold change: 3.75± 0.70; P-value=0.004) (Figure 3).
Figure 2.
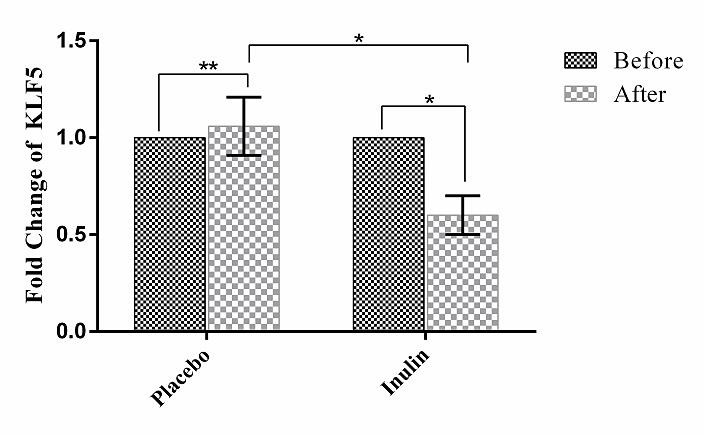
The PBMC levels of KLF5. X-axis represents the study groups and Y-axis shows fold changes of KLF5. Statistical analysis was done by One Sample T test and Independent Sample T test. Each point representsmean±SD. * P-value< 0.05; ** P-value> 0.05.
Figure 3.
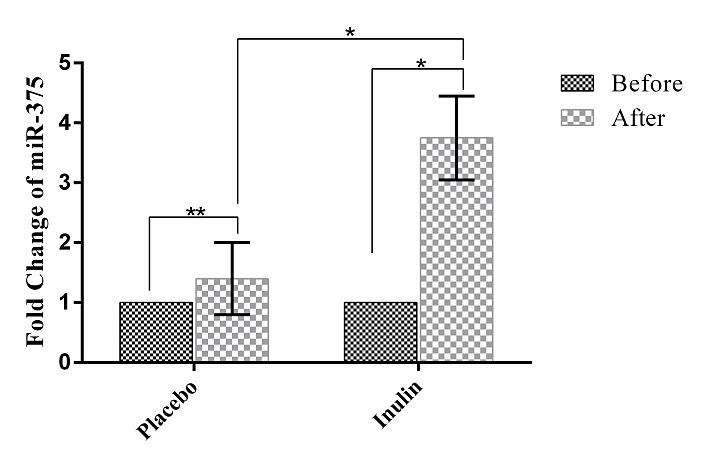
The plasma levels of miR-375. X-axis represents the study groups and Y-axis shows fold changes of miR-375. Statistical analysis was done by One Sample T test and Independent Sample T test. Each point represents mean±SD. * P-value< 0.05; ** P-value> 0.05.
Discussion
Our findings provide strong evidence for modulation of glycemic indices via miR-375 as an important regulator for KLF5 mRNA expression in type 2 diabetic patients after HP inulin supplementation. The findings of the present study indicated that HP inulin supplementation decreased FPG and KLF5 mRNA expression significantly. Interestingly, miR-375 expression increased remarkably in the intervention group compared with the placebo group. Additionally, our study revealed that the levels of FPG, WC and HC decreased in inulin group compared to the placebo one.
Based on our understanding, the present study is the first study that investigated the effects of HP inulin supplementation on KLF5 mRNA expression via miR-375 up-regulation. The decrease of FPG level in our study is in agreement with earlier results.26,27 In contrast, in some previous studies no beneficial effects were reported for FPG level after inulin- type fructans supplementation in diabetic patients.28,29 These conflicting effects could be due to different ethnic background, various doses and time intervals of inulin supplementation. Regarding the effect of inulin supplementation on fasting insulin, HbA1c and HOMA-IR indices we did not observe any significant changes in line with pioneering studies.30,31 According to a new systematic review and meta- analysis, there were no significant improvement in fasting insulin after inulin-type fructans’ supplementation.32
Similar to few previous studies8,33,34 we observed no statistical significant changes in lipid profile following HP inulin supplementation. Nevertheless, in few studies positive effects of prebiotics on lipid profile were reported.35,36 The contradictory results obtained from several studies may be due to the diversity in type and dose of supplements and differences in baseline values of lipid profile.
Other studies have shown that fructooligosaccharides’ consumption was associated with anthropometric indices improvement and the promotion of weight loss.20,37-40 However, conflicting results have also been reported.30 In this study inulin supplementation resulting in WC, HC decreases in intra-group analysis but in between-group assessments no significant differences were observed.
An interesting finding was the effect of inulin supplementation on KLF5 mRNA expression. It has been previously indicated that plasmatic level of miR-375 is considered as a biomarker for β-cell function. This miRNA is a key regulator for securing intestinal epithelium safety and multiple metabolic processes via controlling various gene expression.41 Indeed, KLF5 is a target gene for miR-375 and has been implicated in glucose homeostasis.42 In the present study, we focused on conserved intestinal evolutionary miRNA (miR-375) in order to evaluate glucose homeostasis. In fact, we found a relation between modulation of gut microbiota and overexpression of miR-375 following inulin consumption by suppressing the KLF5 expression in PBMCs. Interestingly, KLF5 is one of the essential transcriptional factors communicating with inflammatory conditions and may contribute to the improvement of glucose homeostasis and diminution of inflammatory cytokines like Tumor necrosis factor alpha (TNF-α) through chemoattractant protein-1 (MCP-1) and transcription factor Nuclear factor-kappa B (NF- κB) pathways.43
Although the exact mechanisms by which inulin- type fructans act on glucose metabolism remain unclear, however several proposed mechanisms have been reported, including:
Ι. Changes in gut microbiota composition particularly increased the number of bifidobacteria and butyrate- producing colon bacteria after feeding inulin.44
ΙΙ. Fermentation of inulin- type fructans in the large bowel into short chain fatty acids(SCFAs) such as acetate, butyrate and propionate modulate inflammation by preventing the production of TNF-α and NF- κB.45
ΙΙΙ. Butyrate and propionate induce intestinal gluconeogenesis which thereby improves glucose homeostasis.32
IV. Increased production of short chain fatty acids in colon may control the expression of some genes and miRNAs which are involved in glucose homeostasis.46
No study is without limitations and the limitations of this study include the small sample size and lack of measurement of some related factors such as inflammatory markers, serum levels of short-chain fatty acids and other genes expression related to glucose homeostasis. The strength of this study is that it was the first study investigating the beneficial effects of inulin supplementation on KLF5 and miR-375 expression in diabetic patients. In order to obtain further conclusive results and determine the exact related mechanisms, more studies with larger sample sizes are needed.
Conclusion
According to the effectiveness of inulin supplementation on glucose homeostasis through effective mechanisms like genes expression, this survey can be considered as a novel therapeutic approach in controlling diabetes. Our results revealed new insights into how KLF5 functions play a role in controlling diabetes. The newly identified KLF5- miR-375 pathway may contribute to diabetes progression. Inhibiting KLF5 may be a potential pharmacological intervention in controlling diabetes. With descriptions above, inulin supplementation maybe considered an adjunctive therapy for diabetic patients.
Acknowledgments
The authors would like to thank all the study participants, as well as nurses and doctors in the diabetes clinics in East Azerbaijan, Iran for their collaboration. The present study did not achieve any grants.
Ethical Issues
The Ethics committee of Tabriz University of Medical Science (Ethic code: IR.TBZMED.REC.1395.671) approved the research protocol of the study and a written informed consent document was obtained from all patients. The study was registered in the Iranian Registry of Clinical Trials website (IRCT ID: IRCT201610212017N31).
Conflict of Interest
The authors would like to gratitude the individuals who participated in this study.
Abbreviations
HP: high performance; KLF5: kruppel like factor 5; FPG: fasting plasma glucose; PBMCs: Peripheral blood mononuclear cells.
References
- 1.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 2.Pourghassem Gargari B, Dehghan P, Aliasgharzadeh A, Asghari Jafar-abadi M. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab J. 2013;37(2):140–8. doi: 10.4093/dmj.2013.37.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin HL, Zheng JJ, Tong DN, Chen WX, Fan XB, Hang XM. et al. Effect of lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62(7):923–30. doi: 10.1038/sj.ejcn.1602792. [DOI] [PubMed] [Google Scholar]
- 4. FAO Technical Meeting on Prebiotics. Food Quality and Standards Service (AGNS), Food and Agriculture Organization of the United Nations (FAO). Rome: FAO Technical Meeting Report; 2007.
- 5.Apolinario AC, de Lima Damasceno BP, de Macêdo Beltrão NE, Pessoa A, Converti A, da Silva JA. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym. 2014;101:368–78. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 6.Vogt L, Meyer D, Pullens G, Faas M, Smelt M, Venema K. et al. Immunological properties of inulin-type fructans. Crit Rev Food Sci Nutr. 2015;55(3):414–36. doi: 10.1080/10408398.2012.656772. [DOI] [PubMed] [Google Scholar]
- 7.Franck A. Technological functionality of inulin and oligofructose. Br J Nutr. 2002;87(S2):S287–91. doi: 10.1079/BJNBJN/2002550. [DOI] [PubMed] [Google Scholar]
- 8.Roshanravan N, Mahdavi R, Alizadeh E, Jafarabadi MA, Hedayati M, Ghavami A. et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: A randomized double-blind, placebo-controlled trial. Horm Metab Res. 2017;49(11):886–91. doi: 10.1055/s-0043-119089. [DOI] [PubMed] [Google Scholar]
- 9.Tsurumaki M, Kotake M, Iwasaki M, Saito M, Tanaka K, Aw W. et al. The application of omics technologies in the functional evaluation of inulin and inulin-containing prebiotics dietary supplementation. Nutr Diabetes. 2015;5:e185. doi: 10.1038/nutd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar M, Verma V, Nagpal R, Kumar A, Gautam SK, Behare PV. et al. Effect of probiotic fermented milk and chlorophyllin on gene expressions and genotoxicity during AFB(1)-induced hepatocellular carcinoma. Gene. 2011;490(1-2):54–9. doi: 10.1016/j.gene.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Wang S. Role of kruppel-like transcription factors in adipogenesis. Dev Biol. 2013;373(2):235–43. doi: 10.1016/j.ydbio.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93(S1):S41–8. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 13.McConnell BB, Kim SS, Yu K, Ghaleb AM, Takeda N, Manabe I. et al. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology. 2011;141(4):1302–13, 1313 e1. doi: 10.1053/j.gastro.2011.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S. et al. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8(8):856–63. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 15.Runtsch MC, Round JL, O’Connell RM. MicroRNAs and the regulation of intestinal homeostasis. Front Genet. 2014;5:347. doi: 10.3389/fgene.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventriglia G, Nigi L, Sebastiani G, Dotta F. MicroRNAs: Novel players in the dialogue between pancreatic islets and immune system in autoimmune diabetes. BioMed Res Int. 2015;2015:749734. doi: 10.1155/2015/749734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzen J, Kumarswamy R, Dangwal S, Thum T. MicroRNAs in diabetes and diabetes-associated complications. RNA Biol. 2012;9(6):820–7. doi: 10.4161/rna.20162. [DOI] [PubMed] [Google Scholar]
- 18.Masotti A. Interplays between gut microbiota and gene expression regulation by miRNAs. Front Cell Infect Microbiol. 2012;2:137. doi: 10.3389/fcimb.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ. et al. Identification of microRNAs associated with ileal and colonic crohn's disease. Inflamm Bowel Dis. 2010;16(10):1729–38. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guess ND, Dornhorst A, Oliver N, Bell JD, Thomas EL, Frost GS. A randomized controlled trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutr Metab (Lond) 2015;12:36. doi: 10.1186/s12986-015-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. IPAQ Group. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms. IPAQ Group, 2005.
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− δδCT method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14(5):844–52. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pescador N, Pérez-Barba M, Ibarra JM, Corbatón A, Martínez-Larrad MT, Serrano-Ríos M. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS One. 2013;8(10):e77251. doi: 10.1371/journal.pone.0077251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagila A, Bhatt M, Poudel B, Mahato P, Gurung D, Prajapati S. et al. Thyroid stimulating hormone and its correlation with lipid profile in the obese nepalese population. J Clin Diagn Res. 2008;2(4):932–7. [Google Scholar]
- 26.Yamashita K, Kawai K, Itakura M. Effects of fructo-oligosaccharides on blood glucose and serum lipids in diabetic subjects. Nut Res. 1984;4(6):961–6. doi: 10.1016/S0271-5317(84)80075-5. [DOI] [Google Scholar]
- 27.Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int J Food Sci Nutr. 2014;65(1):117–23. doi: 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- 28.Alles MS, de Roos NM, Bakx JC, van de Lisdonk E, Zock PL, Hautvast JG. Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am J Clin Nutr. 1999;69(1):64–9. doi: 10.1093/ajcn/69.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Luo J, Rizkalla SW, Alamowitch C, Boussairi A, Blayo A, Barry JL. et al. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose metabolism. Am J Clin Nutr. 1996;63(6):939–45. doi: 10.1093/ajcn/63.6.939. [DOI] [PubMed] [Google Scholar]
- 30.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM. et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62(8):1112–21. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Van Yperselle M, Rizkalla SW, Rossi F, Bornet FR, Slama G. Chronic consumption of short-chain fructooligosaccharides does not affect basal hepatic glucose production or insulin resistance in type 2 diabetics. J Nutr. 2000;130(6):1572–7. doi: 10.1093/jn/130.6.1572. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Prabhakar M, Ju J, Long H, Zhou HW. Effect of inulin-type fructans on blood lipid profile and glucose level: A systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2017;71(1):9–20. doi: 10.1038/ejcn.2016.156. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen A, Sandström B, Van Amelsvoort JM. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br J Nutr. 1997;78(2):215–22. doi: 10.1079/bjn19970141. [DOI] [PubMed] [Google Scholar]
- 34.Letexier D, Diraison F, Beylot M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr. 2003;77(3):559–64. doi: 10.1093/ajcn/77.3.559. [DOI] [PubMed] [Google Scholar]
- 35.Dehghan P, Pourghassem Gargari B, Asgharijafarabadi M. Effects of high performance inulin supplementation on glycemic status and lipid profile in women with type 2 diabetes: A randomized, placebo-controlled clinical trial. Health Promot Perspect. 2013;3(1):55–63. doi: 10.5681/hpp.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo F, Chimienti G, Riezzo G, Pepe G, Petrosillo G, Chiloiro M. et al. Inulin-enriched pasta affects lipid profile and Lp(a) concentrations in italian young healthy male volunteers. Eur J Nutr. 2008;47(8):453–9. doi: 10.1007/s00394-008-0748-1. [DOI] [PubMed] [Google Scholar]
- 37.Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A. et al. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin Nutr. 2009;28(2):182–7. doi: 10.1016/j.clnu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen C, Lefevre S, Peters V, Patterson M, Ghatei MA, Morgan LM. et al. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. Appetite. 2013;66:44–53. doi: 10.1016/j.appet.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Cicek B, Arslan P, Kelestimur F. The effects of oligofructose and polydextrose on metabolic control parameters in type-2 diabetes. Pak J Med Sci. 2009;25(4):573–8. [Google Scholar]
- 40.Kaminskas A, Abaravičius JA, Liutkevičius A, Jablonskienė V, Valiūnienė J, Bagdonaitė L. et al. Quality of yoghurt enriched by inulin and its influence on human metabolic syndrome. Vet Med Zoot. 2013;64(86):23–8. [Google Scholar]
- 41.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H. et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-t cell crosstalk. Nat Immunol. 2011;12(3):239–46. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 42.Gutiérrez-Aguilar R, Benmezroua Y, Vaillant E, Balkau B, Marre M, Charpentier G. et al. Analysis of klf transcription factor family gene variants in type 2 diabetes. BMC Med Genet. 2007;8:53. doi: 10.1186/1471-2350-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumekawa M, Fukuda G, Shimizu S, Konno K, Odawara M. Inhibition of monocyte chemoattractant protein-1 by kruppel-like factor 5 small interfering RNA in the tumor necrosis factor- α-activated human umbilical vein endothelial cells. Biol Pharm Bull. 2008;31(8):1609–13. doi: 10.1248/bpb.31.1609. [DOI] [PubMed] [Google Scholar]
- 44.Delzenne NM, Daubioul C, Neyrinck A, Lasa M, Taper HS. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br J Nutr. 2002;87(S2):S255–9. doi: 10.1079/BJNBJN/2002545. [DOI] [PubMed] [Google Scholar]
- 45.Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-κB activation by suppressing proteasome activity: Down-regulation of proteasome subunit expression stabilizes IκBα. Biochem Pharmacol. 2005;70(3):394–406. doi: 10.1016/j.bcp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Roshanravan N, Mahdavi R, Jafarabadi MA, Alizadeh E, Ghavami A, Saadat YR. et al. The effects of sodium butyrate and high-performance inulin supplementation on the promotion of gut bacterium Akkermansia muciniphila growth and alterations in miR-375 and KLF5 expression in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Eur J Integr Med. 2018;18:1–7. doi: 10.1016/j.eujim.2017.12.011. [DOI] [Google Scholar]