Abstract
Introduction
Clinical studies suggest that HIV-1-infected patients are more likely to use or abuse addictive drugs than is the general population. We hypothesized that HIV-1 proteins impact novelty-seeking behavior and enhance the transcriptional response to nicotine in genes implicated in both novelty-seeking behavior and drug addiction.
Methods
We assessed the effects of HIV-1 proteins on novelty-seeking behavior by comparing baseline activity differences of HIV-1Tg and F344 control rats in the open-field test. One day after behavioral testing, all rats began daily subcutaneous injections of either nicotine (0.4 mg/kg, base) or saline (the same for each rat) for 27 days. At the end of treatment, the prefrontal cortex, nucleus accumbens, and ventral tegmental area were collected for RNA expression analysis of genes in the receptor families for dopamine, GABA, glutamate, and serotonin.
Results
Significant strain difference was detected in the distance moved in the center, such that HIV-1Tg rats traveled greater distance in the center of the arena than did F344 rats. Quantitative RT-PCR analysis showed that mRNA from Drd3 and Grm2 in the prefrontal cortex and Drd5 and Gabra6 in the ventral tegmental area was significantly upregulated, whereas that of Drd5 in the nucleus accumbens was downregulated in HIV-1Tg rats compared with F344 rats. Further, more addiction-related genes were significantly modulated by nicotine in each brain region in the HIV-1Tg rats than in the control animals.
Conclusions
HIV-1 proteins may affect novelty-seeking behavior and modulate the expression of genes related to drug addiction and novelty-seeking behavior.
Implications
HIV-1 viral proteins and chronic nicotine treatment impact the expression of genes involved in novelty-seeking behavior and addiction in three brain regions of the HIV-1 transgenic rat. These findings implicate that HIV-1 proteins may be involved in novelty-seeking behavior and in modulating the expression of genes related to drug addiction and novelty seeking.
Introduction
According to the United Nations Program on HIV/AIDs, approximately 16 million persons utilize drugs of abuse; among them, about 3 million worldwide are living with HIV-1 infection.1 It has been hypothesized that HIV-1 infection alters the structure and function of the central nervous system (CNS) reward pathways, as the infection is associated with drugs of abuse at both the cellular and molecular levels.2,3 It is speculated that these alterations in the CNS make such individuals more vulnerable to the rewarding effects of drugs of abuse.4–8 Although this hypothesis has been supported by clinical evidence showing that the percentage of HIV-infected patients who use various addictive substances such as alcohol, nicotine, morphine/heroin, methamphetamine, and cocaine is greater than that in the general population,6 the molecular mechanisms remain largely unknown.
HIV-1 infection involves the actions of viral proteins on targeted cells of the immune system, such as macrophages and T lymphocytes.9–11 Like the peripheral immune system, the CNS is highly vulnerable to HIV-1 infection. The viral proteins penetrate the CNS during the early phases of the infection and selectively target dopamine-rich regions, particularly the mesocortical and mesoaccumbens dopamine circuits.12–14 Further, the HIV-1 proteins Tat and gp120 are toxic to dopaminergic neurons, causing dopamine depletion in subcortical structures15,16 and contributing to the development of neurologic complications of HIV infection.5,17,18 These protein-induced alterations could contribute to a higher risk of substance abuse development because of the increased sensitivity of the brain to the pleasurable effects of psychostimulants.17 Thus, it is reasonable to assume that HIV-infected patients are more prone to escalate their drug-intake behavior as a result of viral protein-induced changes in dopamine-rich brain regions. The prefrontal cortex–ventral tegmental area–nucleus accumbens (PFC-VTA-NAc) neural circuits are critical for elicitation of reward perception, goal-directed behavior, and habit formation19,20 and are targeted by HIV-1 proteins.21,22 However, it is less clear how viral protein-induced changes in the PFC–VTA–NAc circuitry of neurotransmitter systems mediate the neural and behavioral responses to drugs of abuse.
In addition to molecular changes, persons with long-standing HIV-1 infection demonstrate a greater change in risk-taking and safety assessment behaviors.23 It has been proposed that an alteration in this equilibrium renders HIV-infected patients more vulnerable to drugs of abuse. Nicotine, in the form of cigarette smoking, is the preferred drug of abuse among HIV-infected patients.24 Clinical studies also show that smokers with HIV-1 infection engage in other risky behaviors such as unprotected sex and have higher rates of abuse of alcohol and club drugs25,26 despite their knowledge of the long-term negative consequences of these actions.
Studies of humans and of animal models of nicotine addiction reveal that novelty seeking as a component of risk behavior can predict the likelihood of compulsive use of cigarettes. Individuals who display high novelty-seeking behavior consume more cigarettes than do low novelty seekers.27–29 Previous experiments with mice have shown that novelty-seeking behavior correlates with consistent nicotine use.30,31 Both nicotine effects and novelty-induced reward feedback can be localized to the mesolimbic circuitry with a prominent effect on dopamine.32,33 Molecular mechanistic studies indicate that both novel stimuli and psychostimulant drugs, such as nicotine, affect behavior by producing a strong neuromodulator response through activating the cholinergic,34–37 GABA, glutamatergic, and serotogenergic systems.38 However, there is limited knowledge of the effects of HIV-1 proteins on the molecular mechanisms underlying long-term changes in novelty-seeking behavior and response to nicotine.
In this study, we used both behavioral and molecular approaches to determine whether long-term HIV infection modifies high-risk behaviors and alters the molecular response to nicotine. The HIV-1Tg rat model used carries a gag-pol-deleted HIV-1 genome under the control of the HIV-1 viral promoter and expresses seven of the nine HIV-1 genes.39 The HIV-1Tg rat displays symptoms similar to those of human HIV patients receiving highly active antiretroviral therapy (HAART) and shows greater sensitivity to many psychostimulants, including morphine, alcohol, and nicotine.40–44 For our behavioral analysis, we measured novelty-seeking behavior using the open-field arena test45–47 to determine the modifying effects of viral proteins on behavior and vulnerability to drug abuse. Subsequently, we examined the expression of genes involved in dopaminergic, GABAergic, and glutamatergic neurotransmitter systems and related to nicotine- and novelty-induced reward behaviors. Such molecular studies were conducted in order to determine how gene expression is altered by viral proteins and chronic nicotine treatment.
Materials and Methods
Animals
Male HIV-1Tg rats and F344 genetic-background control rats (Harlan Industries, Cortland, NY) were used at 7–8 weeks of age. All rats were group-housed in standard plastic rat cages, maintained in a temperature (20oC–22oC)- and humidity (45%–55%)-controlled environment with a 12-hour light/dark cycle. Food and water were provided ad libitum. All experimental procedures were conducted during the light cycle and approved by the University of Virginia Animal Care and Use Committee.
Drug, Treatment and Behavioral Testing
(−)-Nicotine hydrogen tartrate (Sigma, St Louis, MO) was dissolved in 0.9% saline, and its concentration was calculated as nicotine free base. Rats from each strain were divided randomly into two groups: saline-treated control and nicotine-treated experimental, designated as follows: F344_Saline (n = 11); F344_Nicotine (n = 12); HIV-1Tg_Saline (n = 9); and HIV-1Tg_Nicotine (n = 11). To determine whether HIV-1 proteins or chronic nicotine treatment modified the function of the central reward system and related behaviors, baseline novelty-seeking behavior was investigated at 8 weeks of age. The open-field test, a widely used method to measure novelty-seeking behavior, was adopted.45 Briefly, novelty-seeking behavior was assessed by recording a unit of activity each time a rat moved from the center of the box to the sides or from the sides to the center by using the Any-Maze video tracking system for 10 minutes (Stoelting Co., Wood Dale, IL). The data on time mobile (total amount of time that the animal was mobile during testing), total distance traveled, and distance traveled in the center and peripheral zones of the open-field arena were automatically tracked by Any-Maze software and analyzed as dependent variables. The test apparatus consisted of a clear plastic box (50 × 50 × 50 cm) raised 28 cm above the ground.45–47 Each rat was placed at the center of the box and allowed to move freely through the apparatus without reinforcements or inhibitions.
One day after the open-field test, rats were injected subcutaneously with either saline or nicotine once per day (0.4 mg/kg/day) for 27 days before brain tissues were collected. The duration of nicotine exposure was chosen according to our recently reported findings48,44 demonstrating that this amount of time can alter mRNA concentrations in the brains of HIV-1Tg rats. This choice was shown to be appropriate, given that our results demonstrated both behavioral and molecular alterations among nicotine-treated in comparison with saline-treated rats. The concentration of nicotine was selected on the basis of our recently reported results.44 Because of the congenital cataracts in HIV-1Tg rats,49 all behavioral experiments were conducted under dimmed red light in order to minimize visual differences between strains.
On day 28, the animals were sacrificed, and different brain regions were collected. Using a rat brain matrix (Kent Scientific, Torrington, CT), 1-mm slices were taken from each brain. The slices containing the prefrontal cortex (PFC; second frontal area), ventral tegmental area (VTA), and nucleus accumbens (NAc) were identified according to the rat brain atlas.50 These regions were chosen because of their implications for the reward system, which can be activated by both nicotine and novel stimuli. Tissue from the regions of interest was collected using a 1.5-mm brain punch (Stoelting, Wood Dale, IL) and stored at −80°C until use. We did not pool brain tissues from multiple animals.
RNA Extraction and Primer Design
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The purity and quantity of total RNA were measured at optical densities of 260 nm and 280 nm with the NanoDrop 2000c (Thermo Scientific, Waltham, MA). The primers for all 17 genes examined were designed using Primer Express (v. 3.0) software (Applied Biosystems). To avoid amplifying genomic DNA, two primers of each gene of interest were designed to span at least one intron. Each pair of primers and their amplicon sequences were tested using the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi) to ensure the specificity of the primers for the targeted genes. Dissociation curves were generated to check the specificity of the primers before including them in the qRT-PCR array. The primer sequences used are shown in Supplementary Table 1.
Quantitative RT-PCR (qRT-PCR) Array
A custom-designed RT-PCR array was used to measure gene expression as described previously. 44,51–53 Briefly, 2 μg of total RNA was reverse transcribed into first-strand cDNA using Superscript II Reverse Transcriptase. The cDNA mixture was incubated at 25°C for 10 minutes, 42°C for 1.5 hour, and 70°C for 15 minutes and then amplified in a volume of 10 μL containing 5.0 μL of 2 × Power SYBR Green PCR Master Mix (Applied Biosystems) and combined sense and antisense primers (2.5 μL). The final concentration was 20 nM in a 384-well plate using the 7900HT Sequence Detection System (Applied Biosystems). The PCR conditions were as follows: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. After the last cycle, a dissociation curve was generated to check for non-specific products.
Statistical Analysis
Graphpad prism (v. 6.01) was used for the statistical analyses, and data are shown as mean ± S.E.M. The unpaired two-tailed t test was employed to determine group differences in the measurements of novelty-seeking behavior. Expression of each gene of interest was first normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and then analyzed using a comparative Ct method.54 The relative expression of each gene was compared between the groups of HIV-1Tg control versus F344 control (to determine viral effects), HIV-1Tg nicotine versus HIV-1Tg saline (to determine the pharmacological effects of nicotine in the presence of viral proteins), and F344 nicotine versus F344 saline (to determine the pharmacological effects of nicotine in the absence of viral proteins) rats using the Student t test. Significant alteration in mRNA expression was defined as a fold change > 20% with a p value < .05 (N = 4–6 per group). According to the literature, a difference of less than 20% between two groups is less likely to be biologically significant.55,56
Results
HIV-1Tg Rats Showed Greater Novelty-Seeking Behavior than F344 Control Rats
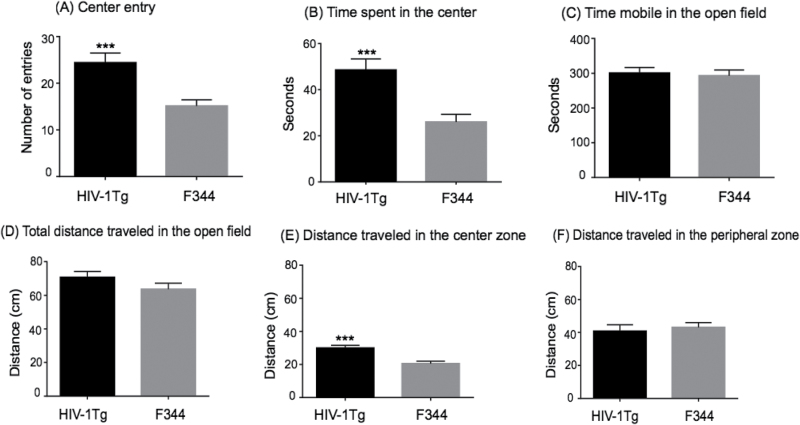
To investigate the role of HIV-1 proteins in novelty-seeking behavior, HIV-1Tg and F344 rats were tested in the open-field arena. Figure 1 shows the strain differences in the measurements of novelty-seeking behavior between the two groups. There was a significant strain difference in the baseline measurements. The HIV-1Tg rats spent more time in the center of the arena (t37 = 3.924; p = .0004) and made more entries into the center (t37 = 3.907; p = .0004) than did the F344 rats (Figure 1A and B). No significant differences were observed in the mobility scores between HIV-1Tg and F344 rats (Figure 1C). Ambulation was also measured as total distance traveled in the open-field arena. Results showed that there was a significant effect of strain on distance traveled in the center zone of the arena, such that HIV-1Tg rats traveled greater distance in the center than did F344 rats (t37 = 4.187; p = .0002) (Figure 1E). Total distance traveled and distance moved in the peripheral zone during the 10-minutes of testing in the open-field was not significantly different between HIV-1Tg and F344 rats (Figure 1D and F). Taken together, these data demonstrate an increase in novelty-seeking behavior in HIV-1Tg rats compared with control animals, suggesting that viral proteins affect the central reward circuit of the brain, which controls novelty-seeking behavior.
Figure 1.
Difference in baseline measures of novelty-seeking behavior in HIV-1Tg and F344 rats in the open-field arena test (N = 9–12 per group). The HIV-1Tg rats spent more time in, exhibited higher numbers of entries into, and traveled greater distance in the center zone of the arena compared with F344 rats. Difference from F344 rats ***p ≤ .001.
Effect of Nicotine or HIV-1 Proteins on Expression of Genes in the NAc that Are Implicated in Drug Addiction and Novelty-Seeking Behavior
As shown in Table 1, our qRT-PCR array analysis revealed that 6 of 17 genes implicated in drug addiction were significantly changed, at p ≤ .05, by nicotine or HIV-1 proteins in the NAc of HIV-1Tg or F344 rats. In the F344 rats, only the GABAA receptor α5 subunit gene (Gabra5) showed significant upregulation, 135%, by nicotine (p = .0001). In the HIV-1Tg rats, nicotine significantly upregulated the expression of Drd5 (132.5%; p = .002), Gabra1 (96%; p = .007), Gabra6 (97.6%; p = .007), and metabotropic glutamate receptor 1 (Grm1) (38%; p = .011) but significantly downregulated the expression of Gabra2 (−18.7%; p = .012) and Grm5 (−30.1%; p = .012). Compared with the effects of nicotine, only the expression of Drd5 was downregulated by HIV-1 proteins (−55.9%; p = .025). Thus, more genes were significantly modulated by nicotine in HIV-1Tg rats than in F344 rats, suggesting that the presence of viral proteins could alter the susceptibility of animals to nicotine addiction.
Table 1.
Effect of Nicotine on Expression of Genes Related to Drug Addiction and Novelty-Seeking Behavior in the Nucleus Accumbens of F344 and HIV-1Tg Rats
| Gene symbol | F344 rat | HIV-1Tg rat | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio (Nic/Sal) | p | Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio (Nic/Sal) | p | Ratio (HIV-1Tg/F344) | p | |
| Drd5 | 0.00046 ± 0.000229 | 0.000348 (0.000236) | 0.757 | .660 | 0.000203 ± 5.11E-05 | 0.000472 ± 0.000157 | 2.325 | .002 | 0.441 | .025 |
| Gabra1 | 0.1380 ± 0.0941 | 0.1231 ± 0.0892 | 0.892 | .785 | 0.0888 ± 0.0117 | 0.1741 ± 0.0534 | 1.961 | .007 | 0.643 | .280 |
| Gabra2 | 0.1926 ± 0.0535 | 0.2217 ± 0.0296 | 1.151 | .272 | 0.1491 ± 0.0108 | 0.1212 ± 0.0133 | 0.813 | .012 | 0.774 | .154 |
| Gabra5 | 0.0170 ± 0.0041 | 0.0363 ± 0.0035 | 2.135 | .0001 | 0.0229 ± 0.0074 | 0.0205 ± 0.0150 | 0.895 | .724 | 1.347 | .186 |
| Gabra6 | 0.000153 ± 0.0001 | 0.000202 ± 0.000135 | 1.320 | .490 | 0.000169 ± 3.49E-05 | 0.000334 ± 9.74E-05 | 1.976 | .007 | 1.105 | .950 |
| Grm1 | 0.0248 ± 0.0112 | 0.0213 ± 0.0019 | 0.859 | .512 | 0.0181 ± 0.0040 | 0.0250 ± 0.0027 | 1.381 | .011 | 0.730 | .197 |
| Grm5 | 0.1730 ± 0.0421 | 0.1620 ± 0.0414 | 0.936 | .658 | 0.1663 ± 0.0337 | 0.1162 ± 0.0211 | 0.699 | .012 | 0.961 | .770 |
Significant p values shown in boldface type.
Effect of Nicotine and HIV-1 Proteins on Expression in the PFC of Genes that Are Implicated in Drug Addiction and Novelty-Seeking Behavior
Of the 17 genes examined in the PFC, we found that the expression of Drd3 (143%; p = .011) and metabotropic glutamate receptor 2 (126%; p = .014) was significantly upregulated by viral proteins in HIV1-1Tg rats (see Table 2). By comparing the saline-treated F344 with HIV-1Tg rats, we found that viral proteins significantly increased the expression of Drd3 (143%; p = .011) and Grm2 (126.2%; p = .014).
Table 2.
Effect of Nicotine on Expression of Genes Related to Drug Addiction and Novelty-Seeking Behavior in the Prefrontal Cortex of F344 and HIV-1Tg Rats
| Gene symbol | F344 rat | HIV-1Tg rat | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio (Nic/Sal) | p | Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio (Nic/Sal) | p | Ratio (HIV-1Tg/F344) | p | |
| Drd1a | 0.00340 ± 0.001 | 0.00321 ± 0.000748 | 0.944 | .723 | 0.0034 ± 0.0001 | 0.0024 ± 0.0004 | 0.717 | .010 | 1.00 | .951 |
| Drd2 | 0.000143 ± 2.46E-05 | 0.000180 ± 4.81E-05 | 1.259 | .189 | 0.000296 ± 0.000139 | 0.000133 ± 1.92E-05 | 0.449 | .032 | 2.07 | .070 |
| Drd3 | 1.80E-05 ± 8.58E-06 | 2.27E-05 ± 1.04E-05 | 1.261 | .439 | 4.37E-05 ± 1.63E-05 | 2.66E-05 ± 6.59E-06 | 0.609 | .038 | 2.43 | .011 |
| Drd5 | 0.000102 ± 6.62E-05 | 7.78E-05 ± 2.34E-05 | 0.763 | .406 | 7.94E-05 ± 1.46E-05 | 4.06E-05 ± 1.61E-05 | 0.511 | .007 | 0.778 | .514 |
| Gabra3 | 0.0398 ± 0.00880 | 0.0534 ± 0.00974 | 1.342 | .040 | 0.0409 ± 0.00694 | 0.0410 ± 0.00785 | 1.002 | .973 | 1.028 | .836 |
| Gabra5 | 0.0430 ± 0.00973 | 0.0381 ± 0.00678 | 0.886 | .337 | 0.0470 ± 0.0200 | 0.0247 ± 0.00263 | 0.526 | .037 | 1.093 | .672 |
| Grm2 | 0.0084 ± 0.0017 | 0.0150 ± 0.00145 | 1.788 | .0001 | 0.0134 ± 0.00282 | 0.0190 ± 0.00441 | 1.418 | .026 | 2.262 | .014 |
Significant p values shown in boldface type.
In the F344 rats, nicotine significantly upregulated the expression of Gabra 3 (34.2%; p = .04) and metabotropic glutamate receptor 2 (Grm2) (78.8%; p = .0001). In the HIV-1Tg rats, nicotine significantly upregulated the expression of Grm2 (41.8%; p = .026) but significantly downregulated the expression of five genes, namely Drd1a (−28.3%; p = .01), Drd2 (−55.1%; p = .032), Drd3 (−39.1%; p = .038), Drd5 (−48.9%; p = .007), and Gabra5 (−47.4%; p = .037).
Effect of Nicotine and HIV-1 Proteins on Expression in the VTA of Genes that Are Implicated in Drug Addiction and Novelty-Seeking Behavior
With the same qRT-PCR approach, we examined expression of the same set of genes in the VTA region of the brains of HIV-1Tg and F344 rats treated with saline or nicotine (Table 3). Nicotine significantly upregulated the expression of Gabra6 (334%; p = .022) but downregulated the expression of Grm1 (−50%; p = .002) and Grm2 (−61.5%; p = .029) in the F344 rats. In HIV-1Tg rats, nicotine significantly downregulated the expression of Drd5, Gabra6, Grm1, Grm2, and Grm5, by −28.8% to −55.5%, with a p value of .041 to .002. In addition, HIV-1 proteins significantly upregulated the expression of Drd5 (110%; p = .002) and Gabra6 (282.4%; p = .006) in the VTA.
Table 3.
Effect of Nicotine on Expression of Genes Related to Drug Addiction and Novelty-Seeking Behavior in the Ventral Tegmental Area of F344 and HIV-1Tg Rats
| Gene symbol | F344 rat | HIV-1Tg rat | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio (Nic/Sal) | p | Saline (Mean ± SD) | Nicotine (Mean ± SD) | Ratio Nic/Sal) | p | Ratio (HIV-1Tg/F344) | p | |
| Drd5 | 0.000667 ± 0.000336 | 0.000496 ± 0.000122 | 0.744 | .267 | 0.0014 ± 0.0002 | 0.000658 ± 0.000248 | 0.470 | .002 | 2.099 | .005 |
| Gabra6 | 7.06E-05 ± 4.84E-05 | 0.000306 ± 0.000204 | 4.334 | .022 | 0.000274 ± 0.000117 | 0.000122 ± 9.27E-05 | 0.445 | .040 | 3.824 | .006 |
| Grm1 | 0.0228 ± 0.0043 | 0.0114 ± 0.0042 | 0.500 | .002 | 0.0236 ± 0.0062 | 0.0168 ± 0.0035 | 0.712 | .041 | 1.035 | .791 |
| Grm2 | 0.0156 ± 0.0081 | 0.0060 ± 0.0045 | 0.385 | .029 | 0.0167 ± 0.0048 | 0.0110 ± 0.0025 | 0.659 | .033 | 1.071 | .952 |
| Grm5 | 0.0397 ± 0.0050 | 0.0339 ± 0.0173 | 0.854 | .451 | 0.0589 ± 0.0269 | 0.0273 ± 0.0064 | 0.463 | .020 | 1.484 | .321 |
Significant p values shown in boldface type.
Discussion
The findings of the current study extend our knowledge of HIV-1 infection such that the viral proteins were shown to affect novelty-seeking behavior in the open-field test by altering gene expression related to major neurotransmitter systems in the brain. HIV-1Tg rats exhibited high novelty-seeking behavior with an abnormal baseline expression of D1- and D2-like dopamine and GABA receptors in the PFC–VTA–NAc neural circuits. We also found that nicotine differentially affects the transcription of excitatory and inhibitory neurotransmitter receptors in HIV-1Tg rats compared with F344 control rats. HIV-1Tg rats presented high levels of novelty-seeking behavior, suggesting that the viral proteins alter the central reward system by changing gene expression related to the response to nicotine.
Among humans, novelty seeking is one of the key personality traits associated with engaging in high-risk behaviors and with the risk of developing substance abuse. Recent studies of drug addiction in neuroAIDS have suggested that the use of addictive drugs is related to high-risk behaviors in persons infected with HIV-1. However, how the virus modifies this association during HIV-1 infection remains largely unclear. To explore this connection, we first investigated whether viral proteins cause any change in novelty-seeking behavior by using HIV-1Tg rats as an animal model that mimics the conditions present in human HIV-1 infection. HIV-1-infected patients receiving HAART show controlled viral replication with a persistent reservoir of viral proteins. These patients demonstrate mild to moderate cognitive deficits and progression of neurodegeneration. The HIV-1Tg rat mimics such patients, as these animals express seven of the nine viral proteins with controlled viral replication. Also, progression of cognitive and motor deficits occurs in such rats, similar to that seen in patients receiving HAART.57–59 Our model attempts to replicate the biology of HIV-1 infected individuals receiving HAART who are chronic smokers. We showed that viral proteins increase novelty-seeking behavior, as HIV-1Tg rats displayed increased time in, made more entries into, and traveled more distance in the center of the arena (see Figure 1) than did the control rats. These results are consistent with clinical findings showing that drug-dependent HIV-1-positive patients exhibit higher scores in novelty seeking and harm avoidance and lower scores in self-directedness relative to healthy controls according to the measurement of personality profile using the Temperament and Character Inventory.60 However, our results show differences from the preclinical results, in that HIV-1Tg rats displayed a low locomotor response to placement in a novel environment.61 Other authors used adult female ovariectomized HIV-1Tg rats and tested novelty-seeking behavior in the activity chamber at 6, 7, and 11 months of age. These HIV-1Tg rats constantly exhibited a weaker response across monthly spaced testing of novelty-seeking behavior compared with control animals.61 The discrepancy between our findings and those of Moran et al.61 may indicate that there are age- and sex-related differences in sensitivity to HIV-1 proteins in the CNS that could result in divergent neurobehavioral adaptations during HIV infection. Similarly, a previous study involving age-dependent motor function in HIV-1Tg rats found that ethanol-treated adult rats demonstrated a significant decrease in locomotor activity in the open-field test compared with water-treated controls.43 However, no difference was observed between ethanol- and water-treated adolescent HIV-1Tg rats in that study.43 Further research is needed to examine whether there are sex- and age-specific differences in the pathogenesis of neurobehavioral diseases in response to HIV-1 infection. Data from total distance traveled in the arena and distance traveled in the peripheral zone of the arena indicated no significant differences between strains; such results may be understood in the context of such extrapolated data on locomotor activity. Namely, HIV-1Tg rats traveled more distance in the center of the arena but may have traveled less distance in the peripheral zone, contributing to the result that both strains traveled similar total distances. The center zone of the arena, a novel environment, may have stimulated the rats’ locomotor activity. However, we would like to note that locomotion has been found to “weakly predict” psychostimulant reward.45 Future studies aimed at more directly measuring reward- or drug-seeking behavior, such as conditioned place preference or drug self-administration, respectively, should be conducted with nicotine.
Next, we extended our behavioral findings by examining potential molecular mechanisms involved in the synergistic effects of HIV-1 proteins and nicotine in the neural circuits of the PFC–VTA–NAc (see Table 4). For this objective, we analyzed the expression patterns of genes implicated in drug addiction and novelty-seeking behavior in HIV-1Tg and F344 rats after repeated nicotine or saline injections. In the saline-treated groups, HIV-1 proteins significantly increased the baseline mRNA concentrations of D2-like dopamine receptor Drd3 in the PFC and D1-like dopamine receptor Drd5 in the VTA, whereas they decreased the baseline expression of Drd5 in the NAc of HIV-1Tg rats compared with the control rats. These results indicate that HIV-1 proteins lead to regionally distinct alterations in the mesocorticolimbic dopaminergic system. The HIV-1 proteins selectively target dopamine-rich regions in the brain and impair baseline dopaminergic synaptic transmission.12,14 The prefrontal dopaminergic circuits of the mesocorticolimbic system are the main targets of HIV-1 proteins and undergo disease-related alterations in synaptic connections that could lead to neurocognitive disorders.15,62–64 Our results provide further evidence for the idea that HIV-1 proteins have a selective effect on PFC dopaminergic activity by enhancing the amounts of D2-like dopamine receptors that may regulate the baseline dopamine quantities in the NAc. Such differences in the mesocortical and mesoaccumbens dopaminergic systems might contribute to the enhanced striatal response to novel stimuli and addictive drugs, such as nicotine, in HIV-1Tg rats.
Table 4.
Summary of Genes Significantly Modulated by Nicotine and HIV-1 Proteins in NAc, PFC, and VTA of HIV-1Tg and F344 Control Rats
| Brain region | Modulation | Nicotine | Viral effect | |
|---|---|---|---|---|
| F344 rats | HIV-1Tg rats | |||
| NAc | Upregulation | Gabra5 | Drd5, Gabra1, Gabra6, Grm1 | — |
| Downregulation | — | Gabra2, Grm5 | Drd5 | |
| PFC | Upregulation | Gabra3, Grm2 | Grm2 | Drd3, Grm2 |
| Downregulation | — | Drd1a, Drd2, Drd3, Drd5, Gabra5 | — | |
| VTA | Upregulation | Gabra6 | — | Drd5, Gabra6 |
| Downregulation | Grm2, Grm5 | Drd5, Gabra6, Grm1, Grm2, Grm5 | — | |
Nac = nucleus accumbens; PFC = prefrontal cortex; VTA = ventral tegmental area.
Nicotine can directly or indirectly activate the central reward pathway by increasing dopamine through a number of mechanisms. Activation of VTA dopaminergic neurons through binding of nicotine to nicotinic acetylcholine receptors (nAChR) results in a direct increase in the extracellular dopamine concentration within the NAc. Nicotine also can indirectly modulate dopaminergic transmission in the central reward circuit by binding to nAChRs on glutamatergic and GABAergic neurons in the VTA.65,66 In our study, nicotine showed a strain-specific pattern of gene expression involved in GABAergic neurotransmission in the PFC–VTA circuits. Chronic nicotine treatment decreased the mRNA of genes encoding GABAergic receptors in HIV-1Tg rats, whereas the mRNA of these genes was increased in F344 rats. Further, a synergistic nicotine effect was found in dopaminergic transmission in the NAc, in that the mRNA level of D2-like dopamine receptor Drd5 was upregulated in HIV-1Tg rats. In contrast, no significant change in mRNA concentrations of dopaminergic receptors was found in the NAc of F344 rats. Further, genes encoding the glutamatergic receptor subunits that were altered by nicotine in the F344 rats were altered in the same direction in HIV-1Tg rats, including upregulation of Grm2 in the PFC and downregulation of Grm1 and Grm2 in the VTA. These results indicate that chronic exposure to nicotine selectively disrupts the inhibitory control of the PFC on the VTA by acting on nAChRs and on local GABA interneurons. These interactions may lead to disinhibition of the dopaminergic neurons in the VTA, which could cause increased dopaminergic transmission in the NAc.67,68 Thus, our results indicate a nicotine-induced uncoupling of PFC-mediated inhibitory control over VTA–NAc reward circuitry, providing a critical neural mechanism for the loss of cognitive control that is observed in HIV-1-infected patients with nicotine dependence. To our knowledge, the present study provides the first demonstration of the synergistic effects of HIV-1 proteins and nicotine on the PFC–VTA–NAc neural circuits, which play a key role in dopamine-driven phenotypes, such as drug addiction and novelty-seeking behavior.
There are a few limitations of this study. First, in our expression analysis, we could not include as many addiction-related genes as we would have preferred. This limit was primarily the result of the small number of genes that could be included in each assay; further, only a small amount of RNA could be extracted from each specific brain region. We did not wish to pool tissues from multiple animals, as this method would increase the number of animals required for the study and fail to detect expression differences among individual animals. Second, for the same reasons, we did not examine expression differences at the protein level for those genes, although we realize that it is important to do so, because the RNA differences we detected may not translate into differences in the amounts of various proteins.
In sum, our current study constitutes another step in the investigation of different implications of nicotine dependence in HIV-1 infected patients. Specifically, we focused on differentiating the effects of nicotine on the PFC–VTA circuits of GABAergic transmission in both the diseased and healthy states. Our results imply that treatments that enhance GABAergic transmission may provide a more effective smoking cessation method for HIV-1-positive smokers. However, further studies should be performed to clarify possible sex- and age-related difference, as well as to account for the activity of other neurotransmitter systems. Additionally, future research utilizing other drugs of abuse may provide useful information regarding the effects of viral proteins on the molecular mechanisms of reward-related behaviors.
Supplementary Material
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
This work was supported, in part, by the China Precision Medicine Initiative (2016YFC0906300), Research Center for Air Pollution and Health of Zhejiang University and US National Institutes of Health grants DA-016149 to SLC and DA-026356 to SLC and MDL.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Drs. Guohua Song and Shaolin Wang for their assistance with sample collection during the experiments. ZY and TN contributed equally to this work.
References
- 1. WHO. HIV/AIDS - Injecting drug use 2014. www.who.int/hiv/topics/idu/en/ Accessed June 1, 2016.
- 2. Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58(13):1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(suppl 2):S62–S69. [DOI] [PubMed] [Google Scholar]
- 4. Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48(8):336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4(3):309–316. [DOI] [PubMed] [Google Scholar]
- 6. Chang SL, Connaghan KP, Wei Y, Li MD. NeuroHIV and use of addictive substances. Int Rev Neurobiol. 2014;118:403–440. [DOI] [PubMed] [Google Scholar]
- 7. Wingo T, Nesil T, Chang SL, Li MD. Interactive effects of ethanol and HIV-1 proteins on novelty-seeking behaviors and addiction-related gene expression. Alcohol Clin Exp Res. 2016;40(10):2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE. Increased sensitivity to cocaine self-administration in HIV-1 transgenic rats is associated with changes in striatal dopamine transporter binding. J Neuroimmune Pharmacol. 2015;10(3):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cicala C, Arthos J, Selig SM, et al. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc Natl Acad Sci U S A. 2002;99(14):9380–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42(2):195–212. [DOI] [PubMed] [Google Scholar]
- 11. Jiang J, Aiken C. Maturation of the viral core enhances the fusion of HIV-1 particles with primary human T cells and monocyte-derived macrophages. Virology. 2006;346(2):460–468. [DOI] [PubMed] [Google Scholar]
- 12. Kumar AM, Fernandez JB, Singer EJ, et al. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15(3):257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norman LR, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Curr Drug Abuse Rev. 2009;2(2):143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obermann M, Kuper M, Kastrup O, et al. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J Neurol. 2009;256(6):948–953. [DOI] [PubMed] [Google Scholar]
- 15. Nath A, Anderson C, Jones M, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14(3):222–227. [DOI] [PubMed] [Google Scholar]
- 16. Gurwell JA, Nath A, Sun Q, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102(3):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. [DOI] [PubMed] [Google Scholar]
- 18. Wang GJ, Chang L, Volkow ND, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(pt 11):2452–2458. [DOI] [PubMed] [Google Scholar]
- 19. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tzschentke TM, Schmidt WJ. Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex. 2000;10(5):488–498. [DOI] [PubMed] [Google Scholar]
- 21. Zhu J, Midde NM, Gomez AM, Sun WL, Harrod SB. Intra-ventral tegmental area HIV-1 Tat1-86 attenuates nicotine-mediated locomotor sensitization and alters mesocorticolimbic ERK and CREB signaling in rats. Front Microbiol. 2015;6:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98(4):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4(4):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds NR. Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev. 2009;21(3 suppl):106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adam BD, Husbands W, Murray J, Maxwell J. Silence, assent and HIV risk. Cult Health Sex. 2008;10(8):759–772. [DOI] [PubMed] [Google Scholar]
- 26. Parsons JT, Kutnick AH, Halkitis PN, Punzalan JC, Carbonari JP. Sexual risk behaviors and substance use among alcohol abusing HIV-positive men who have sex with men. J Psychoactive Drugs. 2005;37(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. [DOI] [PubMed] [Google Scholar]
- 28. Mahoney JJ, III, Thompson-Lake DG, Cooper K, Verrico CD, Newton TF, De La Garza R., II A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. J Psychopharmacol. 2015;29(1):50–56. [DOI] [PubMed] [Google Scholar]
- 29. Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. [DOI] [PubMed] [Google Scholar]
- 30. Abreu-Villaça Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhães AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2006;167(1):175–182. [DOI] [PubMed] [Google Scholar]
- 31. Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36(3):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Düzel E, Bunzeck N, Guitart-Masip M, Düzel S. NOvelty-related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev. 2010;34(5):660–669. [DOI] [PubMed] [Google Scholar]
- 33. Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. [DOI] [PubMed] [Google Scholar]
- 34. Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3(9):351–359. [DOI] [PubMed] [Google Scholar]
- 35. Meeter M, Murre JM, Talamini LM. Mode shifting between storage and recall based on novelty detection in oscillating hippocampal circuits. Hippocampus. 2004;14(6):722–741. [DOI] [PubMed] [Google Scholar]
- 36. Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4(3):193–202. [DOI] [PubMed] [Google Scholar]
- 37. Wilson FA, Rolls ET. Neuronal responses related to the novelty and familarity of visual stimuli in the substantia innominata, diagonal band of Broca and periventricular region of the primate basal forebrain. Exp Brain Res. 1990;80(1):104–120. [DOI] [PubMed] [Google Scholar]
- 38. Vianna MR, Alonso M, Viola H, et al. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem. 2000;7(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98(16):9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Homji NF, Mao X, Langsdorf EF, Chang SL. Endotoxin-induced cytokine and chemokine expression in the HIV-1 transgenic rat. J Neuroinflammation. 2012;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao J, Wang S, Wang J, et al. RNA deep sequencing analysis reveals that nicotine restores impaired gene expression by viral proteins in the brains of HIV-1 transgenic rats. PLoS One. 2013;8(7):e68517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li MD, Cao J, Wang S, et al. Transcriptome sequencing of gene expression in the brain of the HIV-1 transgenic rat. PLoS One. 2013;8(3):e59582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarkar S, Chang SL. Ethanol concentration-dependent alterations in gene expression during acute binge drinking in the HIV-1 transgenic rat. Alcohol Clin Exp Res. 2013;37(7):1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain. 2015;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13(4):367–375. [DOI] [PubMed] [Google Scholar]
- 46. Welker WI. Free and forced exploration of a novel situation by rats. Psychological Reports. 1957;3(1):95–108. [Google Scholar]
- 47. Wingo T, Nesil T, Choi JS, Li MD. Novelty seeking and drug addiction in humans and animals: from behavior to molecules. J Neuroimmune Pharmacol. 2016;11(3):456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Z, Nesil T, Connaghan KP, Li MD, Chang SL. Modulation effect of HIV-1 viral proteins and nicotine on expression of the immune-related genes in brain of the HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2016;11(3):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2(4):319–328. [DOI] [PubMed] [Google Scholar]
- 50. Paxinos G, Watson C. The Rat Brain in Stereotxic Coordinates. San Diego, CA: Academic Press Inc; 2005. [Google Scholar]
- 51. Cui WY, Zhao S, Polanowska-Grabowska R, et al. Identification and characterization of poly(I:C)-induced molecular responses attenuated by nicotine in mouse macrophages. Mol Pharmacol. 2013;83(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao J, Dwyer JB, Mangold JE, et al. Modulation of cell adhesion systems by prenatal nicotine exposure in limbic brain regions of adolescent female rats. Int J Neuropsychopharmacol. 2011;14(2):157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei J, Wang J, Dwyer JB, et al. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14(1):91–106. [DOI] [PubMed] [Google Scholar]
- 54. Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270(1):41–49. [DOI] [PubMed] [Google Scholar]
- 55. Wang J, Gutala R, Sun D, et al. Regulation of platelet-derived growth factor signaling pathway by ethanol, nicotine, or both in mouse cortical neurons. Alcohol Clin Exp Res. 2007;31(3):357–375. [DOI] [PubMed] [Google Scholar]
- 56. Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Res Mol Brain Res. 2004;132(2):168–180. [DOI] [PubMed] [Google Scholar]
- 57. Agbottah E, Zhang N, Dadgar S, et al. Inhibition of HIV-1 virus replication using small soluble Tat peptides. Virology. 2006;345(2):373–389. [DOI] [PubMed] [Google Scholar]
- 58. Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218(1–2):94–101. [DOI] [PubMed] [Google Scholar]
- 59. Viganò A, Trabattoni D, Schneider L, et al. Failure to eradicate HIV despite fully successful HAART initiated in the first days of life. J Pediatr. 2006;148(3):389–391. [DOI] [PubMed] [Google Scholar]
- 60. Fassino S, Leombruni P, Amianto F, Abbate-Daga G. Personality profile of HIV outpatients: preliminary results and remarks on clinical management. Psychother Psychosom. 2004;73(6):361–365. [DOI] [PubMed] [Google Scholar]
- 61. Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239(1):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93(24):13473–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. [DOI] [PubMed] [Google Scholar]
- 64. Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4(8):e6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol. 2009;192:209–233. [DOI] [PubMed] [Google Scholar]
- 66. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychophar macology. 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang D, Gao M, Xu D, et al. Impact of prefrontal cortex in nicotine-induced excitation of ventral tegmental area dopamine neurons in anesthetized rats. J Neurosci. 2012;32(36):12366–12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6(1):4–16. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.