Abstract
Purpose: Metformin is one of the most popular drugs tested against nonalcoholic fatty liver disease (NAFLD). The present study aimed to investigate whether calcium-vitamin D3 cosupplementation will intensify the effect of metformin on the prevention of high-fat, high-fructose (HFFr) diet-induced hepatic steatosis.
Methods: Male wistar rats (210±16 g) were assigned into the following seven groups: a Control group to receive a standard chow and six HFFr-fed groups to receive diets containing either normal (0.5% calcium and 1000 IU/kg vitamin D3) or high amount of calcium and vitamin D3 (2.4% calcium and 10000 IU/kg vitamin D3) (CaD), in combination with gastric gavage administration of either saline or 25 or 200 mg/kg body weight/day metformin. After 60 days, rats were assessed with respect to their anthropometric, metabolic and hepatic parameters, as well as their hepatic AMP-activated protein kinase (AMPK) phosphorylation.
Results: Metformin and CaD, either alone or in combination, caused a significant reduction in HFFr diet-induced high serum aspartate aminotransferase (AST), hepatic steatosis and lipid accumulation without effect on insulin resistance and AMPK phosphorylation. In addition, slightly (and non-significantly) better effects of the combination in ameliorating steatosis and hepatic cholesterol content were observed.
Conclusion: Taken together, our results suggest that metformin and CaD could protect against the onset of HFFr diet-induced NAFLD in an insulin and AMPK-independent manner, without any marked additional benefits of their combination.
Keywords: AMP-activated protein kinase, Calcium, Metformin, Nonalcoholic fatty liver disease, Vitamin D3
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the most common form of chronic liver disease in the world, which is characterized by abnormal triglyceride accumulation in hepatocytes, not due to excess alcohol consumption or other causes of secondary hepatic steatosis.1,2 A growing body of evidence suggests a bidirectional relationship between NAFLD and components of metabolic syndrome,3,4 although insulin resistance may still play an important role in the pathogenesis of NAFLD.5 It is believed that insulin resistance and adiposity are associated with an imbalance between delivery and export of free fatty acid to the liver.1,5 Therefore, it is not surprising that several studies have investigated the efficacy of natural or pharmacological insulin sensitizers on the prevention and management of NAFLD.
Among pharmacological insulin sensitizers, metformin has acquired a fundamental role in the management of many disorders associated with insulin resistance. Metformin is believed to exert its anti-diabetic effects through inhibition of mitochondrial respiratory chain complex I,6,7 resulting in a fall in the ATP/AMP ratio and activation of AMP-activated protein kinase (AMPK), a master kinase regulating cellular energy homeostasis.8 The activation of AMPK in the liver could both induce catabolic pathways, such as β-oxidation of fatty acids, and suppress anabolic pathways like lipogenesis, thus potentially leading to reduced hepatic steatosis.8,9 As some studies have shown that metformin could protect against the development of steatosis in animal models,10,11 however human studies are controversial.12
There is now some evidence that vitamin D could be a natural insulin sensitizer, especially in combination with calcium.13,14 Dietary calcium or VitD3 (cholecalciferol) intake, as well as calcitriol administration have been shown to prevent adiposity, insulin resistance14,15 and hepatic fatty changes16,17 and to be related to AMPK activation in different tissues in animal models.18,19 On the other hand, Shalata et al.20 reported that vitamin D could intensify the effects of sitaglibtin/metformin drugs on treatment of steatosis and on decreasing hepatic triglyceride and malondialdehyde (MDA) content. So the hypothesis that combined calcium and vitamin D3 supplementation augments the protective effects of metformin on the onset of diet-induced steatosis was tested in the present study, and the role of AMPK signaling in mediating observed effects was also determined.
Materials and Methods
Animals and diets
Male Wistar rats (210±16 g) were housed at a room temperature of 22±2°C under a 12-h light–dark cycle. The rats were acclimated to the new conditions for one week before receiving their experimental diets. Then, rats were randomly assigned to two groups to feed on either a standard chow (Control, n=6) or high-fat, high-fructose (HFFr) diet (n=42) for a period of 60 days. Rats had continuous access either to plain tap water or to water containing 20% fructose solution, respectively. The HFFr-fed rats were nourished ad libitum with a normal calcium (0.5%) and VitD3 (1000 IU/kg) diet or a high calcium (2.4%) and VitD3 (10000 IU/kg) diet (CaD). These rats were also randomized to receive, by gastric gavage, either 1 ml of saline alone (S) or 25 or 200 mg/kg body weight metformin, once a day. The standard chow contained recommended levels of calcium (0.5%) and VitD3 (1000 IU/kg).21The highest amount of vitamin D3(10000 IU/kg diet) was selected as the optimal level of vitamin D3 intake without any adverse effect on rats.22 Details of Control and HFFr diets are provided in Table 1. The HFFr diet used has been shown to induce NAFLD, previously.23 Throughout the experiment, daily feed intake and weekly body weight measurements were taken to monitor the health of the rats. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of Urmia University of Medical Sciences. In addition, all animal protocols were approved by the Ethics Committee of Urmia Medical Sciences University.
Table 1. Macronutrient composition and energy contents of the control and high fat high fructose (HFFr) diets .
| - | Constituent | Control | HFFr |
| Macronutrients (% by weight) | Carbohydrate | 52.8 | 47.9 |
| Starch | 52.8 | 30.4 | |
| Fructose | 0 | 17.5 | |
| Fat | 5 | 27.8 | |
| Soybean oil | 5 | 2.8 | |
| Hydrogenated oil | 0 | 5 | |
| Sheep tallow | 0 | 20 | |
| Protein | 20 | 11.5 | |
| Macronutrients (% Kcal) | Carbohydrate | 59.7 | 37.3 |
| Fat | 14.5 | 53 | |
| Protein | 25.8 | 9.7 | |
| Energy (Kcal/g) | 3.1 | 4.72 |
Evaluation of animal and organ weights
At the end of the 60th day of the diet, the animals were anesthetized by an ip injection of a mixture of ketamin (60 mg/kg) and xylazin (10 mg/kg). Then, after blood sampling, the livers and visceral fat from both epididymal and perirenal areas were rapidly excised and weighed. Next, the livers were stored at -80 for further analysis.
Serum analysis
At the end of the treatment period and after a 16 h fast with free access to water, blood samples were collected via portal vein from anaesthetized animals. Blood samples were centrifuged and serum was frozen at -80°C for subsequent analysis. Serum fasting glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides, total and high-density lipoprotein (HDL) cholesterol concentrations were determined by an enzymatic method using individual commercial kits (Pars azmun, Tehran, Iran) and an automatic biochemical analyzer (BT 4500, Biotechnica, Italy). Non-HDL-C was calculated as total cholesterol minus HDL-C. Serum insulin (Bioassay Technology Laboratory, China) and complement C1q/tumor necrosis factor-α related protein-3 (CTRP3) (zellBio, Germany) levels were quantified with ELISA kits. In addition, we used the homeostasis model assessment of insulin resistance (HOMA-IR) method to estimate insulin resistance as [Fasting insulin×Fasting glucose/405].
Hepatic lipid quantitation and lipid peroxidation
The hepatic lipids were extracted according to the method described by Folch et al.24 Hepatic triglyceride and cholesterol content was quantified using commercially colorimetric assay kits (Pars azmun, Iran). Results were given as mg triglyceride or cholesterol per gram liver. Hepatic malondialdehyde (MDA), a product of lipid peroxidation, was also measured to evaluate the degree of oxidative stress, as described by Yousefi et al.25
Histological examination
Immediately after removal, fragments of liver tissues were fixed in a solution of 10% buffered formaldehyde. Sections of the formalin-fixed and paraffin-embedded tissues were stained with hematoxylin and eosin to semi-quantitatively assess the fatty degeneration using the NAFLD activity score (NAS). The histological features were numerically scored according to the percentage of distributions and blinded regarding treatment groups. Scores for steatosis (score 0 to 3), lobular inflammation (score 0 to 3), and ballooning (score 0 to 2), were also summed to produce the NAS, thus they ranged from 0 to 8.
Western blot analysis
Powdered liver tissue, was homogenized (10% w/v) in homogenization buffer containing 50 mM Tris-Hcl, 150 mM Nacl, 5mM Sodium Pyrophosphate (NaPPi), 50mM NaF, 1mM EDTA, 1mM dithiothreitol (DTT), 0.1%SDS (w/v), 1% TXT-100 (v/v), and protease inhibitor cocktail. Tissue homogenates were centrifuged at 1000g for 10 min at 4 °C and supernatants were stored in 50 µl aliquots at - 80 °C for further analysis. Bradford Protein Assay kit was used to evaluate the protein content of the supernatant. SDS-polyacrylamide gel electrophoresis, followed by transfer to nitrocellulose membranes was performed using 50 μg of homogenate protein. Then membranes were blocked in 5% non-fat milk in Tris-buffered saline Tween-20 and incubated overnight with rabbit antibodies against phospho-AMPK (p-AMPKThr172) and AMPK (1:1000 dilution - Cell Signaling Technology Inc., Danvers, Massachusetts, USA) in 5 % BSA (wt/vol). After extensive washing, the membranes were incubated with a peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000 dilution, Cell Signaling Technology Inc. Danvers, Massachusetts, USA) in 5% skim milk (wt/vol). After washing, antibodies were visualized using the BM Chemiluminescence kit (Roche; Germany). Densitometric analyses of immunoblots were performed using Image J software (National Institute of Health, Bethesda, Maryland).26
Statistical analysis
All data are presented as mean ± standard error (SE). Data were tested by one-way analysis of variance (ANOVA, SPSS19). When ANOVA results were significant (p<0.05), the post hoc Tukey test was also applied to find where differences existed.
Results and Discussion
Body and Tissue Weights
The initial body weight of rats was similar (p-value=0.19). At the end of the experimental period, the HFFr+S rats had a near doubling of visceral fat weight compared to the Control group (6.9±1.1 g vs. 3.4±0.4 g, p-value=0.009), while it was slightly and non-significantly lower in the other groups, especially in the rats gavaged with the high dose of metformin (200 mg/kg/day). After 60 days, no significant differences were found between the groups regarding final body weight and liver/body weight percentage (Table 2). It means that the excess energy intake of HFFr rats have led to a greater adiposity, but not to a higher body weight.
Table 2. Body and tissue weight-related measurements .
| - | Control | HFFr+S | +Met25 | +CaD+Met25 | +Met200 | +CaD+Met200 | +CaD+S |
| Initial Body Weight (g) | 213.3±6.5 | 218.7±6.8 | 208±5.6 | 203±3.9 | 201.6±6 | 203±5.4 | 221.1±9 |
| Final Body Weight (g) | 288.3±13.6 | 278.8±11.1 | 243.7±9.4 | 255.1±7 | 238.7±9.5 | 244.5±13.1 | 262.3±6.5 |
| Liver/ body weight (%) | 3.02±0.05 | 2.9±0.05 | 3±0.07 | 3.1±0.09 | 3.1±.1 | 3.1±0.09 | 2.9±0.1 |
| Visceral fat weight (g) | 3.4±0.4 | 6.9±1.1* | 5.5±0.6 | 5.1±0.3 | 4.6±0.5 | 4.7±0.3 | 5.1±0.5 |
| Visceral fat/body weight (%) | 1.1±0.13 | 2.4±0.3* | 2.3±0.2 | 2.01±0.14 | 1.91±0.14 | 2.02±0.14 | 1.94±0.2 |
HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3. Data are mean ± SE. n = 6–7 in each group. (*) P<0.05 versus the Control group. (one way ANOVA, Tukey post hoc test).
Serum Biochemistry
Compared to the Control rats, the HFFr+S rats displayed a non-significant increase in glucose levels and HOMA-IR scores and a decrease in insulin levels. The CaD+S rats had also greater glucose and lower insulin levels compared to the metformin-gavaged rats (not significantly in some cases), although no meaningful differences were observed in HOMA-IR between the groups (p-value=0.34).
Vitamin D and calcium have been linked to the improvement of insulin sensitivity, activation of the Ca2+ -mediated apoptotic pathway in adipose tissues, 14 inhibition of lipogenesis and promotion of lipid oxidation. 17,27 The beneficial effects of metformin are also thought to be through some mechanisms, including activation of AMPK, 28 and increasing insulin receptor activation, glucose uptake 29 and leptin sensitivity in liver. 30 In the present study, we tested the hypothesis that whether or not CaD augments the ameliorating effects of metformin on insulin resistance, as the major pathogenic factor of NAFLD. Surprisingly, to the contrary of our hypothesis, neither metformin nor CaD did not alter the increased levels of HOMA-IR induced by HFFr diet, so exerted their protective effects independent of insulin action. The inability of metformin therapy to reduce indexes of insulin resistance has been reported in both humans31,32and animal studies,10,33 that in part may be due to the unaltered insulin signaling within skeletal muscle and whole-body, and gluconeogenesis after metformin treatment.31,34 In this line, Linden et al.35,36 reported that metformin (300 mg/kg/day) lowered adiposity, hemoglobin A1c levels, hepatic triglycerides and markers of hepatic de novo lipogenesis in diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats, which were not accompanied by an improvement in the both fasting and postchallenge glycemic control and in the increased serum levels of triglyceride and free fatty acids. Indeed, there are some other studies which have found no effect exerted by either dietary calcium and/or vitamin D on glucose intolerance.37-41
In this study, HFFr diet, calcium-vitamin D3 supplementation and oral gavage administration of metformin did not alter the serum concentrations of triglycerides, total cholesterol and Non-HDL-C (p-value>0.05). The rats in the CaD+S group exhibited clearly higher levels of serum HDL-C compared to the groups receiving recommended levels of calcium and vitamin D3. Moreover, the levels of serum CTRP3 was significantly higher in the +Met200 group compared to the other groups (except the +CaD+Met200 group). There was no effect of HFFr diet and no additive effect of metformin and CaD on serum HDL-C and CTRP3 (Table 3).
Table 3. Serum Biochemical Parameters .
| - | Control | HFFr+S | +Met25 | +CaD+Met25 | +Met200 | +CaD+Met200 | +CaD+S |
| Triglycerides (mg/dl) | 49.3±3.8 | 41.2±4.1 | 35.1±2.7 | 48.5±2.9 | 44.2±5.2 | 45.8±6.2 | 38±6.8 |
| Cholesterol (mg/dl) | 63±4.7 | 59.6±1 | 66±3.8 | 64.2±4.5 | 56.1±4.1 | 62.6±2.3 | 65.8±3.8 |
| HDL-C (mg/dl) | 20±0.9 | 20.3±0.4a | 22.1±1.1a,c | 23.3±0.7a,b | 22.2±1.4a,c | 26±0.8b,c | 26.6±0.8b |
| Non-HDL-C (mg/dl) | 41.7±4.2 | 39.6±1.4 | 43.8±3.1 | 44.1±3.3 | 33.8±3.6 | 36.6±2.1 | 39.1±4.6 |
| Glucose (mg/dl) | 114±8.3 | 170.5±9a,b | 135±16.2a | 152.8±19.9a,b | 167.2±20.7a,b | 154.5±14.9a,b | 216.8±5.8b |
| Insulin (mg/dl) | 13.01±0.9 | 12.4±0.3a,b | 13.4±0.7a | 13.2±0.5a | 12.1±0.8a,b | 14.4±0.4a | 10.38±0.5b |
| HOMA-IR | 3.8±0.4 | 5.2±0.3 | 4.9±0.4 | 5.4±0.8 | 4.9±0.6 | 5.5±0.6 | 5.3±0.2 |
| CTRP3 (ng/ml) | 128.4±18.1 | 127.6±22.3a | 131.8±10a | 140.3±16.1a | 235.6±12.9b | 168.2±21.4a,b | 98.1±16.6a |
HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3, HDL-C: High-density lipoprotein cholesterol, HOMA-IR: Homeostatic model assessment of insulin resistance, CTRP3: Complement C1q/tumor necrosis factor-α related protein-3. Data are mean ± SE. n = 6–7 in each group. (*) P<0.05 versus the Control group. Values with different letters are significantly different (P<0.05) (one way ANOVA, Tukey post hoc test).
CTRP3 (also known as cartonectin, cartducin, CORS-26) is a novel adipokine and a member of CTRP superfamily, which has been shown to reduce glucose levels, hepatic steatosis and gluconeogenesis, by its regulatory effects on lipid and glucose metabolism.42,43 Moreover recent studies have suggested that some beneficial effects of CTRP3 could be mediated through AMPK signaling.44-46 The reduced levels of circulating and tissue expression of CTRP3 are reported in both human and rodent models of obesity and diabetes.43,47-49 Nonetheless, choi et al.50 reported an elevated circulating CTRP-3 concentrations in diabetic compared to non-diabetic adults and in the another study no difference was found between patients with and without metabolic syndrome.51 In the present study, although neither the HFFr diet nor CaD supplementation did not alter the circulating levels of this adipokine, metformin administration at a dose of 200 mg/kg led to the highest levels of CTRP3, but not the most improvement in AMPK activation and steatosis. This was, in part, in accordance with findings of Tan and coworkers52 who for the first time investigated the effects of metformin therapy on CTRP3 levels. They demonstrated lower serum and omental adipose tissue CTRP3 in women with PCOS, which was increased after 6 months of metformin therapy.
Moreover, in the present study, the HFFr+S rats showed higher levels of serum ALT and AST compared to the Control group (131.9±17.6 IU/l vs. 65.2±8.1 IU/l, p-value= 0.02 and 239.8±27.9 IU/l vs. 171.5±12 IU/l, p-value=0.12, respectively). CaD supplementation and oral gavage of metformin significantly prevented the increase in the serum concentration of AST, although ALT levels reduced non-significantly, without any differences between groups. (Figure 1).
Figure 1.

Effect of experimental diets on liver enzymes. HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase. Data are mean ± SE. n = 6–7 in each group. (*) P<0.05 versus the Control group. Values with different letters are significantly different (P<0.05) (one way ANOVA, Tukey post hoc test).
Hepatic lipid content and lipid peroxidation
The HFFr+S rats exhibited the expected increases in the hepatic fat deposition (hepatic triglyceride, 5.75±0.59 mg/g tissue weight vs. 1.83±0.25 mg/g tissue weight, p-value< 0.001 and hepatic cholesterol 1.2±0.13 mg/g tissue weight vs. 0.88±0.06 mg/g tissue weight, p-value=0.04, respectively), while it was significantly attenuated in the other groups. There was no marked differences in the hepatic triglyceride and cholesterol content between CaD-supplemented and/or metformin-gavaged groups.
HFFr diet did not increase the lipid peroxidation in livers, which was determined by measurement of hepatic MDA contents, although the concentration of hepatic MDA was significantly decreased in the +Met200 group compared to the HFFr+S group (Figure 2).
Figure 2.
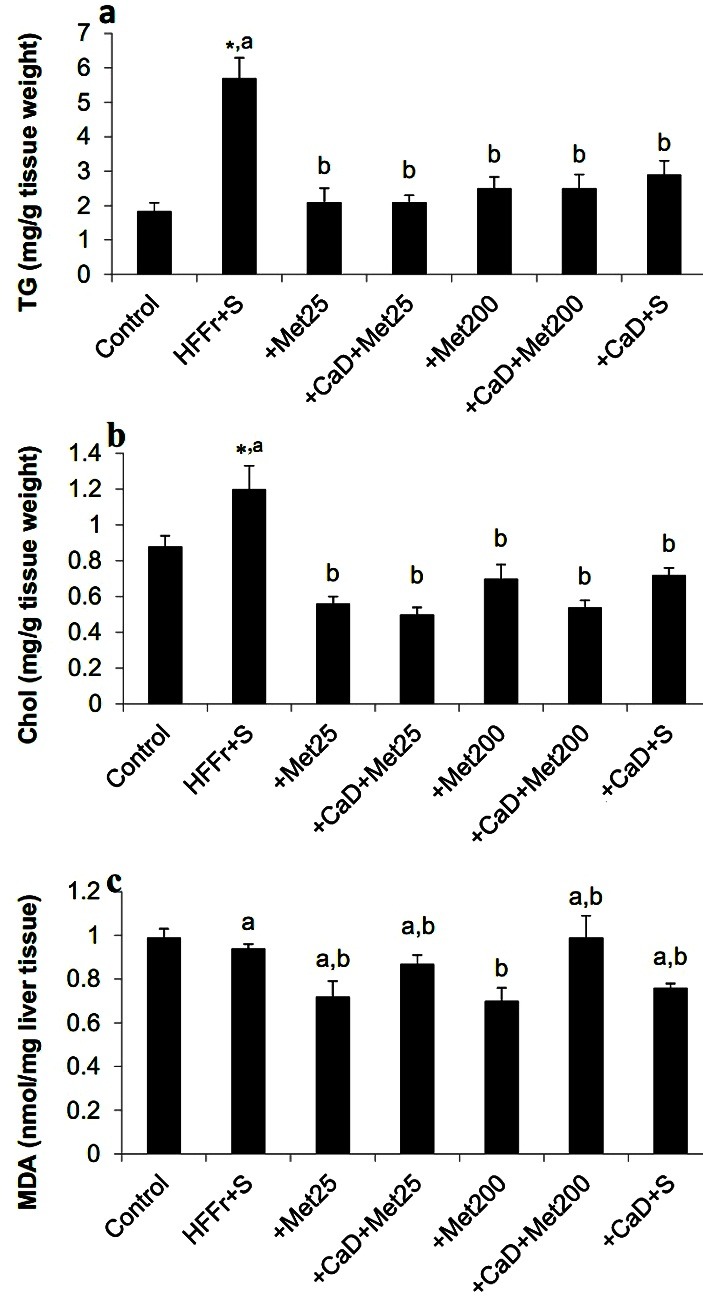
Effect of experimental diets on hepatic lipid content and malondialdehyde. a hepatic TG, b hepatic Chol, c hepatic MDA content. HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3, TG: Triglyceride, Chol: Cholesterol, MDA: Malondialdehyde. Data are mean ± SE.
n = 6–7 in each group. (*) P<0.05 versus the Control group. Values with different letters are significantly different (P<0.05) (one way ANOVA, Tukey post hoc test).
Liver Histology
The livers from HFFr+S rats displayed evidence of fatty liver, with macro and microvesicular steatosis. Although steatosis and NAS scores were significantly attenuated in the other five groups, especially in the metformin groups, compared to that in the HFFr+S group, in accordance with the hepatic triglyceride content. The hepatic histological examination of liver fragments showed no sign of inflammation, and only slightly ballooning degeneration, as is shown in Figure 3.
Figure 3.

a: Histopathological assessment after haematoxylin–eosin staining of liver sections. Sections from liver tissues of the HFFr+S rats showed hepatic steatosis and only slightly ballooning degeneration. Other five groups, especially the metformin-gavaged groups, showed lower fat accumulation (magnification×400). b: The NAFLD activity score (NAS) was determined based on histopathological analysis (steatosis, inflammation and ballooning). HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3. Data are mean ± SE. n = 6–7 in each group. (*) P<0.05 versus the Control group. Values with different letters are significantly different (P<0.05) (one way ANOVA, Tukey post hoc test).
Excessive fat accumulation in hepatocytes is the earliest and the most common manifestation of NAFLD.53In the present study, although liver weight did not differ between groups, serum markers of liver function, hepatic lipid content and steatosis scores were raised in the HFFr+S rats and similarly attenuated in the treated groups in an insulin-independent manner. However, metformin, especially in the dose of 25 mg/kg/day, showed slightly more favorable effects on steatosis and hepatic lipid content. Our present results demonstrated that using the combination of metformin and CaD, was no more effective than each of them alone on the different parameters studied. Although they slightly intensified the hepatoprotective effects of each other on HFFr diet-induced steatosis and hepatic cholesterol accumulation, but not on the increased serum liver enzymes levels and hepatic lipid peroxidation. These results are consistent with the findings of some earlier studies which have demonstrated the protective effects of each of metformin,10,11 calcium16 or 1,25 (OH)2 vitamin D3,17 separately, and CaD54 against the development of steatosis in animal models.
Calcium-vitamin D has been shown to increase the effects of metformin therapy on symptoms of women with polycystic ovary syndrome (PCOS).55,56 Nevertheless, according to the best of our knowledge, no report exists on the effect of combined calcium-vitamin D and metformin on the development of diet-induced NAFLD. Shalata et al. reported that vitamin D could intensify the effects of sitaglibtin/metformin drugs on treatment of steatosis.20 Moreover, a very recent study demonstrated the synergistic protective effects of vitamin D (6 ng/kg SC) and metformin (100 mg/kg) on the improvement of some metabolic and structural abnormalities and, hepatic steatosis induced by 8 weeks of high fat diet feeding in wistar rats.57 In this study no possible mechanism was mentioned for the observed synergistic effects, except a speculation of the involvement of AMPK.
Western blot analysis
There was no statistical difference between the seven groups with respect to the hepatic phosphorylation of AMPK (p-value>0.05) (Figure 4).
Figure 4.

Western blotting analysis of AMPK and p-AMPK expression in the livers of experimental rats. Image shows demonstrative bands of the analyzed proteins in the livers. Bars represent the ratio of phosphorylated AMPKα to total AMPKα. HFFr: High-Fat, High-Fructose diet, S: Saline, +Met25: HFFr+25 mg/kg body weight metformin, +Met200: HFFr+200 mg/kg body weight metformin, +CaD: HFFr+2.4% Calcium plus 10000 IU/kg vitamin D3, AMPK: AMP-activated protein kinase. Data are mean ± SE. n = 6–7 in each group. There were no significant differences between groups (P>0.05).
The suggested key role of AMPK in mechanism of metformin action prompted us to examine the involvement of AMPK in the observed findings. Here, we demonstrated that the phosphorylation of AMPK was not affected by neither HFFr diet nor different doses of metformin and/or calcium-vitamin D3. These findings are discordant from a large body of literature, which suggests that a high-fat diet exposure could decrease hepatic p-AMPK and metformin and calcitriol are activators of this intracellular energy sensor.19,44,58,59 In contrast, and in agreement with our findings, Foretz et al.60 reported that mice lacking hepatic AMPK exhibited normal blood glucose levels and gluconeogenic gene expression compared to those in wild-type mice. The maintenance of the antidiabetic effects of metformin in these animals as well as the reduction of intracellular ATP content clearly revealed that metformin suppressed gluconeogenesis independently of the liver-kinase B1 (LKB1)/AMPK pathway and via lowering hepatic energy status. This finding has been subsequently confirmed by other investigators,35,36,61 who disclosed the AMPK-independent effects of metformin in the management of type 2 diabetes and NAFLD, and suggested that metformin could ameliorate hepatic steatosis through silent mating type information regulation 2 homolog1 (SIRT1) -mediated effects on the autophagy machinery. In this study, we did not assess inflammatory pathways and fatty acid metabolism-related gene expressions, which could provide a direction for future research.
Conclusion
In conclusion, our results suggest that both metformin and calcium-vitamin D3 provide similar hepatoprotective effects in an insulin and AMPK-independent manner, with slightly additional protective benefits of their combination on the steatosis scores and hepatic cholesterol content. Ultimately, the increased CTRP3 levels after metformin administration was also observed in the present study.
Acknowledgments
The authors would like to express their gratitude towards Dr. Amir Abbas Farshid and Dr. Ali Asghar Tehrani for conducting the pathological assessments. The authors would also like to thank the Pharma chemie, Arya and Daana Pharmaceutical Companies and Beyza 21 Feed Mill Company. Financially, this study was supported by Urmia University of Medical Sciences.
Ethical Issues
This study was conducted in accordance with the guide for care and use of laboratory animals approved by the Animal Ethical Committee of Urmia University of Medical Sciences.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Cl Lab Sci. 2011;48(3):97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american association for the study of liver diseases, american college of gastroenterology, and the american gastroenterological association. Am J Gastroenterol. 2012;107(6):811–26. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright P, Byrne CD. Bidirectional relationships and disconnects between nafld and features of the metabolic syndrome. Int J Mol Sci. 2016;17(3):367. doi: 10.3390/ijms17030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F. et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–44. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 5.Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221–39. doi: 10.2147/CEG.S62831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex i. J Biol Chem. 2000;275(1):223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 7.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3:607–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J. et al. Role of amp-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114(2):147–52. doi: 10.1172/jci22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y. et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS One. 2012;7(9):e43056. doi: 10.1371/journal.pone.0043056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab Invest. 2012;92(7):1020–32. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Xu Q, Hong T, Tong G, Feng W, Shen S. et al. Comparative efficacy of anti-diabetic agents on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized and non-randomized studies. Diabetes Metab Res Rev. 2016;32(2):200–16. doi: 10.1002/dmrr.2713. [DOI] [PubMed] [Google Scholar]
- 13.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. Pittas AG, LThe role of vitamin d and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sergeev IN, Song Q. High vitamin d and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res. 2014;58(6):1342–8. doi: 10.1002/mnfr.201300503. [DOI] [PubMed] [Google Scholar]
- 15.Nobre JL, Lisboa PC, Santos-Silva AP, Lima NS, Manhaes AC, Nogueira-Neto JF. et al. Calcium supplementation reverts central adiposity, leptin, and insulin resistance in adult offspring programed by neonatal nicotine exposure. J Endocrinol. 2011;210(3):349–59. doi: 10.1530/joe-11-0172. [DOI] [PubMed] [Google Scholar]
- 16.Hidayat M, Prahastuti S, Tiono H, Dianawati D. Effect of calcium against weight gain and improved histopathologic fatty liver on male wistar rats that fed high fat food. Obes Res Clin Pract. 2013;7:15–6. doi: 10.1016/j.orcp.2013.08.051. [DOI] [Google Scholar]
- 17.Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin d attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest. 2012;42(11):1189–96. doi: 10.1111/j.1365-2362.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 18.He YH, Li ST, Wang YY, Wang G, He Y, Liao XL. et al. Postweaning low-calcium diet promotes later-life obesity induced by a high-fat diet. J Nutr Biochem. 2012;23(10):1238–44. doi: 10.1016/j.jnutbio.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Cao JT, Liang Y, Zhao YC, Lin XH, Li XC. et al. Calcitriol attenuates cardiac remodeling and dysfunction in a murine model of polycystic ovary syndrome. Endocrine. 2016;52(2):363–73. doi: 10.1007/s12020-015-0797-1. [DOI] [PubMed] [Google Scholar]
- 20.Shalata A, Vintfield Y, Krichevsky L, Svzalb S, Grosovski M, Djibre A. et al. The effect of vitamin d and sitaglibtin/metformin combination on hepatic fat and triglyceride content in mo-1 mice with fatty liver disease. J Hepatol. 2013;58(1):S531. [Google Scholar]
- 21.National Research Council. Nutrient requirements of laboratory animals, 4th ed. C: The National Academy Press; 1995. [Google Scholar]
- 22.Fleet JC, Gliniak C. Modeling human vitamin D status in experimental rodents. FASEB J. 2007;21(6):A1110–A1110. [Google Scholar]
- 23.Shojaei Zarghani S, Soraya H, Zarei L, Alizadeh M. Comparison of three different diet-induced non alcoholic fatty liver disease protocols in rats: A pilot study. Pharm Sci. 2015;22(1):9–15. doi: 10.15171/PS.2016.03. [DOI] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 25.Yousefi K, Soraya H, Fathiazad F, Khorrami A, Hamedeyazdan S, Maleki-Dizaji N. et al. Cardioprotective effect of methanolic extract of marrubium vulgare l. On isoproterenol-induced acute myocardial infarction in rats. Indian J Exp Biol. 2013;51(8):653–60. [PubMed] [Google Scholar]
- 26.Soraya H, Farajnia S, Khani S, Rameshrad M, Khorrami A, Banani A. et al. Short-term treatment with metformin suppresses toll like receptors (tlrs) activity in isoproterenol-induced myocardial infarction in rat: Are ampk and tlrs connected? Int Immunopharmacol. 2012;14(4):785–91. doi: 10.1016/j.intimp.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Wang L, Yan J, Liu S. Calcium ameliorates obesity induced by high-fat diet and its potential correlation with p38 mapk pathway. Mol Biol Rep. 2012;39(2):1755–63. doi: 10.1007/s11033-011-0916-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B. et al. Metformin inhibits hepatic gluconeogenesis through amp-activated protein kinase-dependent regulation of the orphan nuclear receptor shp. Diabetes. 2008;57(2):306–14. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- 29.Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab. 2003;88(3):1323–32. doi: 10.1210/jc.2002-021394. [DOI] [PubMed] [Google Scholar]
- 30.Tang X, Li J, Xiang W, Cui Y, Xie B, Wang X. et al. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J Endocrinol. 2016;230(2):227–37. doi: 10.1530/joe-16-0142. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson HK, Hallsten K, Bjornholm M, Tsuchida H, Chibalin AV, Virtanen KA. et al. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: A randomized controlled study. Diabetes. 2005;54(5):1459–67. doi: 10.2337/diabetes.54.5.1459. [DOI] [PubMed] [Google Scholar]
- 32.Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K. et al. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol. 2009;44(7):853–60. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 33.Eskens BJM, Vink H, VanTeeffelen JWGE. Improvement of insulin reistance in diet-induced obese mice by sulodexide, an endothelial glycocalyx mimetic. J Endocrinol Diabetes Obes. 2014;2:1027. [Google Scholar]
- 34.Basu R, Shah P, Basu A, Norby B, Dicke B, Chandramouli V. et al. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes. 2008;57(1):24–31. doi: 10.2337/db07-0827. [DOI] [PubMed] [Google Scholar]
- 35.Linden MA, Lopez KT, Fletcher JA, Morris EM, Meers GM, Siddique S. et al. Combining metformin therapy with caloric restriction for the management of type 2 diabetes and nonalcoholic fatty liver disease in obese rats. Appl Physiol Nutr Metab. 2015;40(10):1038–47. doi: 10.1139/apnm-2015-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden MA, Fletcher JA, Morris EM, Meers GM, Kearney ML, Crissey JM. et al. Combining metformin and aerobic exercise training in the treatment of type 2 diabetes and nafld in oletf rats. Am J Physiol Endocrinol Metab. 2014;306(3):E300–10. doi: 10.1152/ajpendo.00427.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkharfy KM, Al-Daghri NM, Yakout SM, Hussain T, Mohammed AK, Krishnaswamy S. Influence of vitamin d treatment on transcriptional regulation of insulin-sensitive genes. Metab Syndr Relat Disord. 2013;11(4):283–8. doi: 10.1089/met.2012.0068. [DOI] [PubMed] [Google Scholar]
- 38.de Wit NJ, Bosch-Vermeulen H, Oosterink E, Muller M, van der Meer R. Supplementary dietary calcium stimulates faecal fat and bile acid excretion, but does not protect against obesity and insulin resistance in c57bl/6j mice. Br J Nutr. 2011;105(7):1005–11. doi: 10.1017/s0007114510004654. [DOI] [PubMed] [Google Scholar]
- 39.Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K. et al. Effects of combined calcium and vitamin d supplementation on insulin secretion, insulin sensitivity and beta-cell function in multi-ethnic vitamin d-deficient adults at risk for type 2 diabetes: A pilot randomized, placebo-controlled trial. PLoS One. 2014;9(10):e109607. doi: 10.1371/journal.pone.0109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geldenhuys S, Hart PH, Endersby R, Jacoby P, Feelisch M, Weller RB. et al. Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin d in mice fed a high-fat diet. Diabetes. 2014;63(11):3759–69. doi: 10.2337/db13-1675. [DOI] [PubMed] [Google Scholar]
- 41.Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high-dose vitamin d supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29(5):620–9. doi: 10.3904/kjim.2014.29.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. Ctrp3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol. 2013;305(3):G214–24. doi: 10.1152/ajpgi.00102.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson JM, Wei Z, Wong GW. C1q/tnf-related protein-3 (ctrp3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285(51):39691–701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J. et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9(3):e91111. doi: 10.1371/journal.pone.0091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, Zhou Y, Yang B, Li L, Yu S, Chen Y. et al. C1q/tumor necrosis factor-related protein-3 attenuates brain injury after intracerebral hemorrhage via ampk-dependent pathway in rat. Front Cell Neurosci. 2016;10:237. doi: 10.3389/fncel.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang CL, Feng H, Li L, Wang JY, Wu D, Hao YT. et al. Globular ctrp3 promotes mitochondrial biogenesis in cardiomyocytes through ampk/pgc-1alpha pathway. Biochim Biophys Acta. 2017;1861(1 Pt A):3085–94. doi: 10.1016/j.bbagen.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Ban B, Bai B, Zhang M, Hu J, Ramanjaneya M, Tan BK. et al. Low serum cartonectin/ctrp3 concentrations in newly diagnosed type 2 diabetes mellitus: In vivo regulation of cartonectin by glucose. PLoS One. 2014;9(11):e112931. doi: 10.1371/journal.pone.0112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower circulating c1q/tnf-related protein-3 (ctrp3) levels are associated with obesity: A cross-sectional study. PLoS One. 2015;10(7):e0133955. doi: 10.1371/journal.pone.0133955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Jiang L, Yang M, Wu YW, Sun SX, Sun JZ. Expression of ctrp3, a novel adipokine, in rats at different pathogenic stages of type 2 diabetes mellitus and the impacts of glp-1 receptor agonist on it. J Diabetes Res. 2014;2014:398518. doi: 10.1155/2014/398518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ. et al. C1q/tnf-related protein-3 (ctrp-3) and pigment epithelium-derived factor (pedf) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61(11):2932–6. doi: 10.2337/db12-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS. et al. Implication of progranulin and c1q/tnf-related protein-3 (ctrp3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8(2):e55744. doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan BK, Chen J, Hu J, Amar O, Mattu HS, Adya R. et al. Metformin increases the novel adipokine cartonectin/ctrp3 in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(12):E1891–900. doi: 10.1210/jc.2013-2227. [DOI] [PubMed] [Google Scholar]
- 53.Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239(1):192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 54. Shojaei Zarghani S, Soraya H, Alizadeh M. Calcium and vitamin D3 combinations improve fatty liver disease through ampk-independent mechanisms. Eur J Nutr 2016. [DOI] [PubMed]
- 55.Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin d and metformin on polycystic ovary syndrome: A pilot study. Taiwan J Obstet Gynecol. 2009;48(2):142–7. doi: 10.1016/s1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- 56.Tehrani HG, Mostajeran F, Shahsavari S. The effect of calcium and vitamin d supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with poly cystic ovarian syndrome. J Res Med Sci. 2014;19(9):875–80. [PMC free article] [PubMed] [Google Scholar]
- 57.Mostafa DK, Nasra RA, Zahran N, Ghoneim MT. Pleiotropic protective effects of vitamin d against high fat diet-induced metabolic syndrome in rats: One for all. Eur J Pharmacol. 2016;792:38–47. doi: 10.1016/j.ejphar.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Yogalakshmi B, Bhuvaneswari S, Sreeja S, Anuradha CV. Grape seed proanthocyanidins and metformin act by different mechanisms to promote insulin signaling in rats fed high calorie diet. J Cell Commun Signal. 2014;8(1):13–22. doi: 10.1007/s12079-013-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of ampka in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339(2):701–7. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 60.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the lkb1/ampk pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–69. doi: 10.1172/jci40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS. et al. Metformin alleviates hepatosteatosis by restoring sirt1-mediated autophagy induction via an amp-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]


