Abstract
Purpose: The size of polymeric nanoparticles is considered as an effective factor in cancer therapy due to enterance into tumor tissue via the EPR effect. The purpose of this work was to investigate the effective parameters on poly(lactic-co-glycolic acid)-paclitaxel (PLGA –PTX) nanoparticles size.
Methods: We prepared PLGA-PTX nanoparticles via single emulsion and precipitation methods with variable paremeters including drug concentration, aqueous to organic phase volume ratio, polymer concentration, sonication time and PVA concentration.
Results: PLGA-PTX nanoparticles were characterized by dynamic light scattering (DLS) and scanning electron microscopy (SEM). The results exhibited that the diameter of nanoparticles enhanced with increasing drug, polymer and PVA concentrations whereas organic to aqueous phase volume ratio and sonication time required to the optimization for a given size.
Conclusion: The precipitation method provides smaller nanoparticles compared to emulsion one. Variable parameters including drug concentration, aqueous to organic phase volume ratio, polymer concentration, sonication time and PVA concentration affect diameter of nanoparticles.
Keywords: PLGA, Paclitaxel, Nanoparticle, Size
Introduction
Cancer is a major global cause of morbidity and mortality which is estimated that the incidence of all new cancer cases will reach 22 million by 2030 in worldwide.1 Chemotherapy is a versatile cancer treatment modality due to its application as first line,2,3 adjuvant4 and/or palliative therapy5 in the fight against different cancers. In addition, chemotherapy is easier to administer and less invasive compared to other clinical cancer treatment modalities such as surgical removal and radiotherapy. Unfortunately, since the efficacy of most chemotherapeutic drugs is dose dependent, severe chemo-induced side events have been observed at higher doses.6-8 Thus targeted delivery of drugs with minimum non-specific exposure is essential for successful chemotherapy. Tumor targeting chemotherapy can be accomplished by exploiting the diseases’ pathophysiology such as unique or overexpressed molecules9 and leaky tumor vasculature.10
Therapeutics can be passively targeted to the hyper-permeable tumor vasculature commonly observed on most cancers. Moreover, the absence of lymphatic drainage in tumors leads to retention of accumulated therapeutic agents within the tumor tissue.11,12 This unusual extravasation, accumulation and retention of expediently sized therapeutic molecules within tumor tissue is called enhanced permeability and retention (EPR) effect.13 In addition, nano-sized drug carriers can simultaneously deliver higher amounts of drugs with lower unspecific toxicity, without loss in therapeutic activity. Examples of these biocompatible nano-scale drug carriers include solid lipid nanoparticles (SLN),14 liposomes,15 micelles,16 nanobubbles17 and polymers.18,19 Among these, an extensively studied family of materials in the fabrication of biocompatible nanostructures is polymers.20,21 Polymeric nanostructures possesses several advantages such as simple synthesis techniques, ability to carry a wide payload of therapeutic agents and biodegradability.22 A wide range of synthetic and natural polymers have been investigated for a variety of biomedical applications such as tissue engineering,23,24 bioimaging25 biosensors26-28 and drug delivery.29,30 Synthetic polymers, notably, PLGA and its co-monomer PLA are widely used in the synthesis of nano-sized drug delivery systems. Flexible synthesis techniques enable the tailoring of nanoparticle properties such as size,31 drug loading32 and in-vivo drug release.33
Among a legion of anti-cancer drugs, paclitaxel is a potent chemo-agent used in the treatment of several solid tumors including ovarian cancer, breast cancer, AIDS related kaposi sarcoma and lung cancer. Further investigations on the efficacy of paclitaxel against gastrointestinal cancer,34 glioblastoma35 and pancreatic cancer36 have yielded promising results. However, a major clinical limitation of paclitaxel is the drugs’ poor solubility in water. Therefore biocompatible nano-sized colloidal structures offer safer alternative paclitaxel delivery vehicles.37-40
Since most synthesis nanoparticle and drug loading techniques are well established, current scientific focus is increasingly being directed towards optimization of various parameters to obtain effective formulations. Therefore, for EPR targeting, it is of great interest to determine the various input parameters which affect the diameter of nanoparticles. Meanwhile, it is also important to be cognizant of the rate limiting size-dependent physiological processes which may affect the intra-tumoral accumulation of nanoparticles. Nanoparticle sizes less than 30 nm are prone to renal filtration41 whilst sizes larger than 250 nm are ideal candidates for phagocytosis.42 Therefore, the effective therapeutic window for EPR targeting may be considered between 50 and 200 nm. Thus the aim of this work was investigate the various parameters which affect the diameter of PTX loaded PLGA nanoparticles for effective EPR targeting. PLGA nanoparticles were chosen for this study due the simplicity and flexibility of synthesis techniques such as nanoprecipitation and emulsion/solvent evaporation.43,44
Materials and Methods
Paclitaxel was purchased from sigma. PLGA (50:50, MW 30000 g mol−1) was bought from Shenzhen Esun Industrial Co., China. Dichloromethane (DCM) and acetone (99%) supplied by Carol Erba. Polyvinyl alcohol (PVA), fully hydrolized (MW 60000 g mol−1) was obtained from Merck (Germany). All solutions were prepared using deionized water.
Preparation of paclitaxel-loaded nanoparticles by single emulsion
Nanoparticles were prepared by single emulsion (O/W) method. The PVA polymer was dissolved in deionized water as aqueous phase under continuous magnetic stirring at 40 °C for 5 h to obtain a homogenous solution. Different amounts of paclitaxel and PLGA were dissolved in DCM and stirred for 1 h at room temperature. Then the organic phase poured in PVA solution (30 ml) and stirred for 30 min at room temperature. Then emulsion was sonicated using probe sonication (Top Sonics Ltd., Co., Iran) for 4 minute. After evaporation of the organic solvent overnight, the nanoparticles were collected by centrifugation (Eppendorf centrifuge) at 12000 rpm for 20 min at room temperature and washed twice with deionized water.
Preparation of paclitaxel-loaded nanoparticles by precipitation
The homogenous solutions of PVA were prepared. Specific amount of PLGA and paclitaxel was dissolved in acetone (5 ml) as the organic phase and stirred for 30 min at room temperature. Next, organic phase was added to the 20 ml of PVA solution while stirring. Afterward, organic phase was evaporated overnight and nanoparticles were collected by centrifugation at 12000 rpm for 20 min at room temperature and washed twice with deionized water.
Characterization of nanoparticles
Scanning Electron Microscopy (SEM)
The morphology and diameter of nanoparticles were carried out using SEM as an accelerating voltage of 20.0 kv (Philips XL-30) after sputtering with gold. The diameter of nanoparticles was measured by randomly choosing 30 nanoparticles by SemAfore (4.01 demo, JEOI., Finland) software as shown in Figure 1a.
Figure 1.
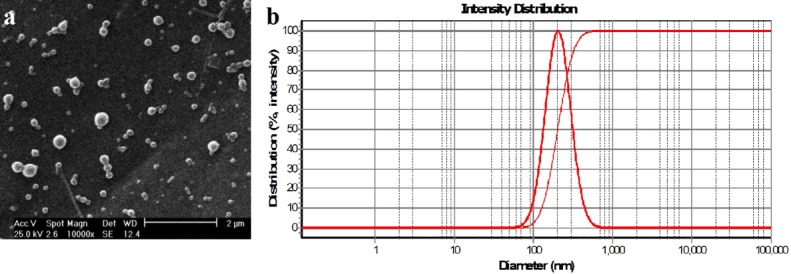
a)SEM image of PLGA-PTX nanoparticles b) DLS result of PLGA-PTX nanoparticles.
Dynamic light scattering (DLS)
The hydrodynamic diameter and the median nanoparticles size were obtained using DLS (Scatter Scope) as shown in Figure 1b.
Results and Discussion
Comparison of two methods single emulsion and precipitation
Table 1 and 2 summarizes experiments conducted in this study to investigate drug and PLGA concentrations, the amounts of PVA and solvent and sonication time which affect PLGA nanoparticles size and morphology. Emulsion solvent evaporation and nanoprecipitation/interfacial deposition are the two most common methods for the preparation of polymeric nanoparticles. These two techniques were originally developed by Vanderhoff et al45 and Fessi et al,46 respectively. In an study PLGA incorporated with procaine nanoparticles was prepared via nanoprecipitaion technique with the size less than 210 nm.32 In another study protein encapsulated in PLGA nanoparticles were prepared by phase separation method with the size around 300 nm for biomedical application.47 Commercial success of the various polymeric nanoformulation products developed by emulsion and nanoprecipitation were documented by Nava-Arzaluz et al48 and Minost et al.49 We investigated the effect of two methods of single emulsion and precipitation on nanoparticles size. The size of polymeric nanoparticles is particularly important in cancer therapy as drug delivery vehicles can enter into the tumor tissue via the EPR effect. Our results indicated that paclitaxel-loaded nanoparticles prepared with precipitation had smaller size (Table 2. No. 11) compare to single emulsion method (Table 1, No. 19). In addition, acetone is used in precipitation method which have less toxic effect than DCM applied in emulsion method.50 Therefore, precipitation method is suggested for medical applications.
Table 1. Applied parameters for preparation of paclitaxel loaded PLGA nanoparticles using single emulsion .
| Number |
PLGA
(mg) |
Solvent
(ml) |
Drug
(mg) |
PVA
(W/V %) |
Sonication time
(min) |
Mean Size
(nm) |
| 1 | 20 | 3 | 1 | 1% | 4 | 250 ± 12 |
| 2 | 20 | 3 | 1.5 | 1% | 4 | 270 ± 22 |
| 3 | 20 | 3 | 3 | 1% | 4 | 353 ± 24 |
| 4 | 20 | 3 | 4.5 | 1% | 4 | 371 ± 39 |
| 5 | 20 | 3 | 6 | 1% | 4 | 402 ± 9 |
| 6 | 60 | 3 | 3 | 1% | 2 | 548 ± 10 |
| 7 | 60 | 3 | 3 | 1% | 4 | 303 ± 15 |
| 8 | 60 | 3 | 3 | 1% | 5 | 546 ± 17 |
| 9 | 60 | 3 | 3 | 1% | 6 | 598 ± 20 |
| 10 | 10 | 3 | 1.5 | 1% | 4 | 250 ± 15 |
| 11 | 30 | 3 | 1.5 | 1% | 4 | 327 ± 17 |
| 12 | 40 | 3 | 1.5 | 1% | 4 | 354 ± 12 |
| 13 | 50 | 3 | 1.5 | 1% | 4 | 381 ± 18 |
| 14 | 60 | 3 | 1.5 | 1% | 4 | 414 ± 21 |
| 15 | 30 | 1 | 1 | 1% | 4 | 478 ± 10 |
| 16 | 30 | 2 | 1 | 1% | 4 | 381 ± 12 |
| 17 | 30 | 3 | 1 | 1% | 4 | 300 ± 18 |
| 18 | 30 | 4 | 1 | 1% | 4 | 412 ± 21 |
| 19 | 30 | 5 | 1 | 1% | 4 | 469 ± 14 |
| 20 | 30 | 6 | 1 | 1% | 4 | 491 ± 11 |
Table 2. Applied parameters for preparation of paclitaxel loaded PLGA nanoparticles using precipitation method .
| Number |
PLGA
(mg) |
Solvent
(ml) |
Drug
(mg) |
PVA
(W/V %) |
Mean Size
(nm) |
| 1 | 20 | 5 | 1 | 1% | 210 ± 17 |
| 2 | 20 | 5 | 1.5 | 1% | 235 ± 10 |
| 3 | 20 | 5 | 3 | 1% | 271 ± 16 |
| 4 | 20 | 5 | 4.5 | 1% | 310 ± 32 |
| 5 | 20 | 5 | 6 | 1% | 342 ± 9 |
| 6 | 20 | 5 | 1 | 0.25% | 130 ± 70 |
| 7 | 20 | 5 | 1 | 0.5% | 192 ± 21 |
| 8 | 20 | 5 | 1 | 1.5% | 271 ± 16 |
| 9 | 20 | 5 | 1 | 2% | 378 ± 29 |
| 10 | 10 | 5 | 1.5 | 1% | 217 ± 14 |
| 11 | 30 | 5 | 1.5 | 1% | 251 ± 12 |
| 12 | 40 | 5 | 1.5 | 1% | 267 ± 21 |
| 13 | 50 | 5 | 1.5 | 1% | 289 ± 18 |
| 14 | 60 | 5 | 1.5 | 1% | 324 ± 17 |
The effect of PVA
The function of PVA concentration as an effective factor on PLGA diameter is shown in Figure 2. In this experiment the applied concentrations of PVA were 0.25, 0.5, 1 and 2 W/V % whereas other parameters were constant. The nanoparticles diameter increased from about 130 nm to 378 nm (Table 2, Nos. 6-9) as the PVA concentration increased from 0.25 to 2 W/V%. It was observed that the size of the nanoparticles enhances in the precipitation method with increasing PVA concentration which can be associated with the deposition of PVA on the surface of paclitaxel-loaded nanoparticles. An increase in nanoparticle size has also been reported as the concentration of PVA enhanced.51
Figure 2.
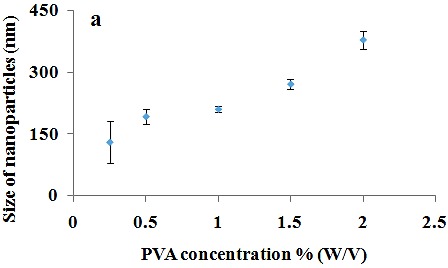
The effect of PVA concentration on nanoparticles size.
The effect of drug concentration
The effect of the amount of drug was also examined on nanoparticles diameter. In this study the different amounts of drug were used whilst other parameters were constant by both emulsion (Table 1, Nos. 1-5) and precipitation (Table 2, Nos. 1-5) methods. As shown in Figure 3, by increasing the amount of drug from 1 to 6 mg, the nanoparticles diameter enhanced from 250 nm to 402 nm and from 210 nm to 342 nm using emulsion and precipitation methods, respectively. This increase may be because of the more content of drug available in the emulsion droplets or adsorption of drug on surface of nanoparticles in single emulsion method. In addition, increase in the amount of paclitaxel leads to larger size of nanoparticles because more solid content form after evaporation in precipitation method. The literature also confirms that increase in content of drug results in larger size of nanoparticles.52,53 Although some reports presented lack of relationship between size of nanoparticles and drug concentration.50,54
Figure 3.
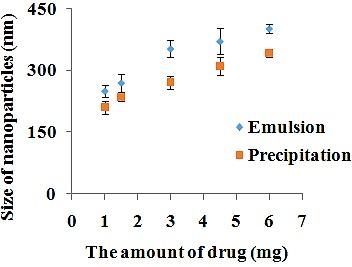
The effect of drug concentration on nanoparticles size by single emulsion and precipitation method.
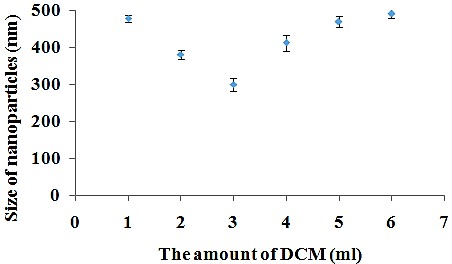
The effect of sonication time on nanoparticles size
Polymeric nanoparticles can be synthesized via two approaches; “bottom up” or “top down”. In “bottom up”, the nanoparticles are formed from continuous deposition of molecular growth species on a nanoparticle nuclei, that is, polymer aggregation. In “Top down” synthesis, external energy sources are used to break down colloidal polymer complex structures into nanoemulsions. A commonly applied technique in the “top down” synthesis is the use of ultra-sound homogenization.55 In this experiment, for nanoparticles prepared by emulsion method, probe sonicator was used for 2, 4, 5 and 6 min (Table 1, Nos. 6-9) and the trend of applied sonication time on mean nanoparticles size was investigated. As shown in Figure 4, the nanoparticles size decreased from 548 to 303 nm when applied sonication time increased from 2 to 4 min. It can be attributed to the high released energy in emulsification process, resulting in the formation of smaller droplets which affect the size of polymeric nanoparticles. In addition, by increasing sonication time from 4 to 6 min, the nanoparticles size increased from 303 to 598 nm. This increase may be because of de-emulsification process56 or agglomeration.57 In a study, size of drug loaded PLGA nanoparticles was optimized by varing sonication time.58 Therefore, sonication time can be considered as a parameter for optimized size of nanoparticles.
Figure 4.
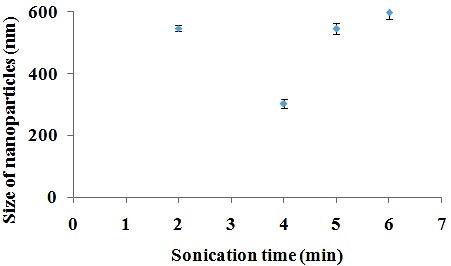
The effect of sonication time on nanoparticles size.
The effect of polymer concentration
In this experiment, by increasing the PLGA concentration from 10 to 60 mg, the mean size of nanoparticles enhanced from about 250 to 414 nm (Table 1, Nos. 2, 10-14) and from about 217 to 324 nm (Table 2, Nos. 2, 10-14) in emulsion and precipitation methods, respectively (Figure 5). In both techniques, it was observed that the size of the nanoparticles has a direct relationship with the PLGA concentration. In single emulsion method, this might be attributed to the increase in viscosity of dispersed phase, resulting in a reduction of the net shear stress and prompting bigger nanodroplets. Besides, PLGA solution cannot be rapidly dispersed into the aqueous phase as the viscosity increases and result in larger nanoparticles.59,60 In precipitation method, frequency of collisions increase which leads to fusion of nanoparticles as concentration of polymer enhances.61
Figure 5.
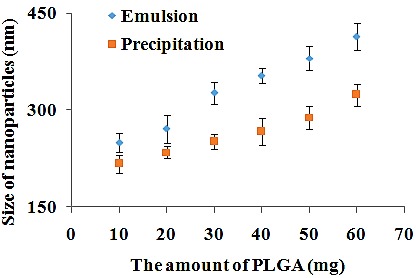
The effect of PLGA concentration on nanoparticles size prepared by single emulsion and precipitation method.
The effect of organic to aqueous phase volume ratio
In our experiments, the nanoparticles size demonstrated an initial decrease from 478 nm to 300 nm as the amount of the organic phase enhanced from 1 to 3 ml (as shown in Figure 6 and Table 1, Nos. 15-17). The reason why the nanoparticles size decreased from 478 nm to 300 may be attributed to viscosity of organic phase. Increasing organic phase results in decrease in concentration of polymer and consequently decreases in size. However, by further increasing the amount of the organic phase from 3 to 6 ml, the particle size enhanced from 300 nm to 491 nm (Table 1, Nos. 17-20). This may be because of an enhanced time for the evaporation of the organic phase. In other words, a higher amount of solvent may cause ostwald ripening of the nanoemulsions before solvent evaporation of organic phase.
Figure 6.
The effect of aqueous to organic phase volume ratio on nanoparticles size.
Conclusion
This study compared two methods for preparation of paclitaxel loaded PLGA nanoparticles including single emulsion and precipitation. The results indicated that precipitation method results in smaller nanoparticles compared to emulsion one. In addition, the effect of the various parameters on the size of the nanoparticles was investigated The results demonstrated that the concentration of the PLGA polymer, drug and PVA had a direct relationship with the size of nanoparticles whereas sonication time and organic phase to aqueous volume ratio needed to the optimization to obtain the nanoparticles with smaller sizes.
Acknowledgments
This work was supported by Tehran University of Medical Sciences, Grant No. 96-01-87-34138.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the human development index (2008-2030): A population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 2.Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: A systematic review. J Thorac Oncol. 2010;5(2):260–74. doi: 10.1097/JTO.0b013e3181c6f035. [DOI] [PubMed] [Google Scholar]
- 3.Mujokoro B, Adabi M, Sadroddiny E, Adabi M, Khosravani M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: A review. Mater Sci Eng C Mater Biol Appl. 2016;69:1092–102. doi: 10.1016/j.msec.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 4.Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015;13:195. doi: 10.1186/s12916-015-0439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassinari D, Fochessati F, Arcangeli V, Sartori S, Agostini V, Fantini M. et al. Carboplatin and gemcitabine in the palliative treatment of stage iv non-small cell lung cancer: Definitive results of a phase ii trial. Tumori. 2004;90(1):54–9. doi: 10.1177/030089160409000113. [DOI] [PubMed] [Google Scholar]
- 6.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 7.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010;144(1):3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001;(30):135–42. doi: 10.1093/oxfordjournals.jncimonographs.a003451. [DOI] [PubMed] [Google Scholar]
- 9.Kue CS, Kamkaew A, Burgess K, Kiew LV, Chung LY, Lee HB. Small molecules for active targeting in cancer. Med Res Rev. 2016;36(3):494–575. doi: 10.1002/med.21387. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura Y, Kimura M, Yamamoto T, Maeda H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J Cancer Res. 1988;79(12):1327–34. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–9. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release. 2012;164(2):138–44. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Martins S, Tho I, Reimold I, Fricker G, Souto E, Ferreira D. et al. Brain delivery of camptothecin by means of solid lipid nanoparticles: Formulation design, in vitro and in vivo studies. Int J pharm. 2012;439(1-2):49–62. doi: 10.1016/j.ijpharm.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152(3):402–10. doi: 10.1016/j.jconrel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–31. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 17.Ayodele AT, Valizadeh A, Adabi M, Esnaashari SS, Madani F, Khosravani M. et al. Ultrasound nanobubbles and their applications as theranostic agents in cancer therapy: A review. Biointerface Res Appl Chem. 2017;7(6):2253–62. [Google Scholar]
- 18.Shah M, Naseer MI, Choi MH, Kim MO, Yoon SC. Amphiphilic pha-mpeg copolymeric nanocontainers for drug delivery: Preparation, characterization and in vitro evaluation. Int J Pharm. 2010;400(1-2):165–75. doi: 10.1016/j.ijpharm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Maleki H, Dorkoosh F, Adabi M, Khosravani M, Arzani H, Kamali M. Methotrexate-loaded plga nanoparticles: Preparation, characterization and their cytotoxicity effect on human glioblastoma U87MG cells. Int J Med Nano Res. 2017;4(1):020. doi: 10.23937/2378-3664/1410020. [DOI] [Google Scholar]
- 20.Murthy SK. Nanoparticles in modern medicine: State of the art and future challenges. Int J Nanomedicine. 2007;2(2):129–41. [PMC free article] [PubMed] [Google Scholar]
- 21.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers. 2010;2(4):522–53. doi: 10.3390/polym2040522. [DOI] [Google Scholar]
- 24.Hosseinzadeh S, Mahmoudifard M, Mohamadyar-Toupkanlou F, Dodel M, Hajarizadeh A, Adabi M. et al. The nanofibrous pan-pani scaffold as an efficient substrate for skeletal muscle differentiation using satellite cells. Bioprocess Biosyst Eng. 2016;39(7):1163–72. doi: 10.1007/s00449-016-1592-y. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Park K, Nam HY, Lee S, Kim K, Kwon IC. Polymers for bioimaging. Prog Polym Sci. 2007;32(8-9):1031–53. doi: 10.1016/j.progpolymsci.2007.05.016. [DOI] [Google Scholar]
- 26.Adabi M, Saber R, Faridi-Majidi R, Faridbod F. Performance of electrodes synthesized with polyacrylonitrile-based carbon nanofibers for application in electrochemical sensors and biosensors. Mater Sci Eng C Mater Biol Appl. 2015;48:673–8. doi: 10.1016/j.msec.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 27.Adabi M, Saber R, Naghibzadeh M, Faridbod F, Faridi-Majidi R. Parameters affecting carbon nanofiber electrodes for measurement of cathodic current in electrochemical sensors: An investigation using artificial neural network. RSC Adv. 2015;5(99):81243–52. doi: 10.1039/C5RA15541J. [DOI] [Google Scholar]
- 28.Samadian H, Zakariaee SS, Adabi M, Mobasheri H, Azami M, Faridi-Majidi R. Effective parameters on conductivity of mineralized carbon nanofibers: An investigation using artificial neural networks. RSC Adv. 2016;6(113):111908–18. doi: 10.1039/C6RA21596C. [DOI] [Google Scholar]
- 29.Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol. 2001;5(4):447–51. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 30.Kamali M, Dinarvand R, Maleki H, Arzani H, Mahdaviani P, Nekounam H. et al. Preparation of imatinib base loaded human serum albumin for application in the treatment of glioblastoma. RSC Adv. 2015;5(76):62214–9. doi: 10.1039/C5RA08501B. [DOI] [Google Scholar]
- 31.Kulkarni SA, Feng SS. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30(10):2512–22. doi: 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- 32.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J Control Release. 1999;57(2):171–85. doi: 10.1016/S0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 33.Corrigan OI, Li X. Quantifying drug release from PLGA nanoparticulates. Eur J Pharm Sci. 2009;37(3-4):477–85. doi: 10.1016/j.ejps.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto J, Matsui T, Kodera Y. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer. 2009;12(2):69–78. doi: 10.1007/s10120-009-0505-z. [DOI] [PubMed] [Google Scholar]
- 35.Terzis AJ, Thorsen F, Heese O, Visted T, Bjerkvig R, Dahl O. et al. Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (taxol) in vitro. Br J Cancer. 1997;75(12):1744–52. doi: 10.1038/bjc.1997.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safran H, Moore T, Iannitti D, Dipetrillo T, Akerman P, Cioffi W. et al. Paclitaxel and concurrent radiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2001;49(5):1275–9. doi: 10.1016/S0360-3016(00)01527-3. [DOI] [PubMed] [Google Scholar]
- 37.Peltier S, Oger JM, Lagarce F, Couet W, Benoit JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006;23(6):1243–50. doi: 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- 38.Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials. 2007;28(12):2137–46. doi: 10.1016/j.biomaterials.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Erdogar N, Esendagli G, Nielsen TT, Sen M, Oner L, Bilensoy E. Design and optimization of novel paclitaxel-loaded folate-conjugated amphiphilic cyclodextrin nanoparticles. Int J Pharm. 2016;509(1-2):375–90. doi: 10.1016/j.ijpharm.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 40.Gupta PN, Jain S, Nehate C, Alam N, Khare V, Dubey RD. et al. Development and evaluation of paclitaxel loaded PLGA:Poloxamer blend nanoparticles for cancer chemotherapy. Int J Biol Macromol. 2014;69:393–9. doi: 10.1016/j.ijbiomac.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 41.Balogh L, Nigavekar SS, Nair BM, Lesniak W, Zhang C, Sung LY. et al. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomedicine. 2007;3(4):281–96. doi: 10.1016/j.nano.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Nicolete R, dos Santos DF, Faccioli LH. The uptake of plga micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int Immunopharmacol. 2011;11(10):1557–63. doi: 10.1016/j.intimp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated plga nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351(1):19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Murakami H, Kobayashi M, Takeuchi H, Kawashima Y. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int J Pharm. 1999;187(2):143–52. doi: 10.1016/S0378-5173(99)00187-8. [DOI] [PubMed] [Google Scholar]
- 45. Vanderhoff JW, El-Aasser MS, Ugelstad J. Polymer emulsification process. Google Patents. 1979.
- 46.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–4. doi: 10.1016/0378-5173(89)90281-0. [DOI] [Google Scholar]
- 47.Swed A, Cordonnier T, Fleury F, Boury F. Protein encapsulation into plga nanoparticles by a novel phase separation method using non-toxic solvents. J Nanomed Nanotechnol. 2014;5(6):241. doi: 10.4172/2157-7439.1000241. [DOI] [Google Scholar]
- 48.Nava-Arzaluz MG, Pinon-Segundo E, Ganem-Rondero A, Lechuga-Ballesteros D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat Drug Deliv Formul. 2012;6(3):209–23. doi: 10.2174/187221112802652633. [DOI] [PubMed] [Google Scholar]
- 49.Minost A, Delaveau J, Bolzinger MA, Fessi H, Elaissari A. Nanoparticles via nanoprecipitation process. Recent Pat Drug Deliv Formul. 2012;6(3):250–8. doi: 10.2174/187221112802652615. [DOI] [PubMed] [Google Scholar]
- 50.Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded plga nanoparticles: Systematic study of particle size and drug content. Int J Pharm. 2007;336(2):367–75. doi: 10.1016/j.ijpharm.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 51.Maaz A, Abdelwahed W, Tekko IA, Trefi S. Influence of nanoprecipitation method parameters on nanoparticles loaded with gatifloxacin for ocular drug delivery. Int J Acad Sci Res. 2015;3(1):1–12. [Google Scholar]
- 52.Krishnamachari Y, Madan P, Lin S. Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int J Pharm. 2007;338(1-2):238–47. doi: 10.1016/j.ijpharm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Snehalatha M, Venugopal K, Saha RN. Etoposide-loaded PLGA and PCL nanoparticles I: Preparation and effect of formulation variables. Drug Deliv. 2008;15(5):267–75. doi: 10.1080/10717540802174662. [DOI] [PubMed] [Google Scholar]
- 54.Derman S. Caffeic acid phenethyl ester loaded PLGA nanoparticles: Effect of various process parameters on reaction yield, encapsulation efficiency, and particle size. J Nanomater. 2015;2015:341848. doi: 10.1155/2015/341848. [DOI] [Google Scholar]
- 55.Sah E, Sah H. Recent trends in preparation of poly(lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J Nanomater. 2015;2015:794601. doi: 10.1155/2015/794601. [DOI] [Google Scholar]
- 56.Español L, Larrea A, Andreu V, Mendoza G, Arruebo M, Sebastian V. et al. Dual encapsulation of hydrophobic and hydrophilic drugs in PLGA nanoparticles by a single-step method: Drug delivery and cytotoxicity assays. RSC Adv. 2016;6(112):111060–9. doi: 10.1039/C6RA23620K. [DOI] [Google Scholar]
- 57.Tripathi A, Gupta R, Saraf SA. PLGA nanoparticles of anti tubercular drug: Drug loading and release studies of a water in-soluble drug. Int J Pharm Tech Res. 2010;2(3):2116–23. [Google Scholar]
- 58.Dangi R, Shakya S. Preparation, optimization and characterization of PLGA nanoparticle. Int J Pharm Life Sci. 2013;4(7):2810–8. [Google Scholar]
- 59.Song X, Zhao Y, Wu W, Bi Y, Cai Z, Chen Q. et al. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: Systematic study of particle size and drug entrapment efficiency. Int J Pharm. 2008;350(1-2):320–9. doi: 10.1016/j.ijpharm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 60.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int J Pharm. 2005;290(1-2):137–44. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 61.Mostafavi SH, Aghajani M, Amani A, Darvishi B, Noori Koopaei M, Pashazadeh AM. et al. Optimization of paclitaxel-loaded poly (d,l-lactide-co-glycolide-n-p-maleimido benzoic hydrazide) nanoparticles size using artificial neural networks. Pharm Dev Technol. 2015;20(7):845–53. doi: 10.3109/10837450.2014.930487. [DOI] [PubMed] [Google Scholar]


