Abstract
Although yeast bloodstream infections (BSIs) are increasingly being reported in patients with hematological malignancies undergoing antifungal therapy, clinical information regarding breakthrough infections is scarce. The aim of this study was to determine the risk factors for and clinical outcomes of breakthrough yeast BSIs in patients with hematological malignancies in the era of newer antifungal agents. Between 2011 and 2014, all consecutive patients with hematological malignancies who developed yeast BSIs were included in a case-control study wherein breakthrough infections (cases) and de novo infections (controls) were compared. Of 49 patients with yeast BSIs, 21 (43%) met the criteria for breakthrough infections. The proportions of Candida krusei and Candida tropicalis in the cases and controls were significantly different (32% [7/22] vs. 3% [1/29], P = .015; 5% [1/22] vs. 38% [11/29], P = .007, respectively). Acute leukemia, presence of a central venous catheter and neutropenia in the 3 days prior to BSI were significant risk factors for breakthrough infections. Six-week mortality rates was 33% [7/21] in the cases and 43% [12/28] in the controls (P = .564). Refractory neutropenia and the Pitt bacteremia score were independent predictors of 6-week mortality. In conclusion, breakthrough infections accounted for a significant proportion of yeast BSIs in patients with hematological malignancies. However, these infections did not increase the risk of death by themselves. Our results suggest that current clinical management of breakthrough yeast BSIs, which includes switching to a different antifungal class and prompt catheter removal is reasonable.
Keywords: breakthrough infection, yeast, bloodstream infection, hematological malignancy
Introduction
Despite recent advances in diagnosis, prophylaxis and treatment, invasive fungal infections (IFIs) remain a leading cause of morbidity and mortality in immunocompromised or critically ill patients.1–3Candida albicans is a major cause of invasive yeast infections, but recently, yeast infections caused by non-albicans Candida species and other rare yeasts have increased.4–7 These less common yeasts usually colonize human skin and mucosal surfaces and are opportunistic pathogens associated with life-threatening infections in immunocompromised hosts. Notably, a significant number of invasive infections caused by these emerging pathogens occurs as breakthrough infections.6,7
Breakthrough invasive candidiasis is an unresolved problem in immunocompromised patients, particularly those with hematological malignancies.3,8 To date, few retrospective studies have investigated the risk factors for and outcomes of breakthrough candidemia compared to de novo candidaemia.9–12 Moreover, most of these studies were confined to candidemia patients and were published more than a decade ago, before the newer broad-spectrum antifungal agents came into use. The change in the epidemiology of IFIs, advances in antifungal agents, and updated strategies for the prevention and treatment of IFIs warrant a reevaluation of breakthrough invasive yeast infections. Therefore, we conducted a study to determine the risk factors for and clinical outcomes of breakthrough yeast bloodstream infections (BSIs) in patients with hematological malignancies in the era of newer antifungal agents.
Methods
Study design, hospital Setting, and patients
We conducted a case-control study from a prospective observational cohort of adult patients with hematological malignancies who developed yeast BSIs between January 2011 and December 2014. All patients aged 18 years or more who were admitted to the Catholic Blood and Marrow Transplantation (BMT) Center, a 1,355-bed tertiary-care teaching hospital located in the Republic of Korea were included in this cohort. All consecutive patients with yeast BSIs were enrolled in the case-control study. Those with breakthrough infections were designated as case patients, while those with de novo infections were included in the control group. In patients with yeast BSIs, blood cultures were usually repeated every 3 days until no infection was detected. During the study period, oral fluconazole tablets (50–400 mg daily), itraconazole oral solution (2.5 mg/kg twice daily), micafungin (50 mg daily) and posaconazole oral suspension (200 mg 3 times daily) were used as primary antifungal prophylaxis. For empirical or targeted therapy against breakthrough IFIs, we preferred to change the antifungal class including amphotericin B deoxycholate (1 mg/kg daily), caspofungin (loading dose of 70 mg, then 50 mg daily), liposomal amphotericin B (3–5 mg/kg daily), and voriconazole (loading dose of 6 mg/kg twice daily, then 3 mg/kg twice daily). Removal of central venous catheters (CVCs) was preferred in all non-neutropenic patients, while in neutropenic patients, infectious disease specialists decided whether to remove the CVC if it appeared to be the source of infection. The study was approved by the Institutional Review Board of Seoul St. Mary's Hospital, and informed consents were waived (KC16RISI0623).
Data collection
The annual usage of antifungal agents for patients admitted to the Catholic BMT Center during the study period was determined by calculating the number of defined daily doses (DDDs) per 1,000 patient-days, as recommended by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology (www.whocc.no/atc_ddd_index/).
Clinical and microbiological data were collected by infectious diseases specialists at the participating BMT Center. The following variables were recorded: patient demographics; type of underlying hematological malignancies; history of previous chemotherapy and hematopoietic stem cell transplantation; comorbidities; risk factors for yeast BSIs in the 4 weeks prior to the first positive blood culture including intensive care unit residence, use of corticosteroid (mean minimum dose of 10 mg daily of prednisone equivalent for >14 days), surgery, hyperalimentation, presence of CVC and neutropenia; prior and concurrent use of antibiotics and antifungals; Pitt bacteremia score at the onset of yeast BSI; clinical characteristics of yeast BSI episodes; antifungal therapy and clinical outcomes.
Definitions
A yeast BSI was defined as at least one blood culture that yields yeast from a patient with compatible clinical signs or symptoms. Candida spp. other than C. albicans, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis were termed ‘uncommon Candida spp.’.13 The day of sampling for the first positive blood culture was considered to be the time of onset of a yeast BSI. Breakthrough infection was defined as an infection occurring in a patient receiving systemic antifungal agents for at least 5 days for any reason before the onset of a yeast BSI.10 Neutropenia was defined as an absolute neutrophil count less than 500 cells/mm3 or expected count of less than 500 cells/ mm3 within the following 2–3 days.14 The definition of septic shock was adapted from the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee.15 The Pitt bacteremia score was calculated to assess the severity of illness at the onset of yeast BSIs.16 The source of the yeast BSI was classified based on clinical evidence of infection regardless of whether causative organisms were recovered from the affected site. Definition of CVC-related BSIs were defined as one of the following criteria: (i) culture of the same organism from both the catheter tip and at least one percutaneous blood culture; or (ii) culture of the same organism from at least two blood samples (one from a catheter hub and the other from a peripheral vein) and growth detected from the catheter hub sample at least 2 h before growth detected from the peripheral vein sample.17 Polymicrobial BSIs were defined as isolation of ≥2 different yeast spp. or a yeast with concomitant bacterial organisms from blood cultures obtained within a 48 h period.18 Time to initiation of appropriate antifungal therapy was defined as the number of days from the onset of a yeast BSI to administration of appropriate antifungal therapy. Appropriate antifungal therapy was defined as a loading or therapeutic dose of a qualifying antifungal agent including azoles, echinocandins and polyenes, based on the results of susceptibility testing, if available. Appropriate doses of antifungal agents for Candida spp. were determined by the updated Infectious Diseases Society of America guidelines.19 The appropriateness of antifungal therapy for yeasts other than Candida spp. was based on the European Society for Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology joint clinical guidelines.20
To evaluate the response to antifungal therapy at 2 weeks, we used a modified version of the Mycoses Study Group and European Organization for Research and Treatment of Cancer guidelines: persistent isolation of yeast spp. from blood specimens and death were termed as ‘treatment failure’.21 Mortality attributable to yeast BSIs was defined as any of the following: (i) blood cultures positive for yeast spp. at the time of death; (ii) failure to resolve signs and symptoms related to yeast BSIs at the time of death; or (iii) death at least 14 days after the onset of yeast BSIs without another explanation.18 The follow-up duration was 100 days after the onset of the yeast BSI, loss to follow-up or death from any cause.
Microbiology and susceptibility tests
Blood cultures were processed using the Bactec Fx automated blood culture system (BD Diagnostics, Sparks, MD, USA). Yeast spp. identification and antifungal susceptibility testing were performed using a commercially available automated system (Vitek®2; bioMérieux, Hazelwood, MO, USA). Interpretation of susceptibility was based on the recent minimal inhibitory concentration breakpoints adopted by the Clinical and Laboratory Standards Institute M27-S4.22
Statistical analyses
Statistical analysis was performed using SPSS version 15.0 (SPSS Korea, Seoul, Korea). Continuous variables were represented using the median and interquartile range (IQR). The χ2 test or Fisher's exact test was used to compare categorical variables. Student's t-test or the Mann–Whitney U-test was used to compare continuous variables. Multivariate analysis was performed by logistic regression analysis for the risk factors associated with breakthrough infections. Cox regression analysis was performed to identify the predictors of 6-week mortality. Variables with P-values of <.20 by univariate analysis were entered in the multivariable model. Kaplan–Meier survival estimates were used to generate survival curves and differences between survival curves were assessed by means of the log-rank test. Two-sided P-values <.05 were considered statistically significant.
Results
Incidence of yeast BSIs and patient characteristics
Among 4,249 adult patients who underwent 10,148 admissions, significant yeast BSIs occurred in 49 patients. The overall incidence yeast BSIs was 22 cases per 1,000 patient-days (4.8 cases per 1,000 hospital admissions). Twenty-one patients (43%) had infections that met the definition for breakthrough infections.
Regardless of the indication of antifungal therapy, fluconazole and itraconazole were most widely used during the study period (Fig. 1). The annual usage of polyenes decreased from 2,510 DDD per patient-day in 2011 to 1,018 DDD per patient-days in 2014, whereas the usage of voriconazole, caspofungin, and micafungin tended to increase slowly.
Figure 1.
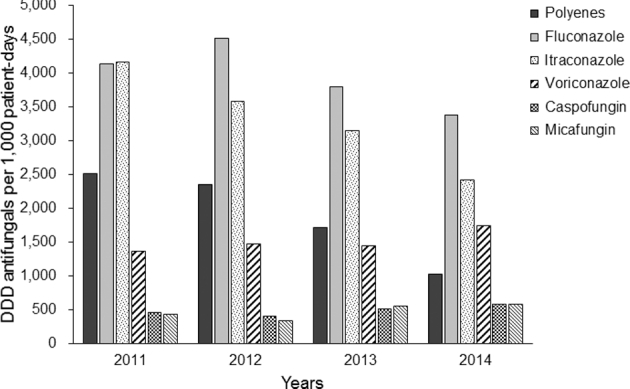
Annual use of antifungal drugs at the Catholic Blood and Marrow Transplantation Center from January 2011 to December 2014. DDD, defined daily doses. Note. Posaconazole was only available through the Korean Orphan Drug Center by early 2014 in Korea, and no statistics could be calculated.
Table 1 shows the study patients’ demographic and clinical characteristics. The two patient groups (cases and controls) did not differ significantly in age, sex, comorbidities, risk factors for yeast BSIs, severity of illness, and source of infections. The total duration of neutropenia was significantly longer in the case group (median, 15 days; IQR, 8–39 days) than the control group (median, 0 days; IQR, 0–3 days; P = .038). Neutropenia in the 3 days prior to a BSI and prior azole exposure in the 4 weeks prior to a BSI were significantly frequent in the case group than the control group. Among the 21 patients with breakthrough yeast BSIs, 15 (71%) were receiving azoles (11 patients on fluconazole, 3 patients on itraconazole, and 1 patient on posaconazole). Three patients (14%) developed breakthrough yeast BSIs while receiving echinocandins (2 patients on caspofungin and 1 patient on micafungin), while another 3 (14%) developed them during polyene therapy (Table 2).
Table 1.
Demographic and clinical characteristics of the patients with breakthrough yeast bloodstream infections (cases) compared to those with de novo yeast bloodstream infections (controls).
| Characteristics | All patients | Cases | Controls | P |
|---|---|---|---|---|
| (n = 49) | (n = 21) | (n = 28) | ||
| Age, median years (IQR) | 58 (49–65) | 54 (50–63) | 62 (49–65) | .84 |
| Male sex | 26 (53) | 13 (62) | 13 (46) | .39 |
| Underlying hematological malignancy | .06 | |||
| Acute leukemia | 20 (41) | 13 (62) | 7 (25) | |
| Myelodysplastic syndrome | 8 (16) | 4 (19) | 4 (14) | |
| Lymphoma | 8 (16) | 2 (10) | 6 (21) | |
| Multiple myeloma | 7 (14) | 1 (5) | 6 (21) | |
| Othera | 6 (12) | 1 (5) | 5 (18) | |
| Treatment of hematological malignancy | .46 | |||
| Chemotherapy | 29 (59) | 14 (67) | 15 (54) | |
| Hematopoietic stem cell transplantation | 6 (12) | 3 (14) | 3 (11) | |
| Supportive therapy | 14 (29) | 4 (19) | 10 (36) | |
| Comorbidities | ||||
| Diabetes mellitus | 13 (27) | 7 (33) | 6 (21) | .51 |
| Chronic kidney disease | 2 (4) | 0 (0) | 2 (7) | .50 |
| Chronic liver disease | 3 (6) | 1 (5) | 2 (7) | 1.00 |
| Prior hospital stay, median days (IQR) | 15 (8–34) | 22 (15–35) | 11 (4–28) | .68 |
| Risk factors for yeast BSIsb | ||||
| ICU residence | 10 (20) | 3 (14) | 7 (25) | .48 |
| Corticosteroidc | 20 (41) | 10 (48) | 10 (36) | .56 |
| Surgery | 7 (14) | 4 (19) | 3 (11) | .44 |
| Hyperalimentation | 38 (78) | 17 (81) | 21 (75) | .74 |
| Central venous catheter | 44 (90) | 21 (100) | 23 (82) | .06 |
| Prior use of antibiotics | 46 (94) | 19 (91) | 27 (96) | .57 |
| Neutropenia in the 3 days prior to a yeast BSI | 30 (61) | 18 (86) | 12 (43) | <.01 |
| Prior azole exposure within 4 weeks prior to a yeast BSI | 20 (41) | 19 (91) | 1 (4) | <.01 |
| Pitt bacteremia score, median (IQR) | 1 (0–3) | 1 (0–2) | 1 (0–3) | .93 |
| Source of yeast BSIs | .19 | |||
| Central venous catheter | 21 (43) | 9 (43) | 12 (43) | |
| Gastrointestinal tract | 12 (24) | 7 (33) | 5 (18) | |
| Unknown | 10 (20) | 5 (24) | 5 (18) | |
| Otherd | 6 (12) | 0 (0) | 6 (22) | |
| Polymicrobial BSIse | 23 (47) | 12 (57) | 11 (39) | .26 |
| First-line antifungal agent | <.01 | |||
| Azole | 10 (20) | 1 (5) | 9 (32) | |
| Echinocandin | 11 (22) | 7 (33) | 4 (14) | |
| Polyene | 28 (57) | 13 (62) | 15 (54) | |
| Time to initiation of appropriate antifungal therapy for yeast BSIs, median days (IQR) | 2 (1–3) | 2 (1–4) | 2 (1–3) | .60 |
| Total duration of antifungal therapy for yeast BSIs, median days (IQR) | 18 (11–27) | 19 (13–30) | 18 (6–27) | .94 |
Note. Data are no. (%) of patients, unless otherwise indicated.
BSI, bloodstream infection; ICU, intensive care unit; IQR, interquartile range.
aSevere aplastic anemia (n = 3), hemophagocytic lymphohistiocytosis (n = 2), and chronic myeloid leukemia (n = 1).
bRisk factors within 30 days prior to a yeast bloodstream infection.
cMean minimum dose of 10 mg/day of prednisone equivalent for >14 days.
dGenitourinary tract (n = 5) and deep soft tissue (n = 1).
eIsolation of ≥2 different species of yeast or a yeast with concomitant bacterial species from blood cultures obtained within a 48-h period.
Table 2.
Summary of breakthrough yeast bloodstream infections in patients with hematological malignancies.
| Patients No. | Sex/age | Underlying hematological malignancy | Existing antifungal agent | Source | Yeast species | Susceptibility to existing antifungal agent | First-line antifungal agent | Outcome at 6 weeks |
|---|---|---|---|---|---|---|---|---|
| 1 | M/48 | Acute leukemia | Micafungin | CVC | Candida krusei | Unknown | Amphotericin B | Died |
| 2 | F/60 | Lymphoma | Fluconazole | Unknown | Candida krusei | Resistant | Amphotericin B | Survived |
| 3 | M/54 | Acute leukemia | Amphotericin B | GI tract | Candida krusei | Unknown | Caspofungin | Survived |
| 4 | M/43 | Myelodysplastic syndrome | Amphotericin B | CVC | Saccharomyces cerevisiae | Susceptible | Caspofungin | Survived |
| 5 | M/63 | Multiple myeloma | Itraconazole | CVC | Candida albicans Candida dubliniensis | Susceptible | Amphotericin B | Died |
| 6 | M/68 | Acute leukemia | Fluconazole | GI tract | Candida krusei | Resistant | Amphotericin B | Survived |
| 7 | M/46 | Acute leukemia | Fluconazole | Unknown | Candid tropicalis | Susceptible | Amphotericin B | Died |
| 8 | M/68 | Myelodysplastic syndrome | Amphotericin B | CVC | Candida pelliculosa | Unknown | Amphotericin B | Survived |
| 9 | F/51 | Myelodysplastic syndrome | Fluconazole | GI tract | Candida guillermondii | Susceptible | Amphotericin B | Died |
| 10 | F/54 | Lymphoma | Fluconazole | GI tract | Candida glabrata | Resistant | Caspofungin | Survived |
| 11 | M/42 | Acute leukemia | Fluconazole | GI tract | Candida krusei | Resistant | Amphotericin B | Survived |
| 12 | F/62 | Acute leukemia | Fluconazole | CVC | Candida albicans | Unknown | Amphotericin B | Survived |
| 13 | F/53 | Acute leukemia | Fluconazole | CVC | Candida krusei | Resistant | Amphotericin B | Survived |
| 14 | M/56 | Acute leukemia | Posaconazole | GI tract | Candida glabrata | Susceptible | Caspofungin | Survived |
| 15 | F/54 | Myelodysplastic syndrome | Itraconazole | CVC | Trichosporon asahii | Resistant | Voriconazole | Survived |
| 16 | M/29 | Severe aplastic anemia | Fluconazole | GI tract | Candida albicans | Susceptible | Caspofungin | Survived |
| 17 | M/51 | Acute leukemia | Caspofungin | Unknown | Pseudozyma aphidis | Resistant | Caspofungin | Survived |
| 18 | F/73 | Acute leukemia | Itraconazole | CVC | Candida krusei | Resistant | Amphotericin B | Died |
| 19 | F/51 | Acute leukemia | Fluconazole | CVC | Candida inconspicua | Resistant | Caspofungin | Survived |
| 20 | M/69 | Acute leukemia | Fluconazole | Unknown | Candida glabrata | Resistant | Amphotericin B | Died |
| 21 | M/56 | Acute leukemia | Caspofungin | Unknown | Candida glabrata | Susceptible | Amphotericin B | Died |
CVC, central venous catheter; GI, gastrointestinal.
Distribution of yeast spp. and risk factors for breakthrough yeast BSIs
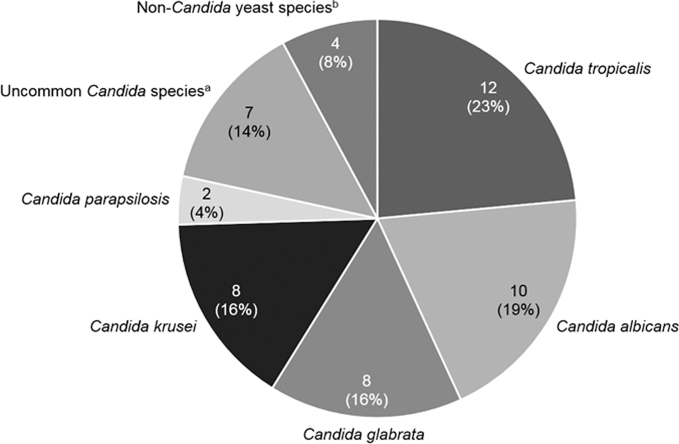
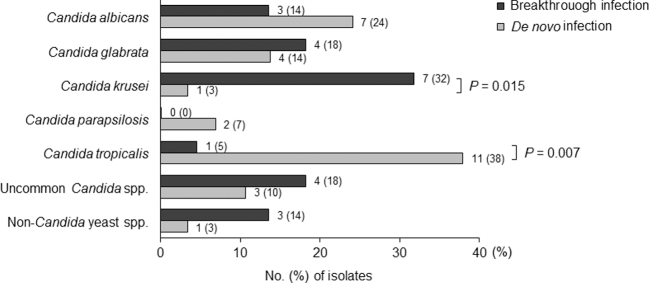
A total of 51 yeast spp. were isolated from 49 patients (Fig. 2). Two patients had polymicrobial BSIs (C. albicans/Candida dubliniensis and C. tropicalis/Candida lusitaniae). C. tropicalis (12/51, 24%) was the most common yeast spp., followed by C. albicans (10/51, 10%), C. glabrata (8/51, 16%), and C. krusei (8/51, 16%). Uncommon Candida spp. accounted for 14% (7/51) of BSIs. Four non-Candida yeast spp. (8%), including Pseudozyma aphidis, Saccharomyces cerevisiae, and Trichosporon asahii, were isolated. There were significant differences in the distribution of yeast spp. between the two patient groups (Fig. 3). C. krusei was more prevalent in the case group than the control group (32% vs. 3%, P = .015), while the opposite was true for C. tropicalis (5% vs. 38%, P = .007). Among the 22 yeast isolates obtained from the case group, 10 isolates (45%) were resistant to the antifungal agent that the patient was receiving when the breakthrough infection occurred, while 7 (32%) were susceptible. Susceptibility profiles of the other five isolates (23%) were not available.
Figure 2.
Distribution of yeast species causing bloodstream infections (BSIs) in patients with hematological malignancies. Note. Data are no. (%) of isolates. aCandida dubliniensis (n = 1), Candida guilliermondii (n = 1), Candida inconspicua (n = 1), and Candida pelliculosa (n = 1) in breakthrough BSIs; Candida famata (n = 1), and Candida lusitaniae (n = 2) in de novo BSIs. bSaccharomyces cerevisiae (n = 1), Trichosporon asahii (n = 1), and Pseudozyma aphidis (n = 1) in breakthrough BSIs; Saccharomyces cerevisiae (n = 1) in de novo BSI.
Figure 3.
Comparison of the distribution of yeast species isolated from breakthrough and de novo yeast bloodstream infections.
In comparison to the control group, breakthrough yeast BSIs occurred more frequently in patients with acute leukemia (62% vs. 25%; P = .018) or neutropenia in the 3 days prior to a yeast BSI (86% vs. 43%; P < .001). Table 3 shows the results of univariate and multivariate analyses of risk factors for breakthrough yeast BSIs. On multivariate analysis, acute leukemia, presence of a CVC and neutropenia in the 3 days prior to a yeast BSI were significantly associated with breakthrough yeast BSIs.
Table 3.
Logistic regression analysis for risk factors associated with breakthrough yeasts bloodstream infections.
| Characteristics | OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Male sex | 1.88 (0.59–5.93) | 0.29 | – | NA |
| Acute leukemia | 6.57 (1.74–24.77) | 0.005 | 6.63 (1.36–32.35) | 0.019 |
| Supportive therapy for hematological malignancies | 2.36 (0.62–8.98) | 0.21 | – | NA |
| Prior hospital stay ≥4 weeks | 1.85 (0.54–6.30) | 0.33 | – | NA |
| Presence of a central venous catheter | 4.35 (0.47–40.40) | 0.19 | 24.70 (1.61–379.68) | 0.021 |
| Neutropenia in the 3 days prior to a yeast BSI | 8.00 (1.91–33.54) | 0.004 | 10.67 (1.88–60.64) | 0.008 |
BSI, bloodstream infection; CI, confidence interval; NA, not available; OR, odds ratio.
Antifungal treatment, removal of CVCs, and clinical outcomes of yeast BSIs
First-line antifungal agents for the treatment of yeast BSIs were significantly different between the cases and controls (P < .001). Polyenes were the most commonly used first-line antifungal agent in both groups (13/21, 62% and 15/28, 54%, respectively). However, the second most frequently used antifungal agent was echinocandins in the case group (7/21, 33%) and azoles in the control group (9/28, 32%). The median time to initiation of appropriate antifungal therapy was 2 days in both the cases (IQR, 1–4 days) and the controls (IQR, 1–3 days) after the onset of yeast BSIs (P = .60). The total duration of antifungal therapy for yeast BSIs did not differ between the cases (median, 19 days; IQR, 13–30 days) and controls (median, 18 days; IQR, 6–27 days) (P = .94).
Among the 44 patients who developed yeast BSIs in the presence of CVCs, the CVC was removed in 12 (57%) of 21 cases and 19 (83%) of 23 controls (P = .10). The CVC was retained in two non-neutropenic patients with other sources of infection and in three neutropenic patients with CVCs-related BSIs who were hemodynamically unstable. The median time for removal of CVCs in the cases and controls was 8 days (IQR, 3.5–19 days) and 3 days (IQR, 2–7 days) after the onset of the yeast BSI, respectively (P = .70). The removal of CVCs within 48 h after the onset of yeast BSIs was observed in 14% (3/21) of cases and 43% (12/28) of controls (P = .06).
The clinical outcomes of the cases and controls are presented in Table 4. Seven of 21 (33%) cases and 12 of 28 (43%) controls were defined as treatment failure at 2 weeks (P = .56). Among the four cases and three controls who showed microbiologic failure at 2 weeks, six patients had removed the CVCs. At 2 weeks, the frequency of death was 14% (3/21) of the cases and 32% (9/28) of controls (P = .19). The crude mortality at 6 weeks was 33% (7/21) for the cases and 43% (12/28) for the controls (P = .56). The mortality attributable to yeast BSIs at 6 weeks and overall mortality was similar for the two groups (29% vs. 32%; P = 1.00; 62% vs. 64%; P = 1.00).
Table 4.
Comparison of clinical outcomes between patients with breakthrough yeast bloodstream infections (cases) and those with de novo yeast bloodstream infections (controls).
| Variables | Cases (n = 21) | Controls (n = 28) | P |
|---|---|---|---|
| Ocular involvementa | 4/10 (40) | 6/11 (55) | .67 |
| Septic shock | 4 (19) | 8 (29) | .52 |
| Continuous renal replacement therapy | 1 (5) | 6 (21) | .21 |
| Mechanical ventilation | 3 (14) | 3 (11) | 1.00 |
| Treatment failure at 2 weeks | 7 (33) | 12 (43) | .56 |
| Microbiologic failureb | 4/18 (22) | 3/19 (16) | .69 |
| Death | 3 (14) | 9 (32) | .19 |
| Mortality at 6 weeks | |||
| Crude | 7 (33) | 12 (43) | .56 |
| Attributable | 6 (29) | 9 (32) | 1.00 |
| Overall mortality | |||
| Crude | 13 (62) | 18 (64) | 1.00 |
| Attributable | 6 (29) | 9 (32) | 1.00 |
Note. Data are no. (%) of patients, unless otherwise indicated.
aData are event/evaluable no. (%) of patients.
bPersistent isolation of yeast species from blood specimens at 2 weeks. Data are event/survivors no. (%) of patients.
Table 5 demonstrates the predictors of 6-week mortality. On univariate analysis, breakthrough infection (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.27–1.72; P = .41) and the retention of CVCs 48 h after the onset of yeast BSIs (HR, 1.19; 95% CI, 0.45–3.13; P = .73) was not associated with higher mortality at 6 weeks. After multivariate analysis, refractory neutropenia (adjusted HR, 38.71; 95% CI, 9.09–164.89) and the Pitt bacteremia score (adjusted HR, 2.15; 95% CI, 1.43–3.23) remained independent predictors of mortality at 6 weeks. The Kaplan-Meier survival curve shows no difference between survival in the two patient groups (Fig. 4). The median (IQR) time to death was 23 days (2–86 days) for the case group and 7 days (4–67 days) for the control group (P = .313).
Table 5.
Predictors of 6-week mortality in all patients with yeast bloodstream infections.
| Characteristics | HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|
| Male sex | 2.05 (0.78–5.39) | .15 | … | .83 |
| Diabetes mellitus | 1.93 (0.76–4.93) | .17 | … | .35 |
| Refractory neutropenia | 11.24 (4.15–30.42) | <.001 | 61.49 (10.25–368.85) | <.001 |
| Source of infection, genitourinary tract | 2.32 (0.67–7.99) | .18 | … | .59 |
| Candida glabrata | 2.09 (0.75–5.86) | .16 | … | .92 |
| Pitt bacteremia score | 1.63 (1.32–2.02) | <.001 | 1.89 (1.14–3.11) | .013 |
| Inappropriate antifungal regimen | 2.50 (1.01–6.15) | .05 | … | .41 |
| Delay in initiation of antifungal therapy >48 h | 2.66 (1.07–6.62) | .04 | … | .37 |
| Retention of a central venous catheter | 2.15 (0.80–5.78) | .13 | … | .99 |
| Breakthrough infection | 2.62 (0.86–7.99) | .09 | … | .28 |
CI, confidence interval; HR, hazard ratio.
Figure 4.
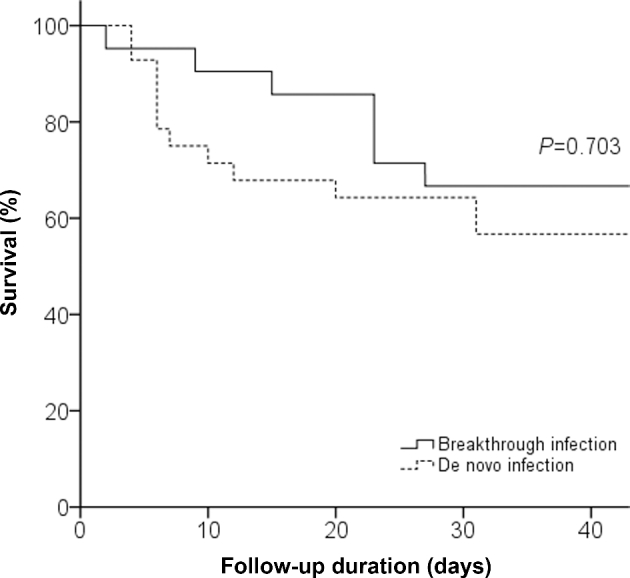
Kaplan–Meier estimates of survival in patients with breakthrough and de novo yeast bloodstream infections.
Discussion
In the present study, breakthrough infections accounted for a significant proportion (43%) of yeast BSIs in patients with hematological malignancies. Compared to de novo infections, breakthrough infections were more often caused by C. krusei. Acute leukemia, presence of a CVC and neutropenia were significantly associated with breakthrough infections. Refractory neutropenia and severity of illness at the onset of yeast BSIs were independent prognostic factors.
Since the first case of breakthrough candidemia was reported in 1993, a number of studies on breakthrough candidemia have been published.10–12,23–25 Most of these studies were performed in patients with cancer. A recent study showed that breakthrough candidemia occurred more frequently in hematological (37%) rather than oncological (10%) patients.8 In another study, breakthrough infection accounted for 53% of all cases of candidemia in patients with hematological malignancies.3 Antifungal prophylaxis and empirical or preemptive antifungal therapy are recommended for patients with anticipated prolonged and profound neutropenia, such as hematopoietic stem cell transplant recipients or patients undergoing intensive chemotherapy for acute leukemia. Therefore, such patients have a high risk of contracting candidemia and are also more frequently exposed to antifungal agents. This could be a possible explanation for the high incidence of breakthrough infections in patients with hematological malignancies.
Although candidemia predominantly originates from sources other than a CVC such as the gastrointestinal tract in neutropenic patients,26 CVCs and other intravascular devices are suggested to be important risk factors in the development breakthrough candidaemia.27 For example, they could provide an entrance site or reservoir for Candida spp. in a biofilm. However, previous studies failed to identify the presence of a CVC as a risk factor for breakthrough candidaemia.10,12,28 The authors presumed that it was impossible to evaluate the role of a CVC in breakthrough candidemia because most patients had a CVC. In our study, a CVC was the source of infection in less than 50% of patients. Even though most patients with yeast BSIs had a CVC, it was a significant factor associated with breakthrough infections. This might be due to some differences in the study design and epidemiology including the subject of study (the general population, patients with cancer or hematological malignancies) and the proportion of breakthrough infections (10 to 45%).
Trends in the distribution of various yeast spp. causing breakthrough infections were consistent with those of previous studies.8,10–12,29,30 Most patients who developed a breakthrough infection were receiving azoles and were infected with azole-resistant Candida spp., especially C. krusei and non-Candida yeast spp. However, we also found breakthrough infections due to strains susceptible to an antifungal agent currently being administered such as C. albicans, which might have resulted from the relatively protective environment of CVCs or suboptimal concentrations of antifungal drugs due to drug interactions or poor intestinal absorption. Therefore, the removal of CVCs should be considered for breakthrough yeast BSIs caused by strains susceptible to a previously administered antifungal agent regardless of the absolute neutrophil count or the source of infection. Like previous studies,8,10,11,25 this study showed no differences in the severity of infection at the onset of BSIs, crude mortality and mortality attributable to yeast BSIs between breakthrough and de novo infections. Therefore, current clinical practice in our institution which includes a switch to a different antifungal class and prompt removal of CVCs is appropriate for treatment of breakthrough yeast BSIs.
In contrast to previous studies, our study involves newer antifungal agents and a relatively homogeneous population. However, this study has some limitations. First, since the study was conducted at a single tertiary-care teaching hospital, the results may not be generalizable to other institutions. Second, antifungal susceptibility could not be determined for all isolates. Third, the number of patients was relatively small.
In conclusion, patients with hematological malignancies had a higher incidence of yeast BSIs than the general population. Breakthrough infections accounted for 43% of all episodes. Some differences existed between the clinical, epidemiological, and in vitro susceptibility data of breakthrough and de novo infections. Current strategies for breakthrough yeast BSIs including a switch to a different antifungal class and prompt removal of CVCs would be reasonable. However, clinical study data on this issue are very limited and large-scale, multicenter studies should be designed. Moreover, evidence-based monitoring for a high-risk group and development of new diagnostic tool for earlier detection of yeast BSIs are warranted to improve the clinical outcomes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Oren I, Paul M. Up to date epidemiology, diagnosis and management of invasive fungal infections. Clin Microbiol Infect. 2014; 20 (Suppl 6): 1–4. [DOI] [PubMed] [Google Scholar]
- 2. Corzo-Leon DE, Satlin MJ, Soave R et al. . Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: A single-centre study with focus on emerging pathogens. Mycoses. 2015; 58: 325–336. [DOI] [PubMed] [Google Scholar]
- 3. Gamaletsou MN, Walsh TJ, Zaoutis T et al. . A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014; 20: O50–57. [DOI] [PubMed] [Google Scholar]
- 4. Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008; 14 (Suppl 4): 5–24. [DOI] [PubMed] [Google Scholar]
- 5. Pagano L, Caira M, Nosari A et al. . Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007; 45: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 6. Chitasombat MN, Kofteridis DP, Jiang Y et al. . Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Infection. 2012; 64: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caira M, Trecarichi EM, Tumbarello M et al. . Uncommon yeast infections in hematological patients: from diagnosis to treatment. Expert Rev Anti Infect Ther. 2011; 9: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 8. Puig-Asensio M, Ruiz-Camps I, Fernandez-Ruiz M et al. . Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015; 21: e491–410. [DOI] [PubMed] [Google Scholar]
- 9. Nucci M, Colombo AL, Spector N et al. . Breakthrough candidemia in neutropenic patients. Clin Infect Dis. 1997; 24: 275–276. [DOI] [PubMed] [Google Scholar]
- 10. Uzun O, Ascioglu S, Anaissie EJ et al. . Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis. 2001; 32: 1713–1717. [DOI] [PubMed] [Google Scholar]
- 11. Kontoyiannis DP, Reddy BT, Hanna H et al. . Breakthrough candidemia in patients with cancer differs from de novo candidemia in host factors and candida species but not intensity. Infect Control Hosp Epidemiol. 2002; 23: 542–545. [DOI] [PubMed] [Google Scholar]
- 12. Nucci M, Colombo AL. Risk factors for breakthrough candidemia. Eur J Clin Microbiol Infect Dis. 2002; 21: 209–211. [DOI] [PubMed] [Google Scholar]
- 13. Chen SC, Marriott D, Playford EG et al. . Candidaemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009; 15: 662–669. [DOI] [PubMed] [Google Scholar]
- 14. Lee DG, Kim SH, Kim SY et al. . Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011; 26: 220–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bone RC, Balk RA, Cerra FB et al. . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine; Chest. 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 16. Rhee JY, Kwon KT, Ki HK et al. . Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009; 31: 146–150. [DOI] [PubMed] [Google Scholar]
- 17. Mermel LA, Allon M, Bouza E et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 49: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SH, Yoon YK, Kim MJ et al. . Risk factors for and clinical implications of mixed Candida/bacterial bloodstream infections. Clin Microbiol Infect. 2013; 19: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pappas PG, Kauffman CA, Andes DR et al. . Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 62: e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arendrup MC, Boekhout T, Akova M et al. . ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014; 20 (Suppl 3): 76–98. [DOI] [PubMed] [Google Scholar]
- 21. Segal BH, Herbrecht R, Stevens DA et al. . Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008; 47: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clinical and Laboratory Standards Institute Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4 Wayne, PA: Clinical and Laboratory Standards Institute, 2012. [Google Scholar]
- 23. Myoken Y, Kyo T, Fujihara M et al. . Clinical significance of breakthrough fungemia caused by azole-resistant Candida tropicalis in patients with hematologic malignancies. Haematologica. 2004; 89: 378–380. [PubMed] [Google Scholar]
- 24. Myoken Y, Kyo T, Sugata T et al. . Breakthrough fungemia caused by fluconazole-resistant Candida albicans with decreased susceptibility to voriconazole in patients with hematologic malignancies. Haematologica. 2006; 91: 287–288. [PubMed] [Google Scholar]
- 25. Gamaletsou MN, Daikos GL, Walsh TJ et al. . Breakthrough candidaemia caused by phenotypically susceptible Candida spp. in patients with haematological malignancies does not correlate with established interpretive breakpoints. Int J Antimicrob Agents. 2014; 44: 248–255. [DOI] [PubMed] [Google Scholar]
- 26. Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001; 33: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 27. Rex JH. Editorial response: catheters and candidemia. Clin Infect Dis. 1996; 22: 467–470. [DOI] [PubMed] [Google Scholar]
- 28. Blumberg EA, Reboli AC. Failure of systemic empirical treatment with amphotericin B to prevent candidemia in neutropenic patients with cancer. Clin Infect Dis. 1996; 22: 462–466. [DOI] [PubMed] [Google Scholar]
- 29. Maschmeyer G, Patterson TF. Our 2014 approach to breakthrough invasive fungal infections. Mycoses. 2014; 57: 645–651. [DOI] [PubMed] [Google Scholar]
- 30. Krcmery VC Jr., Babela R. Mortality associated with breakthrough candidemia among patients with and those without cancer. Clin Infect Dis. 2002; 35: 898; author reply 899. [DOI] [PubMed] [Google Scholar]