Abstract
Introduction
Pictorial warning labels (PWL) that use photographs and the personal details of real people whose health has been affected by smoking (testimonial PWL) provide factual information about the consequences of tobacco use.
Methods
Nine hundred and twenty-four adult current smokers participated in an online experiment that tested responses to four types of warning labels: (1) non-testimonial text warning labels (currently on packs in the United States); (2) non-testimonial PWL (previously proposed by the United States Food and Drug Administration); (3) image only testimonial PWL (created for study); (4) image + personal details testimonial PWL (created for study). Participants were randomly assigned to condition and then exposed to up to five warning labels addressing different health effects. Differences between conditions were assessed using emotional responses and a set of intention measures immediately following exposure, and self-reported behavior change at 5-week follow-up.
Results
Compared to the non-testimonial text warning labels, all PWL elicited stronger emotional responses and intentions to forgo cigarettes and avoid the warning labels. Non-testimonial PWL and image + personal details testimonial PWL elicited stronger intentions to quit, whereas image only testimonial PWL generated a greater amount of quitting activity in the weeks following exposure. There were no significant differences in responses when comparing the non-testimonial PWL with both types of testimonial PWL.
Conclusions
PWL that use images of real people convey factual information about the health effects of tobacco use. These testimonial PWL may be a promising alternative to the images previously proposed for use on PWL in the United States.
Implications
In the United States, the PWL developed by the Food and Drug Administration (FDA) in 2011 were found by the courts to be unconstitutional, in part because they were deemed to present an opinion rather than fact. Findings from this experimental study indicate that PWL that use the images and personal details of real people to convey factual information about the health effects of tobacco use may satisfy the FDA’s requirement for a set of PWL that (1) have the potential to positively impact the determinants of smoking cessation behavior, (2) meet legislative requirements under the Family Smoking Prevention and Tobacco Control Act and (3) may be more acceptable to the courts than the previously proposed and now dismissed PWL that carried non-factual images.
Introduction
In 2009, the US Family Smoking Prevention and Tobacco Control Act (FSPTCA) mandated the Food and Drug Administration (FDA) to create nine color pictorial warning labels (PWL), which were to replace the text-only warning labels that had appeared on cigarette packs in the United States since 1984.1 Consistent with standards recommended by the World Health Organization’s Framework Convention on Tobacco Control,2 the PWL were to cover 50% of the front and back of the pack with a prescribed textual warning statement and a color image depicting the negative health consequences of smoking.1 In June 2011, FDA issued its final rule specifying the set of nine PWL. However, these PWL were quickly subject to legal challenges from the tobacco industry, culminating in a ruling from the US Court of Appeals for the DC Circuit that the PWL were unconstitutional because they limited tobacco companies’ right to freedom of speech.3 Rather than challenge this decision, FDA withdrew the proposed PWL and announced their intention to generate a new set of PWL following additional research.4
Scholars have analyzed the previous court rulings for insights into the label elements that, if modified, may help to minimize how vulnerable FDA’s next iteration of PWL are to similar legal challenges.5–8 One consideration is the extent to which the labels are judged to present factual information versus an opinion, an issue on which the courts disagreed when reviewing the FDA’s original PWL.5,6 For instance, in the case of “Discount Tobacco City & Lottery Inc. v. United States”9 in the court of the Sixth Circuit, the PWL were deemed factual and accurate, and consequently, were subject to the most lenient form of legal review and were upheld.6 By comparison, when the Court of Appeals for the DC Circuit decided that the PWL were not purely factual but were intended to evoke an emotional response (“R.J. Reynolds Tobacco Co. v. FDA”10), a stricter form of review was applied, and the evidence required to defend the policy was beyond that currently available to the FDA.6 Kraemer and Baig5 have therefore argued that it is critical that the images used in the next iteration of PWL are deemed to present factual information rather than an opinion. One way to achieve this may be to use photographs that accurately represent the health consequences of smoking.5,6 In the current study we test the potential effectiveness of a set of PWL that feature photographs of real people whose health has been affected by smoking, which we call “testimonial PWL.” We propose that these photographs comprise factual information, and so may be more amenable to the courts than were the images originally used by FDA, which included created or staged photographs of people whose health or wellbeing had been affected by their own or others’ smoking, simulations of diseased body parts, and animations (see5).
Testimonial (or narrative) messages have been the subject of considerable recent attention from health communication scholars, who have aimed to document and explain the persuasive benefits associated with such messages. Two recent meta-analyses have confirmed that narratives can have persuasive effects on attitudes, intentions, and behaviors,11,12 due to their ability to garner attention and facilitate comprehension by illustrating the potential consequences of an event or behavior to the audience (as per exemplification theory13–15), or to transport audiences into the world of the narrative, thereby reducing counterarguing and increasing emotional responding (as per transportation theory16,17). In other domains of tobacco control communications, such as television advertisements18–20 and newspaper articles,21 there is evidence that personal testimonials can be particularly effective in certain circumstances, such as when encouraging smokers of lower socioeconomic status to call a quitting helpline.18 However, past research has produced more mixed evidence regarding the potential effectiveness of testimonial warning labels, with some indication that effects may depend on the amount of testimonial information presented. Using measures of perceived effectiveness, credibility, and relevance, a handful of studies have shown that images depicting the “lived experiences” of the sufferers of tobacco-related illnesses do not perform as well as images depicting diseased organs and body parts,22–26 although the use of “lived experiences” images can still confer some benefit over alternatives such as symbolic images.22,23,27 In one of these studies, PWL were most effective when the image was accompanied by a textual message that took a didactic rather than testimonial form,24 whereas another study found that effectiveness was increased by the addition of a brief narrative statement that provided the name, age, and a quote from the person in the image.25
In the current study, we use the term testimonial PWL to indicate that the label features just the image, or image and personal details of a real person, whereas non-testimonial PWL are those that contain created or staged images. Although other definitions of “testimonial” have previously been used,24,25 we believe this term captures the essence of what is communicated by these warning labels: one person’s testimony (in visual and/or visual plus textual format) of their experience with the health consequences of tobacco use. Specifically, we tested the effectiveness of two types of testimonial PWL. The image only testimonial PWL contained only the image of the real person whose health had been affected by smoking. Importantly, these PWL were designed to be compliant with the formatting requirements specified under the FSPTCA.1 By comparison, the image + personal details testimonial PWL supplemented the image with a brief statement providing the person’s name, age, and health status. Although these PWL are not entirely compliant with the FSPTCA, the addition of a testimonial statement was found to enhance effectiveness in the study by Hammond and colleagues,25 and it may be that such information is required to clearly convey the factual nature of these images. Given that our objective was to support the FDA in their search for alternative PWL that (1) have the potential to positively impact the determinants of smoking cessation behavior, (2) meet legislative requirements under the FSPTCA and (3) may be more acceptable to the courts than the previously proposed and now dismissed non-testimonial PWL, our primary aim was to test whether both types of testimonial PWL were more effective than the non-testimonial text warning labels (TWL) that currently appear on packs in the United States, and were at least as effective as the non-testimonial PWL that were originally proposed by the FDA.
Method
Sample
In October–December 2014, participants were recruited through Survey Sampling International’s (SSI) US panel.28 SSI’s panel is comprised of individuals who voluntarily opt-in to be a member of the panel and receive small financial incentives for completing surveys. Prospective panel members are sourced using online banners, invitations, and messages on online communities, social networks and websites of all types, but are then subject to rigorous quality controls before being added to the panel.28 As with other non-probability online panels, the SSI panel cannot be considered representative of the population, and so we do not suggest our parameter estimates represent the national population statistically. However, the patterns of responses observed in this large and varied sample are expected to reflect those in the population to a meaningful extent.
Eligible participants were 18–60 year old current smokers who had smoked at least 100 cigarettes and had completed fewer than three online surveys about cigarette smoking or other tobacco products in the past 3 months. As part of a larger experimental study, 3055 participants were randomly assigned to one of 17 experimental conditions. However, only four of these conditions were relevant to the current study, and so the sample is limited to the N = 924 participants randomized to one of those conditions.
Stimuli
For each experimental condition, we created five warning labels with five different “themes,” meaning they each focused on a different health effect of tobacco use (Table 1). Given that our primary objective was to provide evidence pertinent to the situation in the United States, the five themes were based on five of the nine warning statements prescribed in the FSPTCA1: “Smoking can kill you”; “Cigarettes cause fatal lung disease”; “Cigarettes cause stroke and health disease”; “Cigarettes are addictive”; and “Tobacco smoke causes fatal lung disease in nonsmokers.”
Table 1.
Warning Label Content
| Condition | Theme 1 (“kill”) | Theme 2 (“fatal lung disease”) | Theme 3 (“heart disease/stroke”) | Theme 4 (“addiction”) | Theme 5 (“fatal lung disease in nonsmokers”) |
|---|---|---|---|---|---|
| Non-testimonial TWL |

|

|

|

|
|
| Non-testimonial PWL |

|
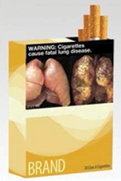
|

|

|

|
| Image only testimonial PWL |

|

|

|

|

|
| Image + personal details testimonial PWL |

|

|

|

|

|
TWL = text warning label; PWL = pictorial warning label. Images and personal details of Terrie (Theme 1), Roosevelt (Theme 3) and Nathan (Theme 5) were used with permission from US Centers for Disease Control and Prevention. Image and personal details of Lena (Theme 2) and Barb (Theme 4) were used with permission from Health Canada. Digital images of the warning labels and cigarette packs were created by Kyle Cassidy, Annenberg School for Communication, University of Pennsylvania. Supplementary Appendix A details the text that appeared on each warning label.
To create the testimonial PWLs, we searched for case studies of individuals whose health had been affected in the way described by each theme. Images and stories of these case studies were sourced and used with permission from the US Center for Disease Control and Prevention’s Tips From Former Smokers mass media campaign (Terrie, Roosevelt, and Nathan) and with permission from Health Canada (Lena and Barb). For each theme, we created two types of testimonial PWL, one which featured just an image of the person (image only testimonial PWL) and one which featured an image plus a brief statement providing the person’s name, age, and health status (image + personal details testimonial PWL). Both types of testimonial PWL also carried the mandatory warning statement.
To create warning labels for the non-testimonial PWL condition, we paired the five mandatory warning statements with the non-testimonial image used by the FDA (see5). The non-testimonial PWL used in our study differed from those proposed by the FDA only in that the format of the warning statement was standardized across themes, and the warning labels did not carry the 1-800-QUIT-NOW phone number.
Stimuli in the non-testimonial TWL condition comprised the four text warning statements that currently appear on cigarette packets in the United States. The content of these statements does not match the five themes used in the other two conditions, but this condition represents the warnings currently encountered by smokers.
As shown in Supplementary Appendix A, for each warning label, participants saw a static image of a front-of-pack view, a back-of-pack view, and a side-of-pack view. Supplementary Appendix A also details the text that appeared on each warning label.
Procedure
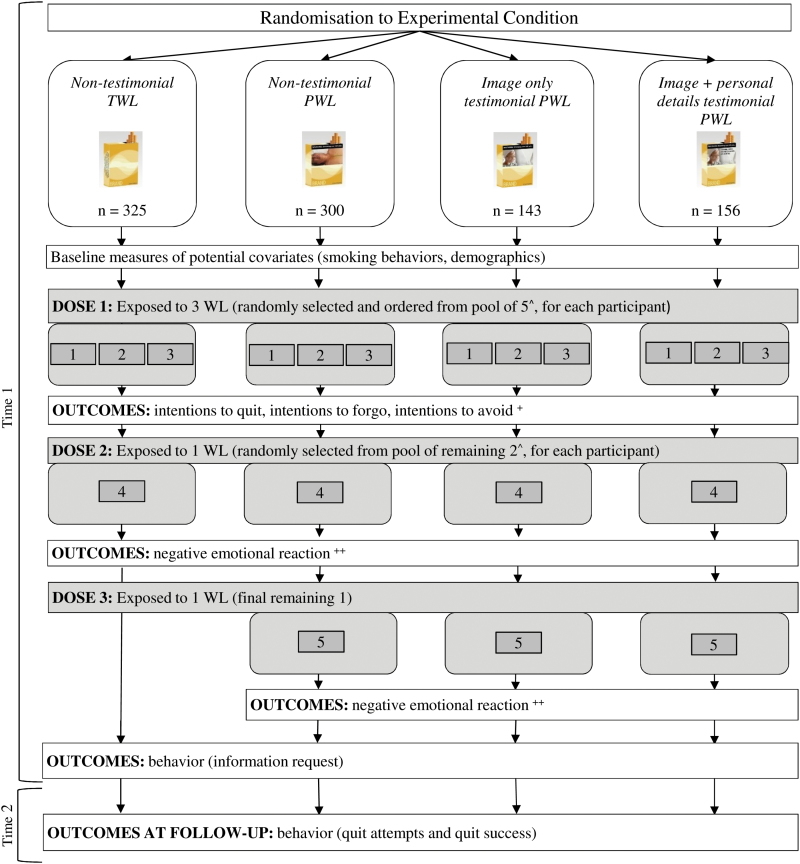
This study was approved by the Institutional Review Board at the University of Pennsylvania. The study comprised an online experimental session (Time 1) during which participants were exposed to the warning labels, and a 5-week follow-up online survey (Time 2). Figure 1 illustrates the procedure. As shown in Figure 1, one feature of this procedure is that participants were exposed to five different warning labels (or four, in the non-testimonial TWL condition) over three separate “doses.” At Time 1, we measured the aggregate impact of warning labels on intentions after Dose 1. In Dose 1 participants were exposed to three of the warning labels in their condition. The three labels were randomly selected from the five themes and presented in random order for each participant. Measuring intentions after exposure to multiple warning labels better enabled us to approximate real world conditions of warning label exposure, in which smokers are repeatedly exposed to multiple warning labels conveying information about different health effects. After measuring intentions, we also collected detailed assessments of the two remaining individual warning labels within each condition (ie, emotional reactions following Doses 2 and 3). By measuring emotional reactions after intentions, we avoided the potential confounding of message processing and overall impact that could have occurred if we instead measured emotional reactions before intentions. Our design avoided this potential confounding, while still allowing us to measure both the aggregate impact of exposure (after Dose 1) and detailed information about reactions to the individual images (after Dose 2 and 3) in each condition. It also meant that our assessment of behavioral outcomes at the end of Time 1 and at Time 2 once again approximated conditions of real world exposure, in that participants had been exposed to four (in the non-testimonial TWL condition) or five different warning labels (all other conditions) (Figure 1).
Figure 1.
Study procedure. ^For participants in the non-testimonial TWL condition, the first three warning labels were selected from a pool of four warning labels (and so the fourth label was the final one remaining). For participants in the non-testimonial PWL and testimonial PWL conditions, the first three warning labels were selected from a pool of five warning labels (Table 1). +Additional outcomes measured following Dose 1 included intentions to seek help when trying to quit (ie, intentions to call a quitline, buy a nicotine replacement product, enrol in a smoking cessation program), unprompted and prompted knowledge of the health effects of smoking, attitudes towards smoking, and self-efficacy to quit. ++Additional outcomes measured following Dose 2 and Dose 3 included engagement with the warning labels, identification with the person shown in the warning label, defensive processing, and perceived effectiveness of the warning labels. Digital images of the warning labels and cigarette packs were created by Kyle Cassidy, Annenberg School for Communication, University of Pennsylvania.
Measures
Warning label effectiveness was assessed using several of the measures recommended by the International Agency for Research on Cancer.29 Given that this experimental study involved such limited exposure to the warning labels, our primary outcomes were affective reactions (negative emotions) and intentions to quit smoking, forgo cigarettes, and avoid the warning labels. However, the follow-up survey also allowed us to assess whether even this limited exposure was associated with self-reported behavior change in the weeks following exposure.
Negative Emotional Reactions
After Doses 2 and 3 (Figure 1), participants completed a set of measures representing potential mediators of the effect of warning label exposure on intentions and behaviors; however, for the purposes of the current study, only the results for the negative emotional reactions are reported. Past research has demonstrated that affective reactions are one of the critical mechanisms through which warning labels contribute to changes in smoking behaviors.7,8,29–33 We measured “negative emotional reactions” using a scale of seven items adapted from Gibson et al.34 The question wording encouraged smokers to consider their emotional reaction to the specific warning label that they had just seen: While looking at the warning on this pack of cigarettes, I felt…(1) disgusted; (2) fearful; (3) guilty; (4) regretful; (5) sad; (6) worried; and (7) angry at myself for being a smoker. Responses were measured using 5-point scales (1 “strongly disagree”–5 “strongly agree”) (α = 0.92 after second dose; α = 0.93 after third dose).
Intention Outcomes
Following Dose 1 (Figure 1), participants rated their willingness to engage in three quitting-related behaviors in the next 30 days: (1) try to quit smoking; (2) reduce the number of cigarettes smoked per day; and (3) quit smoking completely (1 “definitely will not”–4 “definitely will”).34 Responses were averaged into an “intentions to quit” scale (α = 0.87).
Longitudinal population surveys have demonstrated that smokers who forgo cigarettes because of warning labels may be more likely to make subsequent quit attempts.30,35–38 Following exposure to the first three warning labels, “intentions to forgo” were measured using the item: If my usual pack of cigarettes looked like these packs of cigarettes, I would hold back from smoking a cigarette when I was about to smoke one (1 “strongly disagree”–5 “strongly agree”).34,35
As a result of the negative feelings that can occur when exposed to warning labels, some smokers attempt to avoid these labels by covering them up or moving their cigarettes to a different container.29,30,39 While such warning label avoidance is sometimes considered an undesirable outcome, longitudinal population studies have shown that rather than being undesirable, warning label avoidance is actually associated with more frequent thoughts about the harms of smoking30 and increased quitting activity,38 most likely because attempts to suppress or avoid certain thoughts actually tend to increase the frequency of those thoughts.40 Following Dose 1, participants indicated how likely they would be to engage in three avoidance behaviors: If my usual pack of cigarettes looked like these packs of cigarettes, I would…(1) cover it up; (2) keep the pack out of sight; and (3) transfer the cigarettes to a different container (1 “strongly disagree”–5 “strongly agree”).34,35 Responses were averaged into an “intentions to avoid” scale (α = 0.86).
Behavioral Outcomes
At the end of Time 1, participants were given the opportunity to read some tips on how to quit smoking. If they requested to read these tips, they were taken to a new page displaying information from websites such as the US Centers for Disease Control and Prevention. We created one binary variable capturing the proportion who “requested quitting info.”
At the beginning of Time 2, participants were reminded that they recently took part in a study in which they viewed and evaluated cigarette packs. They were then asked whether they had changed or had thought about changing their smoking behavior, since participating in that study. Response options included: (1) I have not made any changes to my smoking behavior; (2) I thought about quitting, but did not make an attempt; (3) I tried to cut down the number of cigarettes, but didn’t make an actual attempt to quit; (4) I decided to quit, but haven’t made an actual attempt yet; (5) I made an attempt to quit, but I’ve relapsed to smoking; and (6) I quit, and I’m still quit.41 We created one binary variable measuring self-reported “quit attempts” ((5) “I made an attempt, but I’ve relapsed to smoking” or (6) “I quit, and I’m still quit”; compared to responses (1)–(4) combined), and a second binary variable measuring “quit success” ((6) “I quit, and I’m still quit”; compared to responses (1)–(5) combined).
Potential Covariates
Potential covariates included age, sex, educational attainment, race, ethnicity, parental status, and annual household income (see Table 2). Readiness to quit was measured at the beginning of the study using the 0–10 Contemplation Ladder scale adapted from Biener and Abrams.42 Six questions from the Fagerstrӧm Test for Nicotine Dependence measured participants’ nicotine dependence.43 Participants also reported whether or not they currently smoked every day, and how many times they had tried to quit smoking in the past year. Two questions adapted from the brief questionnaire of smoking urges measured cigarette cravings.44,45 In addition, at the end of Time 1, we asked participants whether they had smoked at any point during the study45 (Table 2).
Table 2.
Sample Characteristics
| Non-testimonial TWL | Non-testimonial PWL | Image only testimonial PWL | Image + personal details testimonial PWL | |||
|---|---|---|---|---|---|---|

|

|

|

|
|||
| Total N = 924 | n = 325 | n = 300 | n = 143 | n = 156 | ||
| % | % | % | % | % | χ2 | |
| Sex (female) | 50.3 | 52.9 | 50.7 | 45.5 | 48.7 | 2.41 |
| Education (low)a | 22.7 | 23.1 | 23.0 | 25.9 | 18.6 | 2.36 |
| Race | 7.84 | |||||
| White | 84.4 | 85.9 | 81.3 | 85.9 | 85.9 | |
| Black | 6.9 | 7.1 | 6.4 | 7.8 | 7.1 | |
| Otherb | 8.7 | 7.1 | 12.4 | 6.3 | 7.1 | |
| Ethnicity (Hispanic) | 9.9 | 8.9 | 10.3 | 9.1 | 11.5 | 0.99 |
| Children in household (any)c | 56.1 | 52.3 | 60.0 | 53.2 | 59.0 | 4.78 |
| Smoking status (daily) | 81.1 | 86.5 | 79.3 | 76.9 | 76.9 | 10.09* |
| Smoking during study (yes) | 19.8 | 17.9 | 22.0 | 20.3 | 19.2 | 1.75 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | |
| Age (18–60) | 38.5 (10.8) | 39.0 (10.9) | 37.7 (10.7) | 39.6 (11.5) | 37.9 (10.3) | 1.33 |
| Annual household income (in $1000s)d | 59.5 (38.7) | 58.3 (38.7) | 59.6 (37.2) | 54.3 (35.6) | 66.3 (43.4) | 2.60 |
| Readiness to quit (0–10 scale)e | 6.2 (2.8) | 6.1 (2.8) | 6.2 (2.8) | 6.4 (3.0) | 5.9 (2.9) | 0.72 |
| Nicotine dependence (0–10 scale)c | 5.3 (2.6) | 5.3 (2.5) | 5.5 (2.6) | 5.0 (2.8) | 5.3 (2.5) | 1.13 |
| Quitting history (N attempts in past year; 0–99)f | 3.1 (8.0) | 2.5 (5.3) | 3.0 (7.7) | 4.0 (10.8) | 3.5 (10.1) | 1.40 |
| Smoking urge at beginning of study (1–5 scale) | 3.8 (0.9) | 3.7 (1.0) | 3.8 (0.9) | 3.7 (0.9) | 3.8 (0.9) | 0.34 |
TWL = text warning label; PWL = pictorial warning label; SD = standard deviation. N varied slightly for some variables due to missing data, but where there was missing data, it applied to less than 1% of all cases. Chi-Squares and F statistics (from ANOVA models) tested differences in the distribution of sample characteristics across the four conditions. Degrees of freedom for Chi-Squares ranged from 3 to 6. Degrees of freedom for F statistics were (df1, df2) = (3, 923). By design, twice as many participants were randomized to the non-testimonial TWL and non-testimonial PWL conditions (control conditions) as to the two testimonial PWL conditions. Images and personal details of Terrie (Theme 1) were used with permission from US Centers for Disease Control and Prevention. Digital images of the warning labels and cigarette packs were created by Kyle Cassidy, Annenberg School for Communication, University of Pennsylvania.
aLow educational attainment was defined as high school or less.
bParticipants who chose more than one race were categorized as “Other” race.
cComparable measures were not available in the US Centers for Disease Control and Prevention’s 2013–2014 National Adult Tobacco Survey (NATS).
dFor comparison with the NATS measure of the percentage of smokers whose total household income was less than $40 000, this variable was dichotomized at <$40 000 (vs. ≥$40 000).
eFor comparison with the NATS measure of the percentage of smokers who were thinking about quitting cigarettes for good, this variable was dichotomized at ≥5, which was the scale point labelled as “I think I should quit smoking but I am not quite ready.”
fFor comparison with the NATS measure of the percentage of smokers who had made at least one quit attempt in the past 12 months, this variable was dichotomized at ≥1 (vs. 0).
***p < .001; **p < .01; *p < .05.
Statistical Analysis
All analyses were conducted using Stata Version 14.1.46 For each outcome, we estimated a linear (for continuous outcomes) or logistic (for binary outcomes) regression model, first with the non-testimonial TWL condition as referent and then again with the non-testimonial PWL condition as referent. With the non-testimonial TWL condition as the referent category, we could examine whether the non-testimonial PWL, image only testimonial PWL, and image + personal details testimonial PWL were more effective than the labels that currently appear on packs. With the non-testimonial PWL condition as the referent category, we could assess the benefit of using testimonial rather than non-testimonial images. Each model was run unadjusted and then adjusted for covariates: in Table 3 we present results from both unadjusted and adjusted models but in text we refer only to the results from adjusted models. Preliminary analyses indicated that of all potential covariates, smoking status (daily vs. non-daily) was the only variable that was unevenly distributed across conditions (Table 2). Given that smoking status was also significantly associated with six of the seven outcome measures (in bivariate models; data not shown) it was included as a covariate in adjusted models. In addition, sensitivity analyses assessed whether the overall pattern of effects was the same for daily and non-daily smokers, by adding an interaction term (condition x smoking status) to the unadjusted regression model for each outcome.
Table 3.
Effects of Non-testimonial TWL, Non-testimonial PWL, Image Only Testimonial PWL, and Image + Personal Details Testimonial PWL Conditions: Results From Unadjusted and Adjusted Linear and Logistic Regression Models
| vs. Non-testimonial TWL | vs. Non-testimonial PWL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| N | % | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Negative emotion (1–5 scale)a | ||||||||||
| Non-testimonial TWL | 324 | 3.05 (1.02) | Ref | Ref | −0.30*** | −0.46, −0.14 | −0.28** | −0.44, −0.12 | ||
| Non-testimonial PWL | 300 | 3.35 (1.07) | 0.30*** | 0.14, 0.46 | 0.28** | 0.12, 0.44 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 3.35 (1.06) | 0.29** | 0.09, 0.49 | 0.28** | 0.08, 0.47 | −0.00 | −0.20, 0.20 | −0.01 | −0.21, 0.19 |
| Image + personal details testimonial PWL | 156 | 3.43 (1.02) | 0.38*** | 0.19, 0.56 | 0.36*** | 0.17, 0.55 | 0.08 | −0.11, 0.27 | 0.08 | −0.11, 0.27 |
| Intention outcomes | ||||||||||
| Intentions to quit (1–4 scale) | ||||||||||
| Non-testimonial TWL | 325 | 2.44 (0.75) | Ref | Ref | −0.20** | −0.32, −0.08 | −0.18** | −0.30, −0.06 | ||
| Non-testimonial PWL | 300 | 2.64 (0.75) | 0.20** | 0.08, 0.32 | 0.18** | 0.06, 0.30 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 2.58 (0.79) | 0.14 | −0.01, 0.29 | 0.11 | −0.04, 0.25 | −0.07 | −0.22, 0.09 | −0.07 | −0.22, 0.08 |
| Image + personal details testimonial PWL | 156 | 2.67 (0.80) | 0.23** | 0.09, 0.38 | 0.20** | 0.05, 0.34 | 0.03 | −0.12, 0.18 | 0.02 | −0.13, 0.17 |
| Intentions to forgo (1–5 scale) | ||||||||||
| Non-testimonial TWL | 325 | 2.33 (1.14) | Ref | Ref | −0.79*** | −0.98, −0.61 | −0.77*** | −0.96, −0.59 | ||
| Non-testimonial PWL | 300 | 3.13 (1.21) | 0.79*** | 0.61, 0.98 | 0.77*** | 0.59, 0.96 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 3.05 (1.21) | 0.72*** | 0.48, 0.95 | 0.69*** | 0.45, 0.92 | −0.08 | −0.31, 0.16 | −0.09 | −0.32, 0.15 |
| Image + personal details testimonial PWL | 156 | 3.05 (1.22) | 0.72*** | 0.49, 0.95 | 0.69*** | 0.46, 0.92 | −0.08 | −0.31, 0.15 | −0.08 | −0.31, 0.15 |
| Intentions to avoid (1–5 scale) | ||||||||||
| Non-testimonial TWL | 325 | 2.21 (1.04) | Ref | Ref | −1.08*** | −1.25, −0.91 | −1.07*** | −1.24, −0.90 | ||
| Non-testimonial PWL | 300 | 3.29 (1.08) | 1.08*** | 0.91, 1.25 | 1.07*** | 0.90, 1.24 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 3.18 (1.02) | 0.98*** | 0.77, 1.18 | 0.96*** | 0.75, 1.17 | −0.10 | −0.32, 0.11 | −0.11 | −0.32, 0.11 |
| Image + personal details testimonial PWL | 156 | 3.32 (1.11) | 1.12*** | 0.92, 1.32 | 1.11*** | 0.90, 1.31 | 0.04 | −0.17, 0.25 | 0.04 | −0.17, 0.24 |
| Behavioral outcomes | N | % | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Requested quitting infob | ||||||||||
| Non-testimonial TWL | 325 | 33.9 | Ref | Ref | 0.91 | 0.65, 1.26 | 0.89 | 0.64, 1.24 | ||
| Non-testimonial PWL | 300 | 36.0 | 1.10 | 0.79, 1.53 | 1.12 | 0.80, 1.56 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 35.0 | 1.05 | 0.69, 1.59 | 1.08 | 0.71, 1.63 | 0.96 | 0.63, 1.45 | 0.96 | 0.63, 1.46 |
| Image + personal details testimonial PWL | 156 | 36.5 | 1.13 | 0.76, 1.68 | 1.15 | 0.77, 1.72 | 1.02 | 0.68, 1.53 | 1.03 | 0.69, 1.54 |
| Quit attemptc | ||||||||||
| Non-testimonial TWL | 325 | 7.4 | Ref | Ref | 0.72 | 0.41, 1.26 | 0.75 | 0.43, 1.32 | ||
| Non-testimonial PWL | 300 | 10.0 | 1.39 | 0.79, 2.44 | 1.33 | 0.76, 2.34 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 15.4 | 2.28** | 1.23, 4.22 | 2.16* | 1.16, 4.01 | 1.64 | 0.91, 2.95 | 1.62 | 0.89, 2.93 |
| Image + personal details testimonial PWL | 156 | 11.5 | 1.64 | 0.86, 3.11 | 1.54 | 0.81, 2.95 | 1.17 | 0.63, 2.18 | 1.16 | 0.62, 2.15 |
| Quit successc | ||||||||||
| Non-testimonial TWL | 325 | 1.5 | Ref | Ref | 0.45 | 0.15, 1.34 | 0.51 | 0.17, 1.53 | ||
| Non-testimonial PWL | 300 | 3.3 | 2.21 | 0.75, 6.53 | 1.95 | 0.66, 5.83 | Ref | Ref | ||
| Image only testimonial PWL | 143 | 7.0 | 4.81** | 1.61, 14.35 | 4.16* | 1.38, 12.56 | 2.18 | 0.89, 5.36 | 2.13 | 0.86, 5.30 |
| Image + personal details testimonial PWL | 156 | 4.5 | 3.01 | 0.94, 9.63 | 2.57 | 0.79, 8.33 | 1.36 | 0.51, 3.65 | 1.32 | 0.49, 3.56 |
B = unstandardized beta coefficient; CI = confidence interval; M = mean; OR = odds ratio; PWL = pictorial warning label; Ref = referent category in regression model; SD = standard deviation; TWL = text warning label. Adjusted models control for the effect of smoking status (non-daily vs. daily). In the adjusted models, smoking status was a statistically significant predictor (p < .05) of negative emotion, intentions to quit, intentions to forgo, quit attempts, and quit success (daily < non-daily).
aParticipants in the non-testimonial PWL, image only testimonial PWL, and image + personal details testimonial PWL conditions provided two ratings each, one after Dose 2 and one after Dose 3. Therefore, models adjusted for clustering at the individual level and used robust standard errors. Because participants in the non-testimonial TWL condition provided only one rating (after Dose 2), sensitivity analyses tested whether effects were of the same magnitude when using just one rating for each participant in the three PWL conditions (ie, only Dose 2, and then only Dose 3). This indicated that coefficients for the comparison between non-testimonial TWL and each PWL condition were larger when using Dose 2 emotion ratings for all participants (range of adjusted B = 0.32–0.40), compared to when using Dose 2 emotion ratings for participants in the non-testimonial TWL condition and Dose 3 emotion ratings for all other participants (range of adjusted B = 0.23–0.32). However, all coefficients were still statistically significant (p < .05).
bMeasured at the end of Time 1 survey.
cMeasured at the beginning of the Time 2 survey.
***p < .001; **p < .01; *p < .05.
Of 924 participants who completed Time 1, 226 (24.5%) did not complete the Time 2 survey. The non-completion rate was similar across the conditions (25.9%, 24.7%, 27.3%, and 18.6%; χ2 = 3.87, p = .276) and the baseline characteristics of those lost to follow-up did not differ significantly between conditions (with one exception: a greater percentage of those lost to follow-up in the non-testimonial PWL condition were from a visible minority group (27.4%) compared with the non-testimonial TWL (13.1%), image only testimonial PWL (5.1%) and image + personal details testimonial PWL (17.2%) conditions, χ2 = 12.82, p = .046) (data not shown). For analyses predicting quit attempts and quit success at Time 2, we therefore conducted an intention-to-treat analysis (N = 924) with those who were lost to follow-up assumed not to have made a quit attempt or have successfully quit. Sensitivity analyses assessed whether the same pattern of effects was observed when limiting the sample to those who completed Time 2 (Supplementary Appendix B).
Results
Our sample of adult established current smokers (Table 1) had a highly similar profile to the sample of adult established current smokers in the 2013–2014 Centers for Disease Control and Prevention’s National Adult Tobacco Survey (NATS)47 in terms of age and the percentage of the sample who were female, Hispanic, lived in households with a total annual income of <$40 000, were thinking about quitting, and had made at least one quit attempt in the past 12 months. Our sample contained fewer respondents with low levels of education (22.7% cf. 48.4%), slightly more respondents who were white (84.4% cf. 77.2%) and fewer who were black (6.9% cf. 14.4%), and slightly more daily smokers (81.1% cf. 75.8%). Intercorrelations among the seven outcomes analyzed ranged from 0.02 (intentions to avoid and quit attempts) to 0.56 (quit attempts and quit success).
As shown in Table 3, the non-testimonial PWL (ie, FDA warning labels) elicited higher levels of negative emotion (B = 0.28, 95% CI [0.12, 0.44]) compared to the non-testimonial TWL (ie, current warning labels), and higher scores on all three intention measures, but particularly so on avoidance intentions (intentions to quit: B = 0.18, 95% CI [0.06, 0.30]; intentions to forgo: B = 0.77, 95% CI [0.59, 0.96]; intentions to avoid: B = 1.07, 95% CI [0.90, 1.24]). There were no significant differences between the non-testimonial TWL and non-testimonial PWL conditions across the three behavioral measures, although the direction of effects consistently favored the non-testimonial PWL (requested quitting info: OR = 1.12, 95% CI [0.80, 1.56]; quit attempt: OR = 1.33, 95% CI [0.76, 2.34]; quit success: OR = 1.96, 95% CI [0.66, 5.83]).
Table 3 also shows that the image only testimonial PWL and image + personal details testimonial PWL both elicited higher levels of negative emotion compared to the non-testimonial TWL (ie, current warning labels) (image only: B = 0.28, 95% CI [0.08, 0.47]; image + personal details: B = 0.36, 95% CI [0.17, 0.55]) and higher scores on intentions to forgo cigarettes (image only: B = 0.69, 95% CI [0.45, 0.92]; image + personal details: B = 0.69, 95% CI [0.46, 0.92]) and intentions to avoid the warning labels (image only: B = 0.96, 95% CI [0.75, 1.17]; image + personal details: B = 1.11, 95% CI [0.90, 1.31]). Only the image + personal details testimonial PWL elicited higher scores on intentions to quit (B = 0.20, 95% CI [0.05, 0.34]). Compared to the non-testimonial TWL, similar proportions of smokers in the two testimonial PWL conditions requested information on quitting at the end of Time 1 (Table 3). However, whereas only 7.4% of smokers exposed to the non-testimonial TWL reported that they had attempted to quit in the weeks preceding the follow-up survey, 15.4% of smokers exposed to the image only testimonial PWL had attempted to quit (OR = 2.16, 95% CI [1.16, 4.01]). Furthermore, 1.5% of participants in the non-testimonial TWL condition reported that they were quit at the time of follow-up, compared with 7.0% in the image only testimonial PWL condition (although we note the particularly wide 95% CI around this effect; OR = 4.16, 95% CI [1.38, 12.56]; Table 3).
Compared to the non-testimonial PWL (ie, FDA warning labels), neither the image only testimonial PWL nor the image + personal details testimonial PWL were any more effective at eliciting any of the outcomes (Table 3).
Sensitivity Analyses
We tested interactions between condition and smoking status, to ensure that the overall pattern of effects was not driven by the uneven distribution of daily smokers across conditions. For all seven outcomes, the overall interaction effect was non-significant (all p’s > .20), indicating that the effect of condition was not moderated by daily versus non-daily smoking status. In addition, as shown in Supplementary Appendix B, the same overall pattern of findings was observed when effects on quit attempts and quit success were examined using only those participants who completed Time 2.
Discussion
These findings indicate that PWL that use the images and personal details of real people to convey factual information about the health effects of tobacco use may be a promising alternative to the fictional photographs, simulations of diseased body parts, and animations that were originally proposed by FDA. Compared to the non-testimonial TWL that currently appear on cigarette packs in the United States, the non-testimonial PWL and both types of testimonial PWL (image only and image + personal details) elicited stronger negative emotional reactions and stronger intentions to forgo cigarettes and to avoid the warning labels. Intentions to quit were also stronger among those exposed to non-testimonial PWL and image + personal details testimonial PWL, but not the image only testimonial PWL. However, the image only testimonial PWL were the only labels to generate significantly greater quitting activity in the weeks following exposure. Compared to those exposed to the current non-testimonial TWL, smokers exposed to the new image only testimonial PWL were more than twice as likely to have attempted to quit in the weeks between exposure and the follow-up survey, and were more than four times as likely to report that they had quit and were still quit.
While the results for the behavioral outcomes could suggest that using images of real people rather than non-testimonial images may enhance the effectiveness of PWL, the more robust finding to emerge from this study is that there is unlikely to be any detrimental effects of replacing the images originally proposed by the FDA with images of real people whose health has been affected by smoking. We found that non-testimonial PWL and both types of testimonial PWL performed similarly on the measures of negative emotion reactions and intentions to quit, forgo cigarettes, and avoid the warning labels. There was a tendency for the image only testimonial PWL to generate more quitting activity than the non-testimonial PWL, although these differences were not statistically significant. Therefore, while further exploration of differences in effectiveness between the non-testimonial and testimonial PWL is required, it remains the case that the use of testimonial images may help to minimize how vulnerable the next iteration of warning labels in the United States are to legal challenges based on the factual nature of the messages.5
For jurisdictions that already have PWL in effect, these findings suggest that it may be worth considering the potential benefits of including testimonial PWL as part of the mix of warning labels in effect at any one time (as has been done in Canada and Australia, among other countries48). Maintaining salience is a key challenge for warning label policies,2,30,49–51 and it is possible that using a mix of different styles of images (ie, non-testimonial and testimonial) may help to reduce the rate at which the impact of the warning labels wears out. It is also possible that testimonial PWL may be more effective among some groups than others,18,24 although further work is required to investigate this. Testimonial PWL may also provide jurisdictions with useful opportunities for enhancing the impact of the warning labels through linkages with mass media campaigns.52 For instance, the successful Tips From Former Smokers mass media campaign in the United States53–55 featured a series of testimonials, three of which were the source of content for the testimonial PWL tested here. If the testimonial television advertisements were aired at the same as the testimonial PWL were appearing on cigarette packs, then past research suggests that the reinforcing effects of being exposed to the same message via two different sources could lead to stronger effects overall.56,57
One limitation of this study is that we did not measure whether smokers exposed to the testimonial PWL were aware that they were viewing images of real people, or if they believed that these photographs were staged. It is therefore difficult to claim that the beneficial effects of the testimonial PWL (particularly the image only testimonial PWL) are due to the fact that smokers knew they were viewing images of real people. Future research should investigate whether such knowledge moderates the impact of the PWL. Participants in the non-testimonial TWL condition were exposed to one less warning label than were participants in the three PWL conditions (four vs. five, respectively). This resulted from our decision to use the four TWL that currently appear on cigarette packs in the United States as stimuli in the non-testimonial TWL condition, an upside of which is that our analyses compare possible future warning labels with the current situation. We are confident that this differential exposure did not drive the overall pattern of effects, given that significant differences were also observed for the intention measures, which in all conditions, were measured following exposure to three warning labels.
Additional limitations associated with the experimental design include that exposure to the warning labels was limited, involved looking at the warning label on a static image of an unbranded cigarette pack, and occurred online in an artificial setting. On the other hand, the inclusion of a follow-up component is a particular strength, as it allowed an initial test of the potential impact of these testimonial PWL on self-reported quitting activities removed in time from immediate exposure to the labels. Additional research is certainly required to replicate these behavioral effects, and such work would be strengthened by the inclusion of more stringent definitions of quit success (eg, sustained cessation) and objectively validated measures of abstinence. Finally, we reiterate that our use of a non-probability online panel to recruit participants means that some uncertainty remains that these effects would be replicated within the broader population of smokers, both within the United States and elsewhere. In particular, this sample was more highly educated that the general population of smokers in the United States. Several other studies have observed that low- and high-education smokers in the United States are equally affected by exposure to PWL,34,58,59 and one study with smokers in Mexico found that highly educated smokers responded more favorably to PWL that carried non-testimonial than testimonial statements.24 Therefore, we do not expect the educational composition of our sample to undermine our conclusions about the potential effectiveness of testimonial PWL.
The findings from this study suggest that warning label images that present a factual account of the impact of tobacco on one individual, warrant consideration by the FDA as they work to develop PWL with the capacity to survive inevitable legal challenges from the tobacco industry. Final decisions about what will constitute a legally acceptable set of warning labels will come from a much larger body of scientific research and robust exchanges among interested parties in legal scholarship and in the courts.5–8 However, the present study suggests that smokers can be affected in important ways following even modest exposure to images of real people whose health has been affected by smoking.
Supplementary Material
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health and the Food and Drug Administration’s Center for Tobacco Products (P50CA179546). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We acknowledge the contribution of Mr Kyle Cassidy at the Annenberg School for Communication, University of Pennsylvania, who created the stimuli used in this study. We thank Professor Robert Hornik, Dr Laura Gibson, Professor Caryn Lerman, Dr Andrew Strasser, and Professor Emily Falk at the University of Pennsylvania, Professor Melanie Wakefield at Cancer Council Victoria, Associate Professor David Hammond at University of Waterloo, and Professor Ellen Goodman at Rutgers, who all provided feedback on the original study design. We also thank staff at Health Canada and at the Office on Smoking and Health at the Centers for Disease Control and Prevention, who arranged access to the images and stories used in the testimonial pictorial warning labels.
References
- 1. United States Public Laws. Family Smoking Prevention and Tobacco Control Act. Public Law 111–31 [H.R. 1256]. 2009.
- 2. World Health Organization. WHO Framework Convention on Tobacco Control. Guidelines for implementation Article 5.3; Article 8; Articles 9 and 10; Article 11; Article 12; Article 13; Article 14 2011. http://whqlibdoc.who.int/publications/2011/9789241501316_eng.pdf Accessed October 28, 2016.
- 3. Tobacco Control Legal Consortium. Cigarette graphic warnings and the divided federal courts 2015. http://publichealthlawcenter.org/sites/default/files/resources/Tobacco-Control-Legal-Consortium-Cigarette-Graphic-Warnings-and-the-Divided-Federal-Courts.pdf Accessed October 28, 2016.
- 4. Dennis B. Government quits legal battle over graphic cigarette warnings. Washington Post 2013. www.washingtonpost.com/national/health-science/government-quits-legal-battle-over-graphic-cigarette-warnings/2013/03/19/23053ccc-90d7-11e2-bdea-e32ad90da239_story.html Accessed October 28, 2016.
- 5. Kraemer JD, Baig SA. Analysis of legal and scientific issues in court challenges to graphic tobacco warnings. Am J Prev Med. 2013;45(3):334–342. [DOI] [PubMed] [Google Scholar]
- 6. Goodman EP. Visual gut punch: persuasion, emotion and the constitutional meaning of graphic disclosure. Cornell Law Rev. 2014;99(3):513–569. [PubMed] [Google Scholar]
- 7. Byrne S, Katz SJ, Niederdeppe J, Mathios AD. Do the ends justify the means? A test of alternatives to the FDA proposed cigarette warning labels. Health Commun. 2015;30(7):680–693. [DOI] [PubMed] [Google Scholar]
- 8. Peters E, Evans AT, Hemmerich N, Berman M. Emotion in the law and the lab: the case of graphic cigarette warnings. Tob Regul Sci. 2016;2(4):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Discount Tobacco City & Lottery, Inc. v. United States. 674 F.3d 509 (6th Cir. 2012).
- 10. R.J. Reynolds Tobacco Co. v. United States Food & Drug Admin. 845 F.Supp.2d 266 (D.C.C. 2012).
- 11. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105–113. [Google Scholar]
- 12. Braddock K, Dillard JP. Meta-analytic evidence for the persuasive effect of narratives on beliefs, attitudes, intentions, and behaviors. Commun Monogr. 2016;83(4):446–447. [Google Scholar]
- 13. Aust CF, Zillmann D. Effects of victim exemplification in television news on viewer perception of social issues. J Mass Commun Q. 1996;73(4):787–803. [Google Scholar]
- 14. Zillmann D. Exemplification theory: judging the whole by some of its parts. Media Psychol. 1999;1(1):69–94. [Google Scholar]
- 15. Zillmann D. Exemplification effects in the promotion of safety and health. J Commun. 2006;56(s1):S221–S237. [Google Scholar]
- 16. Gerrig RJ. Experiencing Narrative Worlds. On the Psychological Activities of Reading. New Haven, CT: Yale University Press; 1993. [Google Scholar]
- 17. Green MC, Brock TC. The role of transportation in the persuasiveness of public narratives. J Pers Soc Psychol. 2000;79(5):701–721. [DOI] [PubMed] [Google Scholar]
- 18. Durkin S, Biener L, Wakefield MA. Effects of different types of antismoking ads on reducing disparities in smoking cessation among socioeconomic subgroups. Am J Public Health. 2009;99(12):2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durkin S, Wakefield MA, Spittal MJ. Which types of televised anti-tobacco campaigns prompt more quitline calls from disadvantaged groups? Health Educ Res. 2011;26(6):998–1009. [DOI] [PubMed] [Google Scholar]
- 20. Leas EC, Myers MG, Strong DR, Hofstetter R, Al-Delaimy WK. Recall of anti-tobacco advertisements and effects on quitting behavior: results from the California smokers cohort. Am J Public Health. 2015;105(2):e90–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HS, Bigman CA, Leader AE, Lerman C, Cappella JN. Narrative health communication and behavior change: the influence of exemplars in the news on intention to quit smoking. J Commun. 2012;62(3):473–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thrasher JF, Carpenter MJ, Andrews JO, et al. Cigarette warning label policy alternatives and smoking-related health disparities. Am J Prev Med. 2012;43(6):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cameron LD, Williams B. Which images and features in graphic cigarette warnings predict their perceived effectiveness? Findings from an online survey of residents in the UK. Ann Behav Med. 2015;49(5):639–649. [DOI] [PubMed] [Google Scholar]
- 24. Thrasher JF, Arillo-Santillan E, Villalobos V, et al. Can pictorial warning labels on cigarette packages address smoking-related health disparities? Field experiments in Mexico to assess pictorial warning label content. Cancer Causes Control. 2012;23(suppl 1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammond D, Thrasher J, Reid JL, Driezen P, Boudreau C, Santillan EA. Perceived effectiveness of pictorial health warnings among Mexican youth and adults: a population-level intervention with potential to reduce tobacco-related inequities. Cancer Causes Control. 2012;23(suppl 1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mutti S, Reid JL, Gupta PC, et al. Perceived effectiveness of text and pictorial health warnings for smokeless tobacco packages in Navi Mumbai, India, and Dhaka, Bangladesh: findings from an experimental study. Tob Control. 2016;25(4):437–443. [DOI] [PubMed] [Google Scholar]
- 27. Huang L-L, Thrasher JF, Reid JL, Hammond D. Predictive and external validity of a pre-market study to determine the most effective pictorial health warning label content for cigarette packages. Nic Tob Res. 2016;18(5):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Survey Sampling International (SSI). Consumer online panel 2016. www.surveysampling.com/audiences/consumer-online/ Accessed October 28, 2016.
- 29. International Agency for Research on Cancer. Methods for Evaluating Tobacco Control Policies. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 30. Yong HH, Borland R, Thrasher JF, et al. Mediational pathways of the impact of cigarette warning labels on quit attempts. Health Psychol. 2014;33(11):1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang A-L, Lowen SB, Romer D, Giorno M, Langleben DD. Emotional reaction facilitates the brain and behavioural impact of graphic cigarette warning labels in smokers. Tob Control. 2015;24(3):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emery LF, Romer D, Sheerin KM, Jamieson KH, Peters E. Affective and cognitive mediators of the impact of cigarette warning labels. Nic Tob Res. 2014;16(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrews JC, Netemeyer RG, Kees J, Burton S. How graphic visual health warnings affect young smokers’ thoughts of quitting. J Market Res. 2014;51(2):165–183. [Google Scholar]
- 34. Gibson L, Brennan E, Momjian A, Shapiro-Luft D, Seitz H, Cappella JN. Assessing the consequences of implementing graphic warning labels on cigarette packs for tobacco-related health disparities. Nic Tob Res. 2015;17(8):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borland R, Yong HH, Wilson N, et al. How reactions to cigarette packet health warnings influence quitting: findings from the ITC Four-Country survey. Addiction. 2009;104(4):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Partos TR, Borland R, Thrasher JF, et al. The predictive utility of micro indicators of concern about smoking: findings from the International Tobacco Control Four Country Study. Addict Behav. 2014;39(8):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li L, Borland R, Fong GT, et al. Smoking-related thoughts and microbehaviours, and their predictive power for quitting: findings from the International Tobacco Control (ITC) China Survey. Tob Control. 2015;24(4):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thrasher JF, Swayampakala K, Borland R, et al. Influences of self-efficacy, response efficacy, and reactance on responses to cigarette health warnings: a longitudinal study of adult smokers in Australia and Canada. Health Commun. 2016;31(12):1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammond D, Fong GT, McDonald PW, Brown KS, Cameron R. Graphic Canadian cigarette warning labels and adverse outcomes: evidence from Canadian smokers. Am J Public Health. 2004;94(8):1442–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wegner DM. Ironic processes of mental control. Psychol Rev. 1994;10(1):34–52. [DOI] [PubMed] [Google Scholar]
- 41. Brennan E, Durkin SJ, Wakefield MA, Kashima Y. Assessing the effectiveness of antismoking television advertisements: do audience ratings of perceived effectiveness predict changes in quitting intentions and smoking behaviours? Tob Control. 2014;23(5):412–418. [DOI] [PubMed] [Google Scholar]
- 42. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. [DOI] [PubMed] [Google Scholar]
- 43. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 44. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nic Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 45. Maloney EK, Cappella JN. Does vaping in e-cigarette advertisements affect tobacco smoking urge, intentions, and perceptions in daily, intermittent, and former smokers? Health Commun. 2016;31(1):129–138. [DOI] [PubMed] [Google Scholar]
- 46. Stata Statistical Software: Release 13 [computer program]. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 47. Centers for Disease Control and Prevention. National Adult Tobacco Survey (NATS) 2016. www.cdc.gov/tobacco/data_statistics/surveys/nats/ Accessed October 28, 2016.
- 48. Canadian Cancer Society. Cigarette package health warnings: international status report (fourth edition) 2014. www.cancer.ca/~/media/cancer.ca/CW/For%20media/Media%20releases/2014/Tobacco%20Warnings%20Oct%202014/CCS-international-package-warnings-report-2014-ENG.pdf Accessed October 28, 2016.
- 49. Hitchman SC, Driezen P, Logel C, Hammond D, Fong GT. Changes in effectiveness of cigarette health warnings over time in Canada and the United States, 2002–2011. Nic Tob Res. 2014;16(4):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hammond D, Wakefield M, Durkin S, Brennan E. Tobacco packaging and mass-media campaigns: research needs for Articles 11 and 12 of the WHO Framework Convention on Tobacco Control. Nic Tob Res. 2013;15(4):817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li L, Borland R, Yong H, et al. Longer term impact of cigarette package warnings in Australia compared with the United Kingdom and Canada. Health Educ Res. 2015;30(1):67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rigotti NA, Wakefield M. Real people, real stories: a new mass media campaign that could help smokers quit. Ann Behav Med. 2012;157(12):907–909. [DOI] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention. Tips From Former Smokers 2016. www.cdc.gov/tobacco/campaign/tips/resources/videos/ Accessed October 28, 2016.
- 54. McAfee T, Davis KC, Alexander RL, Pechacek TF, Bunnell R. Effect of the first federally funded US antismoking national media campaign. Lancet. 2013;382(9909):2003–2011. [DOI] [PubMed] [Google Scholar]
- 55. McAfee T, Davis KC, Shafer P, Patel D, Alexander R, Bunnell R. Increasing the dose of television advertising in a national antismoking media campaign: results from a randomised field trial. Tob Control. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brennan E, Durkin S, Cotter T, Harper T, Wakefield M. Mass media campaigns designed to support new pictorial health warnings on cigarette packets: evidence of a complementary relationship. Tob Control. 2011;20(6):412–418. [DOI] [PubMed] [Google Scholar]
- 57. Thrasher JF, Murukutla N, Pérez-Hernández R, et al. Linking mass media campaigns to pictorial warning labels on cigarette packages: a cross-sectional study to evaluate effects among Mexican smokers. Tob Control. 2013;22(e1):e57–e65. [DOI] [PubMed] [Google Scholar]
- 58. Cantrell J, Vallone DM, Thrasher JF, et al. Impact of tobacco-related health warning labels across socioeconomic, race and ethnic groups: results from a randomized web-based experiment. PLOS ONE. 2013;8(1):e52206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hammond D, Reid JL, Driezen P, Boudreau C. Pictorial health warnings on cigarette packs in the United States: an experimental evaluation of the proposed FDA warnings. Nic Tob Res. 2013;15(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.