Abstract
Purpose: Onopordon acanthium L. is known for its medicinal properties. Our recent study showed that its seed extract is a novel natura angiotensin-converting-enzyme inhibitor (ACEI). This study was carried out to investigate its possible antihypertensive effects in patients receiving losartan.
Methods: This uncontrolled clinical trial was carried out among 20 patients (30-60y) with uncontrolled hypertension despite receiving 50 mg losartan (stage I & II) in two hospitals in Iran. After completing informed consent, patients were treated by 2 capsules [each 1g of Onopordon acanthium seed extract (OSE)] as add-on therapy, two times per day.
Results: 18 patients completed the study (50.94 ±8.37y). Mean systolic blood pressure (SBP) at the baseline was 151.9 ± 13.74mmHg and at the end of the study, it was 134.6 ± 18.25 mmHg and mean diastolic blood pressure (DBP) was 97.41 ± 10.36 at the baseline and was 85.71 ± 7.481 after 8 weeks. OSE significantly reduced SBP and DBP at the end of 8 weeks (P=0.003, 95% CI: -19.7, -15.1; P=0.0006, 95% CI: -10.23, -13.15; respectively). No evidence of hepatic or renal toxicity was detected.
Conclusion: Based on the results of this study OSE has antihypertensive property with no significant adverse effects. However, because of the low number of samples, this medication may be not safely administered. The results of this study could be the basis for further studies with larger sample size. IRCT registration number: IRCT2013020712391N.
Keywords: Onopordon acanthium L, Hypertension, Blood pressure, Angiotensin converting enzyme, Persian medicine
Introduction
Hypertension (HTN) is a global concern and affects approximately 75 million adults in the United States and if left untreated increases risk of stroke, myocardial infarction, vascular disease, and chronic kidney disease.1,2 Diagnosis and treatment of high blood pressure are essential to prevent mortality and morbidity.2,3 Hypertension may be treated by using routine drugs such as angiotensin-converting-enzyme (ACE) inhibitors, beta-blockers, diuretics, calcium channel blockers, alpha-blockers, and peripheral vasodilators,3,4 improving lifestyle factors including weight loss, quitting smoking, reducing sodium intake, regular exercise and limiting alcohol consumption.5 These recommendations may be used alone or in combination with others4. Losartan is an oral medication that belongs to a class of drugs called angiotensin receptor blocker (ARBs) which was approved by the U.S. Food and Drug Administration (FDA) in April 1995. Losartan blocks the angiotensin receptor, relaxes muscle cells and dilates blood vessels and reduces blood pressure.6,7
Our recent study showed that the OSE is a novel natural inhibitor agent and inhibit angiotensin-converting-enzyme by 80.2 ± 2 % at concentration of 330 μg/ml, and exerted antioxidant activity (IC50 value of 2.6 ± 0.04 mg/ml).8 This plant has been well known under the name "Khaje Bashi"9 and has long been used in folk medicine as a hypotensive, cardiotonic and diuretic agent.10 Also it has been noted in Persian Medicine (PM) literatures as diuretic, diaphoretic, antipyretic, analgesic. Cotton thistle or Scotch thistle are its common names. It is a flowering plant belonging to the Compositae (Asteraceae) family and is widely naturalized almost globally, particularly in Europe and Western Asia and is a vigorous biennial plant with coarse, spiny leaves and conspicuous spiny-winged stems.11 In modern medicine, O. acanthium has been reported to be a bactericide, cardiotonic, hypotensive and hemostatic agent and is used against hypotonicity.12-14 This species has several bioactive components among which sesquiterpene lactones have been found to have numerous biological properties including antibacterial, anti-inflammatory, anti-malarial, and hypotensive effects.15 Since the antihypertensive effect of O. acanthium seed extract has not been studied on patients in follow up of our previous in vitro study, we conducted this trial to investigate the possible antihypertensive effects of OSE on patients with stage I-II hypertension who were under treatment with losartan, a chemical ACE inhibitor, as add-on therapy.
Materials and Methods
Plant material
O. acanthium L. dried seeds were purchased from the local market in Tehran (Grand Bazaar), Iran. The seeds were identified by Prof. Gholamreza Amin and were kept under the voucher number PMP–714 at the herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences.
Preparation of the extract and drug formulation
The extract was prepared in the laboratory of traditional pharmacy at faculty of Traditional Medicine, Tehran university of medical sciences via maceration method using ethanol as the solvent (1:8) at three time points (24, 48 and 72 h). The three extracts were mixed, filtered and evaporated. The total evaporated extract was freeze-dried and grinderies to obtain a powder for preparing the capsule. Each capsule contained 1g of the freeze-dried powder equal to 11 gram of dried seeds, and all the physiochemical quality control (QC) tests were performed on the capsules.
Toxicology evaluation
To measure the toxic dose of total extract of OSE (Khaje Bashi plant), rats were used and kept at the Animal house of the Faculty of Pharmacy, Tehran University of Medical Sciences. Animals were kept in standard light (12 hours of light and 12 hours of darkness), room temperature (25-35 °C) with adequate water and food. 12 male albino NMRI mice were divided into three groups (4 animals per group). The whole extract of the plant was taken in dried form and stored in a dark bottle in the refrigerator until it was tested. Due to its low solubility, the extract was dissolved in dimethyl sulfoxide (DMSO) at a desired concentration and diluted with normal saline at a rate of 1:3 and made as a monotonous suspension.
The solution was injected intraperitoneally (i.p.). Animals received the same amount of DMSO in different dose groups. Animals’ condition (lethargy and movement) and animal death were investigated for 24-48 hours. In each group, mortality rate was reported and plotted against the dose used. The dose that causes 50 percent of deaths in animals or “Lethal Dose, 50%” (LD50) was calculated based on the curve.
Considering that no studies have been done on the toxicity of this plant, in the first step, 0.5, 1, 2, 3.5 and 5 g/kg of body weight were used to find the dose range, but no mortality or change was observed in animals. In the next step, doses of 6-20 g/kg were used, and in dose 20, all animals died. In the next stage, the range of 6-13.5 g/kg was tested in 6 groups of animals. The results were calculated as a percentage of death for each group. LD50 was calculated using non-linear sigmoid regression and Probit method. Meanwhile, the relationship between effective dose and effect was analyzed using Pearson's relationship and according to the results of these experiments, the lethal dose (LD50 value of 8.44 ± 0.04 g/kg) was determined. According to these results, the extract of this plant was classified in "practically non-poisonous ".
Clinical trial design
This open-labeled, non-randomized, uncontrolled clinical trial, a pilot study, was performed on 20 patients, who were under losartan treatment (50mg/d) for at least 6 weeks before starting the study and their blood pressure constantly remained higher than 140/90 (stage I&II) hypertension according to the seventh report of the joint national committee (JNC VII) report.16 Sample size was calculated as follow: based on predicted 14 mmHg reduction of systolic blood pressure (d=14) at the end point (after 8 weeks), standard deviation 20 (σ=20, power 80%, confidence interval (CI) of 95% (Z1-α/2=1.96) and taking into account a correlation of 0.5 between frequent measurements and considering 20 % loss to follow, 20 patients were enrolled to determine the effective dose of therapy. Each individual took 2 capsules, two times a day (2 g/BD) and blood pressure was checked every other week. If blood pressure has increased more than 15 mmHg, the patients would have dropped out the study.
The primary outcome measure was systolic and diastolic blood pressure that was measured using aneroid sphygmomanometer (F. Bosch, Model: 0123, Germany). Metabolic parameters (lipid profile, liver function tests, BUN, Cr, FBS) were measured two times during the study (at the beginning and at the end of study) at Noor laboratory to evaluate the general health status of the patients.
Participants
The patients (30-60 y) were selected from Imam Khomeini & Amir-alam hospitals of Tehran University of medical sciences. Patients with history of malignant hypertension, blood pressure higher than 200/140 mmHg and whom needed multi-drug treatment or emergency IV drug infusion, history of secondary hypertension, end organ damages (EOD), sudden increase of blood pressure to greater than 15 mmHg at any time during the study, cardiac arrhythmias, symptomatic valvular heart diseases (except mitral valve prolapse) were excluded from the study. Other exclusion criteria were: diabetes type 1& 2, liver disorders, pregnancy breast feeding, cured or uncured malignancies during the last 5 years, serum potassium >5.2 or <3.5 mEq/L in the first visit, drug or alcohol abuse. The procedure was explained to all patients and written informed consents were obtained. Patients were examined at the beginning of the study and then every two weeks, and their arterial blood pressure was measured. The researcher's contact number was also provided to patients in order to report any problems between every two visits. In addition, patients were asked at each session about possible complications such as coughing, severe headache, visual impairment, any types of arrhythmias, and orthostatic hypotension, and were recorded. Demographic and baseline data, medical history and any concomitant medications of enrolled patients were recorded. The study protocol was approved by the ethics committee of Research Institute for Islamic and Complementary Medicine of Iran University of Medical Sciences at 09/10/2012 with reference number 732/P26/M/T.
Statistical methods
The changes in blood pressure were analyzed using repeated measures ANOVA analysis. P value <0.05 was considered significant. The LD50 was calculated using nonlinear sigmoid regression and Probit technique. Moreover, the correlation between effect and administered dose was analyzed using Pearson correlation test.
Results and Discussion
Cytotoxicity of the extract was performed in mice by injecting different doses of OSE (0.5-13.5 g/kg) to measure the minimum toxic dose of OSE and LD50 was 8.44 ± 0.04 g/kg. Sampling began on May 21, 2013 and continued for five months, till October 23, 2013. 54 patients were assessed. Only 24 patients met the inclusion criteria whom four were not willing to participate in the study. At the end, 18 patients (of 20) completed the study and two patients were left the study due to increased blood pressure more than 160/100 mmHg in one patient after 2 days and another, after 3 days from starting point. Based on the ethical issues and respect to the patients’ right, they were excluded and researcher referred both of them to the cardiologist to be treated with two or more drugs (Figure 1).
Figure 1.
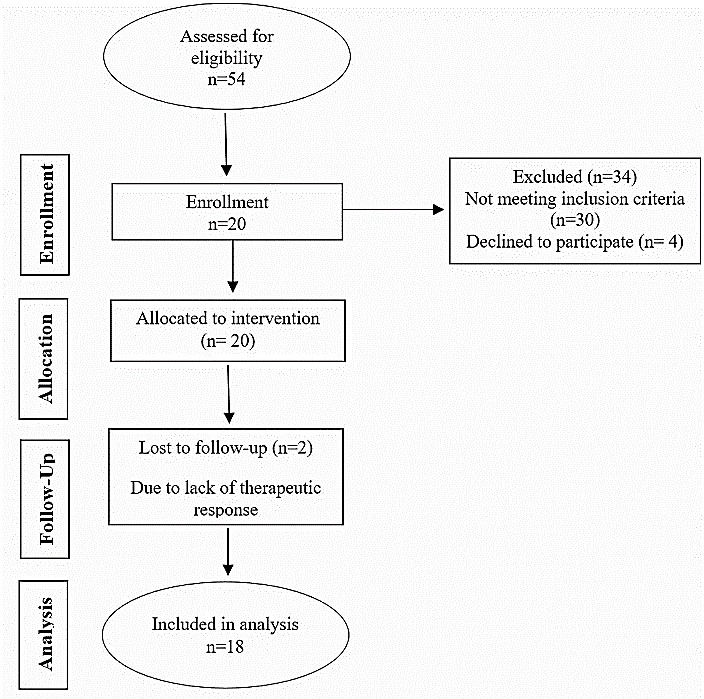
The trial flowchart
Patients took 2 capsules two times a day (2 g/BD) and were visited every other week and systolic and diastolic blood pressure and any possible side effects were recorded (Table 1).
Table 1. Blood pressure changes (before and after the study) .
| Blood pressure(mmHg) | Time | Mean ± SD |
| Systolic blood pressure (SBP) | at baseline | 151.9 ± 13.74 |
| after 8 weeks | 134.6 ± 18.25 | |
| Diastolic blood pressure (DBP) | at baseline | 97.41 ± 10.36 |
| after 8 weeks | 85.71 ± 7.48 |
After treatment for at least 8 weeks, systolic and diastolic blood pressure decreased significantly (P=0.003, 95% CI: -19.7, -15.1; P=0.0006, 95% CI: -10.23, -13.15; respectively) (Table 2). Moreover systolic and diastolic blood pressure decreased after 45 days treatment (P=0.025, 95% CI: -15.4, -10.6; P=0.034, 95% CI: -7.71, -7.85; respectively). At the end of the study, OSE decreased systolic and diastolic blood pressure 17.3 and 11.7 mmHg, respectively. (Figure 2, A & B).
Table 2. Baseline characteristics of participants and changes in systolic and diastolic BP.
| Demographic characteristics | ||
| Age | y (mean ± SD) | 50.94 ± 8.73 |
| BMI | kg/m2(mean ± SD) | 30.4 ± 4.84 |
| Male | n (%) | 7 ( 35) |
| Sample size | n | 20 |
| Blood pressure(mmHg) | ||
| Systolic blood pressure (SBP) | After 2 weeks | ~ -11 |
| After 4 weeks | ~ -9.9 | |
| After 6 weeks | ~ -13 | |
| After 8 weeks | ~ -17.3 | |
|
Diastolic blood pressure
(DBP) |
After 2 weeks | ~ -4.3 |
| After 4 weeks | ~ -4.59 | |
| After 6 weeks | ~ -7.79 | |
| After 8weeks | ~ -11.7 | |
Figure 2.
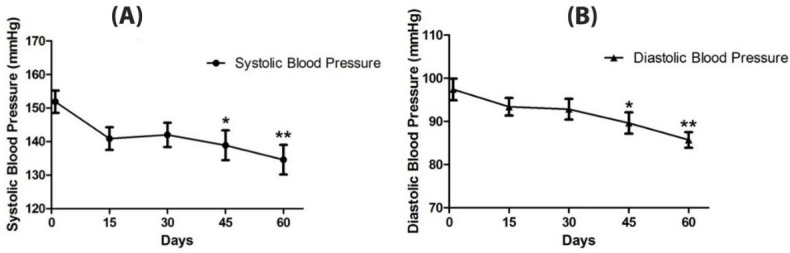
Blood pressure changes during treatment. Systolic (A) and diastolic blood pressures (B) have been measured at multiple time points: on the first day of investigation, 15, 30, 45, and 60 days after treatment (mean ± SEM). Decline of blood pressure at different time points have been compared with the blood pressure at the beginning of the investigation. There was a significant decline of blood pressure beyond 45 days of treatment when compared with the blood pressure before treatment (the asterisks indicate P < 0.05, repeated measures ANOVA analysis).
We did not observe hepatic or renal toxicity as the result of O. acanthium extract consumption in patients during the study.
The results of these tests showed no significant changes (CBC, FBS, TG, Chol, LDL, HDL, SGOT, SGPT, BUN, Cr). As shown in the following diagrams, although these changes were not significant, in some cases, such as glucose, cholesterol, triglyceride, LDL, VLDL, and BUN, there was a tendency to reduction. Based on these results, HDL levels show a modest increase. The levels of sodium and creatinine were almost constant. The amount of potassium and liver enzymes were in the normal range (Figures 3 & 4). In general the changes of liver enzymes levels before and after treatment was not significant (P=0.1481; P=0.1400, respectively) (Figure 5).
Figure 3.
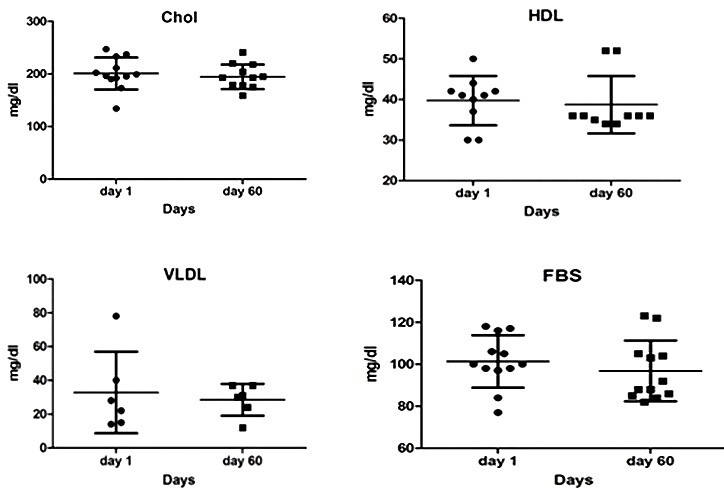
Changes in metabolic factors compared with the beginning of the intervention. After analysis, student t-test did not change significantly. P <0.05 was considered as a significant level.
Figure 4.
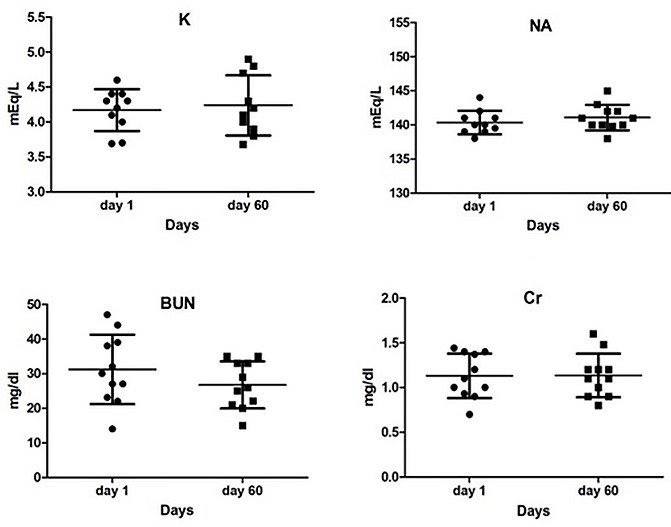
Changes in electrolytes and renal tests compared with the beginning of the intervention. After analysis, student t-test did not change significantly. P <0.05 was considered as a significant level.
Figure 5.
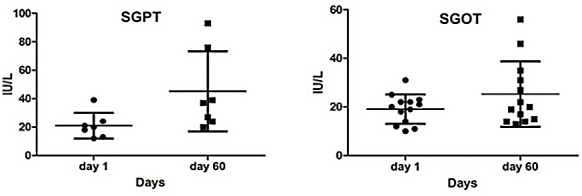
Changes in the liver function tests compared to the beginning of the intervention. After analysis, student t-test did not change significantly. P <0.05 was considered as a significant level.
Regarding the side effects, we detected mild dyspnea in two patients after 2 weeks. Also two patients showed dizziness and feeling of heaviness in the head in the first month of treatment that resolved later.
These data indicated that 4 g/day of O. acanthium seed extract for 8 weeks significantly decreases systolic and diastolic blood pressure in patients with stage I and II primary essential hypertension under losartan treatment. In line with our recent findings in which a novel compound was extracted and identified from O. acanthium named Onopordia, OSE showed antioxidant and angiotensin converting enzyme inhibitor (ACEI) activity (80.2 ± 2 %). Its potential of blood pressure decreasing can be due to its different components such as sesquiterpenes and flavonoid.8 There is no study on evaluating the effects of O. acanthium on blood pressure; however, some studies around the world have evaluated the hypotensive effect of some popular and well-known hypotensive herbal medicines.
Asgary et al., in a study in Iran, administered Achillea wilhelmsii to 120 patients for 8 weeks and showed significant decline of systolic and diastolic blood pressure (P=0.005 and P=0.003, respectively).17 Walker et al., administered 500 mg/day of dried, full-spectrum aqueous-alcoholic extract of hawthorn (Crataegus laevigata) leaves and flowers to 36 patients with essential HTN for 10 weeks and found that diastolic blood pressure decreased in 19 patients. However the difference was not significant (P=0.08).18 In a follow up study Walker et al., evaluated the hypotensive effect of Crataegus laevigata, 1200mg/day for 16 weeks on 76 patients and indicated significant decrease in diastolic (P=0.03) but no significant decrease in systolic blood pressure (P=0.32).19 Consistent with these findings Ried, et al. indicated that treatment with aged garlic extract for 12 weeks lowered systolic blood pressure 10.2 mmHg.20 Furthermore Susalit, et al., investigated the hypotensive effect of olive (Olea europaea) leaf extract, 500 mg/daily for 8 weeks on 232 patients and indicated the extract lowered 10 mmHg systolic blood pressure.21 Moreover, several other herbal agents were studied independently and showed hypotensive effect. Examples are Sour Cherry (Prunus cerasus L.), Nigella sativa seed extract, pomegranate juice, Hibiscus sabdariffa and canola oil with sunflower oil.22-29 In this study, OSE lowered systolic blood pressure by 17.3 mmHg that is more potent than all studied hypotensive agents. Similar effects have been reported about aged garlic extract and olive leaf extract that showed a reduction of about 10 mmHg of systolic blood pressure. The reasons for such discripency is not clear but it may be due to the presence of both ACEI and diuretic activity in OSE. This finding was in line with previous report by Sharifi, et al., that revealed OSE has more hypotensive effect than other 50 herbal agents which has been assessed.8
Several limitations are inherent to the present study. Recruiting hypertensive patients categorized in the first and second stage of hypertension who were also treated with losartan was very difficult. The majority of hypertensive patients had to use two or more drugs to control their blood pressure, due to lack of good response to one-drug therapy. The limitation of recruiting hypertensive patients in this study was consistent with the one in the other studies. The withdrawal of the patients was also an obstacle to obtain the desired results with higher power of analysis.
Conclusion
OSE synergistically with diuretic and plasma ACE inhibitor activity reduced blood pressure (both systolic and diastolic) in the patients under treatment with losartan, and did not show remarkable side effects in patients with primary hypertension. Because of the low number of samples, this medication may be not safely recommended. Further clinical trials is needed for increasing certainty with larger sample size and placebo.
Acknowledgments
This study was part of a postgraduate thesis entitled: ''Investigating the Effect of Onopordon Acanthium Seed on Primary Hypertension Reduction in Patients under Treatment by Losartan''; and was supported by a grant from Tehran University of Medical Sciences. The funding source had no involvement in any part of the study. We would like to thank the participants for their contribution to the maintenance of our patient record without which this project would have been impossible. Special thanks to Mr. Hadi Salehi, for his kindly efforts for extraction and drug preparation. We would like to thank Dr. Mohsen Amin assistant Prof. at Faculty of Pharmacy, Tehran University of Medical Sciences for his help in data analysis and manuscript editing.
Ethical Issues
The study protocol was approved by the ethics committee of Research Institute for Islamic and Complementary Medicine of Iran University of Medical Sciences at 09/10/2012 with reference number 732/P26/M/T.
Conflict of Interest
The authors have declared no conflicts of interest.
References
- 1.Chockalingam A. Impact of world hypertension day. Can J Cardiol. 2007;23(7):517–9. doi: 10.1016/S0828-282X(07)70795-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chockalingam A. World hypertension day and global awareness. Can J Cardiol. 2008;24(6):441–4. doi: 10.1016/S0828-282X(08)70617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sever PS, Messerli FH. Hypertension management 2011: Optimal combination therapy. Eur Heart J. 2011;32(20):2499–506. doi: 10.1093/eurheartj/ehr177. [DOI] [PubMed] [Google Scholar]
- 4.Kalra S, Kalra B, Agrawal N. Combination therapy in hypertension: An update. Diabetol Metab Syndr. 2010;2:44. doi: 10.1186/1758-5996-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF. et al. Guidelines for management of hypertension: Report of the fourth working party of the british hypertension society, 2004-bhs iv. J Hum Hypertens. 2004;18(3):139–85. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 6.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK. et al. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312(5770):117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A. et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108(36):14849–54. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifi N, Souri E, Ziai SA, Amin G, Amini M, Amanlou M. Isolation, identification and molecular docking studies of a new isolated compound, from onopordon acanthium: A novel angiotensin converting enzyme (ace) inhibitor. J Ethnopharmacol. 2013;148(3):934–9. doi: 10.1016/j.jep.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Amin G. The most common iranian traditional medicinal plants. 2nded. Tehran: Tehran University of Medical Sciences and Research Center for Medical Ethics and History; 2008. [Google Scholar]
- 10.Ugur A, Sarac N, Duru ME. Chemical composition and antimicrobial activity of endemic onopordum caricum. Middle-East J Sci Res. 2011;8(3):594–8. [Google Scholar]
- 11.Stace C. New flora of the british isles. 3rd ed. UK: Cambridge University Press; 2010. [Google Scholar]
- 12.Khalilov L, Khalilova A, Shakurova E, Nuriev I, Kachala V, Shashkov A. et al. Pmr and 13c nmr spectra of biologically active compounds. Xii. Taraxasterol and its acetate from the aerial part of onopordum acanthium. Chem Nat Compd. 2003;39(3):285–8. doi: 10.1023/A:1025478720459. [DOI] [Google Scholar]
- 13. Reichard S, Seebacher L. Addendum to a report, Analysis and Assessment of the Invasive risk of Onopordum Illyricum. University of Washington, College of Forest Resources, Center for Urban Horticulture; 2001.
- 14.Tyumkina T, Nuriev I, Khalilov L, Akhmetova V, Dzhemilev U. Pmr and 13 c nmr spectra of biologically active compounds. Xiii.* structure and stereochemistry of a new phenylpropanoid glycoside isolated from "onopordum acanthium" seeds. Chem Nat Compd. 2009;45(1):61–5. doi: 10.1007/s10600-009-9254-9. [DOI] [Google Scholar]
- 15.Esmaeili A, Saremnia B. Preparation of extract-loaded nanocapsules from "onopordon leptolepis" Dc. Ind Crop Prod. 2012;37(1):259–63. doi: 10.1016/j.indcrop.2011.12.010. [DOI] [Google Scholar]
- 16.National High Blood Pressure Education P . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2004. [PubMed] [Google Scholar]
- 17.Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of achillea wilhelmsii. Drugs Exp Clin Res. 2000;26(3):89–93. [PubMed] [Google Scholar]
- 18.Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: A randomized double-blind pilot study of mild, essential hypertension. Phytother Res. 2002;16(1):48–54. doi: 10.1002/ptr.947. [DOI] [PubMed] [Google Scholar]
- 19.Walker AF, Marakis G, Simpson E, Hope JL, Robinson PA, Hassanein M. et al. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: A randomised controlled trial. Br J Gen Pract. 2006;56(527):437–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Ried K, Frank OR, Stocks NP. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas. 2010;67(2):144–50. doi: 10.1016/j.maturitas.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T. et al. Olive (olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with captopril. Phytomedicine. 2011;18(4):251–8. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Ataee-jafari A, Hosseini S, Heshmat R, Parviz M, Raeeszade S, Yoosefi M. et al. Effect of sour cherry (prunus cerasus l.) on cardovascular risk factors in patients with diabet type ii. Iran J Diabetes Lipid Disord. 2006;5(4):365–70. [Google Scholar]
- 23.Ashraf R, Khan RA, Ashraf I, Qureshi AA. Effects of allium sativum (garlic) on systolic and diastolic blood pressure in patients with essential hypertension. Pak J Pharm Sci. 2013;26(5):859–63. [PubMed] [Google Scholar]
- 24.Saberi M, Kazemi-saleh D, Boloorian V. Effect of olive leaf on mild to moderate and common drug resistant hypertension. J Med Plants. 2009;3(27):52–9. [Google Scholar]
- 25.Dehkordi FR, Kamkhah AF. Antihypertensive effect of nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22(4):447–52. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 26.Sohrab G, Sotoode G, Siasi F, Neyestani T, Rahimi A. Effect of pomegranate juice consupmtion on blood pressure in type 2 diabetic patients. Iran J Endocrinol Metab. 2009;9(4):399–405. [Google Scholar]
- 27.Wahabi HA, Alansary LA, Al-Sabban AH, Glasziuo P. The effectiveness of hibiscus sabdariffa in the treatment of hypertension: A systematic review. Phytomedicine. 2010;17(2):83–6. doi: 10.1016/j.phymed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa l. Tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140(2):298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 29.Seied-ebrahimi S, Shidfar F, heydari I, Haghighi L, Gohari M-r, Hoseini S. Comparison of the effect of canola oil with sunflower oil on blood pressure,lipid profile, apoproteins, lipoprotein (a), total antioxidant capacity and crp in hyperlipidemic postmenopausal women. Iran J Nutr Sci Food Technol. 2011;2:21–9. [Google Scholar]


