Abstract
Introduction
Novel nicotine delivery systems represent an evolving part of the tobacco harm reduction strategy. The pharmacokinetic (PK) profile of nicotine delivered by P3L, a pulmonary nicotine delivery system, and its effects on smoking urges and craving relief in relation to Nicorette inhalator were evaluated.
Methods
This open-label, ascending nicotine levels study was conducted in 16 healthy smokers. Three different nicotine delivery levels, 50, 80, and 150 µg/puff, delivered by the P3L system were evaluated consecutively on different days after the use of the Nicorette inhalator. Venous nicotine PK, subjective effects, and tolerability were assessed.
Results
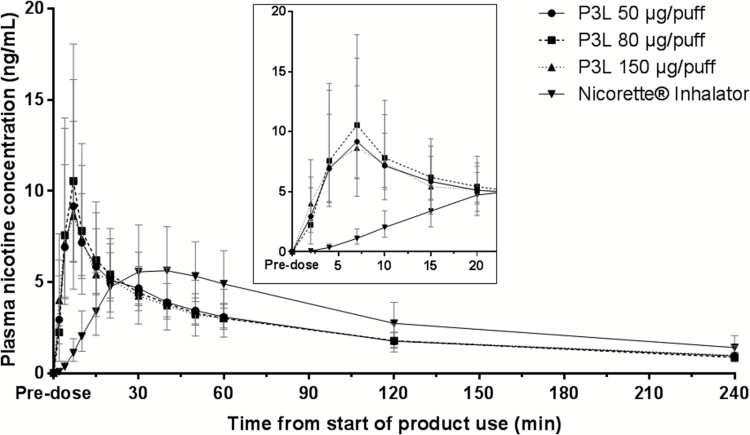
Geometric least-squares means for maximum plasma nicotine concentration (Cmax), generated by the mixed-effect model for exposure comparison, were 9.7, 11.2, and 9.8 ng/mL for the 50, 80, and 150 µg/puff P3L variants, respectively, compared to 6.1 ng/mL after Nicorette inhalator use. Median time from product use start to Cmax was 7.0 minutes for all P3L, compared to 30.0 minutes for the Nicorette inhalator. Craving reduction was slightly faster than with the Nicorette inhalator as assessed with the visual analog scale craving score. The mean Questionnaire of Smoking Urges -brief total scores did not differ for both products. P3L was well tolerated.
Conclusions
At all three nicotine levels tested, the inhalation of the nicotine lactate aerosol delivered with the P3L provided plasma nicotine concentrations higher and faster compared to the Nicorette inhalator. The plasma nicotine concentration–time profile supports a pulmonary route of absorption for P3L compared to the oromucosal absorption of the Nicorette inhalator.
Implications
The combination of nicotine and lactic acid with the P3L device shows potential over existing nicotine delivery systems by delivering nicotine with kinetics close to published data on conventional cigarettes and without exogenous carrier substances as used in current electronic nicotine delivery systems. Altogether, the PK profile, subjective effects, and safety profile obtained in this study suggest P3L is an innovative nicotine delivery product that will be acceptable to adult smokers as an alternative to cigarettes.
Introduction
Reducing exposure to toxicants and thereby providing a potentially safer delivery of nicotine compared to cigarettes are among the strategies to reduce the harm caused by tobacco smoking-related diseases.1,2 As part of the tobacco harm reduction strategy, novel nicotine delivery systems constitute potential alternatives to cigarettes (CC). However, in order to enhance the chances that smokers successfully transition from CC to a novel nicotine delivery system, such a system must be well tolerated and acceptable to them.
Consumer acceptability and effectiveness of a nicotine delivery system as a substitute for CC may be attributed to a nicotine pharmacokinetic (PK) profile that is comparable to CC.3,4 Thus, the development of an inhaled nicotine delivery system with absorption kinetics similar to those of a cigarette would be an advancement in pursuing harm reduction through nicotine maintenance.5
In recent years, new electronic nicotine delivery systems (ENDS) were introduced to the market and continue to gain interest as CC smoking replacements.6,7 However, they are not able to deliver nicotine as efficiently as a CC.8,9 This might explain their low satisfaction scores for some smokers and rates of reverting to CC smoking.10 Nevertheless, latest ENDS developments seem to increase nicotine delivery efficiencies.11 Other non-tobacco-based nicotine delivery systems, currently available as nicotine replacement therapy (NRT), such as the Nicorette inhaler system (Johnson & Johnson), exhibit slow nicotine absorption,12,13 which contribute to their limited capacity to act as smoking substitutes.14
In contrast to currently available electronic nicotine delivery systems, for example, electronic cigarettes, the novel nicotine delivery system P3L does not contain exogenous carrier compounds such as propylene glycol, used in e-cigarettes vaporizing nicotine-containing liquids. The aerosol from P3L is generated by combining vapors of a weak base such as nicotine with a weak acid to form particles of a neutral salt molecule. This technology for the pulmonary delivery of nicotine was first described by Rose et al.15 and clinically validated in a study yielding a rapid increase in plasma nicotine concentration, subjective satisfaction, and craving relief after exposure to several doses of an aerosol formed by nicotine and pyruvic acid vapors administered by inhalation.3 In addition, a repeated dose inhalation toxicity study in rats evaluating nicotine pyruvate containing aerosols concluded that coadministration of nicotine with pyruvic acid does not increase the biological effects (OECD 412 end points) observed with nicotine alone.16
The herein described P3L system is the result of the further development of the above-described technology into a handheld device that consists of a pen-size holder and a charger unit. The holder component includes a cartridge containing nicotine and the acid, each in separated cavities, and electronics ensuring controlled heating of the cartridge at around 100°C. The total amount of delivered nicotine salt is controlled through a ventilation mechanism. P3L deploys lactic acid as the acid component as it exhibits, in contrast to pyruvic acid, intrinsic stability during moderate controlled heating which enables constant aerosol formation, independent from real-world variability of ambient temperatures. Lactic acid has a desirable safety profile (a weak organic acid, water soluble, constituent of normal cells and found in food and medicinal products) and the ability to generate an aerosol upon mixing with nicotine vapor.
The use of nicotine lactate in an inhaler device has recently been reported as a pressurized metered dose inhaler (pMDI) system containing additional propellants and delivering standardized nicotine sprays.17 Besides this, pMDIs in general require adequate actuation coordination in contrast to the individually self-controllable and ritual-driven aerosol delivery of the P3L system.
The objectives of the study presented in this article were to evaluate in healthy smokers the plasma nicotine PK profile, subjective effects assessed by urge to smoke, craving relief and product evaluation, and the safety and tolerability of the nicotine-containing aerosol delivered by the P3L system in relation to the Nicorette inhalator.
Methods
Study Design
The study design was an open-label study to determine nicotine PK profiles and pharmacodynamics effects (ie, subjective effects), safety, and tolerability of the nicotine-containing aerosol delivered by P3L at three different ascending nicotine levels in relation to the Nicorette inhalator. Considering that this was the first clinical study with P3L, a dose escalation design was selected for safety considerations. The Nicorette inhalator was selected as comparator, as it presents the closest available nicotine delivery technology and device that has received regulatory approval. The study was conducted at Christchurch Clinical Studies Trust Ltd., New Zealand, between October and December 2015. The study was approved by an Independent Ethics Committee (Southern Health and Disability Ethics Committee, HDEC, Ministry of Health, Wellington, New Zealand) and by the New Zealand Medicines and Medical Devices Safety Authority (MedSafe, Wellington, New Zealand). The study was conducted in accordance with the principles of the current Declaration of Helsinki,18 the Notes for Guidance on Good Clinical Practice (CPMP/ICH/135/95),19 and the Guideline on the Regulation of Therapeutic Products in New Zealand, part 11,20 and was registered at www.clinicaltrials.gov (NCT02532374). All participants provided written informed consent to participate in the study.
Subjects
Subjects were recruited via the clinical site’s database and by advertisement. The sample size was empirically based. A sample of 12 subjects was targeted for the analysis of this study to optimize the precision about the mean and variance for the study objectives.21
Male and female healthy Caucasian smokers with a minimum age of at least 21 years were eligible to participate if they had smoked cigarettes for ≥3 consecutive years and ≥10 commercially available nonmenthol cigarettes per day for the last 4 weeks prior to screening. Menthol cigarette smokers were excluded to remove a potential source of variability for this first study with the P3L system.
Subjects were ineligible if they had a body mass index of <18.5 or >32 kg/m2, a urinary cotinine level of <200 ng/mL at screening, or medical conditions requiring medication or other medical interventions. The subjects were provided financial compensation for their time and the inconvenience of participating in the study.
Procedures
The study consisted of a screening period, one day of admission, four separate days of on-site product use with 1–3 days in between each product use, and a 7-day safety follow-up period (Supplementary Figure 1).
During the admission-baseline period, subjects were asked to familiarize themselves with P3L (nicotine aerosol level 50 µg/puff) and the Nicorette inhalator using three to five inhalations of each product. During the study, subjects were confined at least 12 hours prior to product use overnight. On the first study visit, each subject used the Nicorette inhalator at a rate of 1 inhalation every 15 seconds on average over approximately 20 minutes, that is, 80 puffs in total. This dosing regimen was selected, as it delivers nicotine amounts in a range similar to P3L, is within the label accepted by health authorities, and has been tested in several clinical studies without revealing an important safety concern.22 The Nicorette inhalator (15 mg; Johnson & Johnson) was purchased from the local pharmacy.
During each subsequent visit, subjects used one of the three variants of P3L delivering different nicotine levels in a single-ascending-level scheme, of which the subjects were aware of, starting with the P3L variant delivering approximately 50 µg nicotine per puff, followed by approximately 80 µg nicotine per puff, and finally the variant delivering approximately 150 µg nicotine per puff. The nicotine delivery levels of each P3L variant were determined using a smoking machine under the Health Canada Intense smoking regimen corresponding to 12 puffs per cartridge and subsequent quantification in the particulate phase extract by means of gas chromatography.23 For the three different P3L variants (50, 80, and 150 µg/puff) the average nicotine amounts per puff measured (n = 5) were 55.6 μg/puff (SD = 7.8), 79.1 μg/puff (6.1), and 155.4 μg/puff (21.0), respectively. Each subject inhaled the aerosol at a rate of 1 inhalation every 30 seconds on average over approximately 6 minutes, that is, 12 inhalations in total. A minimum 12-hour nicotine washout period before each product use was implemented. The duration of the inhalation and the puff volumes for each subject during product usage were not controlled.
The P3L system (version PMD 1.1) comprises of a charger and a holder into which the cartridge and the mouth piece are inserted. In the cartridge, a nicotine/menthol mixture and lactic acid are deposited on porous host materials inside two separate cavities. One cavity of the cartridge contained 14 µL of a nicotine/menthol mixture (3.56 mg l-menthol, Sigma Aldrich, in 10 μL S-nicotine, Siegfried), and the second cavity contained 20 µL l-lactic acid (Purac PF 90; Purac Bioquimica SA, Spain). The cartridge was inserted into the holder within 24 hours before the respective product use. Product assembly was carried out at the clinical site’s pharmacy.
The holder includes a heating element that heats the cartridge at a constant temperature of around 100°C, electronics ensuring temperature control, charging and heater activation, and a battery. The charger recharges the holder, with energy capacity sufficient to deliver an aerosol over a period of approximately 6 minutes. The mouthpiece has holes allowing dilution of the mainstream aerosol with different number of ventilation holes designed to deliver the differing nicotine amounts per puff. Based on the working principle of the P3L aerosol generation technology, the total amount of nicotine salt in the final aerosol is produced through a per puff dilution with air through the ventilation inlets in the mouthpiece. This mechanism allowed to create the different nicotine delivery versions of the investigated P3L system.
Measures
Baseline Characteristics
The recorded baseline characteristics of subjects included age, sex, weight, height, smoking history, and current combustible cigarette brand used. Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence questionnaire in its revised version,24 as updated in 2012.25
Pharmacokinetics
The PK profiles of nicotine in plasma were determined from single use of the three variants of P3L and the Nicorette inhalator. Fifteen venous blood samples were collected for each subject during each product use following the same collection window: 45, 30, and 15 minutes prior start of product use and 2, 4, 7, 10, 15, 20, 30, 40, 50, 60, 120, and 240 minutes after start of product use. Plasma nicotine concentrations were determined by high-performance liquid chromatography tandem mass spectrometry with a 0.2 ng/mL lower limit of quantification.
Subjective Effects Assessment
A visual analog scale (VAS)-craving assessment was used to assess the level of craving based on response to the question “How strong is your craving for cigarettes?” on a scale from “no craving” to “strong craving” by measuring the number of millimeters (0–100 mm) from the “no craving” dot to the point at which the drawn line intersected the scale.26 The level of craving was scored within 1 hour prior to each product use (t0) and 4, 10, 20, 30, 40, 60, 120, and 240 minutes after start of each product use. Urge to smoke was assessed using the brief, 10-item version of the Questionnaire of Smoking Urges (QSU-brief).27 The QSU-brief items are rated on a 7-point scale, ranging from 1 = strongly disagree to 7 = strongly agree. Higher scores indicate greater urge to smoke. Two factors and a total score were derived. Factor 1 includes items representing the desire and intention to smoke with smoking perceived as rewarding. Factor 2 includes items representing an anticipation of relief from the negative effects of smoking with an urgent desire to smoke. The QSU-brief was completed within 1 hour prior to each product use start (t0) and 10, 20, 30, 40, 60, 120, and 240 minutes after start of each product use. The modified Cigarette Evaluation Questionnaire (mCEQ) measuring domains of reinforcement28 was completed within 1 hour after each product use start (t0). The following domains were evaluated: smoking satisfaction, psychological rewards, aversion, enjoyment of respiratory tract sensations, and craving reduction.
Safety and Tolerability Monitoring
Safety variables monitored in this study included adverse events (AEs), vital signs (systolic and diastolic blood pressure, pulse rate, and respiratory rate), spirometry (FEV1), electrocardiogram data, concomitant medication, clinical chemistry, hematology, urine analysis safety panel, physical examination, and respiratory symptoms (cough assessment). The cough assessment was conducted at baseline (admission visit) and 24 hours after each product use (t0) by means of a questionnaire composed of a VAS, three Likert-type scales, and one open question.29
Data analyses
Data were analyzed for all enrolled subjects who did not have major protocol deviations, who completed at least one P3L product use period, and for whom at least one PK parameter could be derived. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
The analyzed nicotine PK parameters included the maximum baseline-corrected plasma concentration (Cmax), time to reach Cmax (tmax), and the baseline-corrected area under the plasma concentration–time curves (i) from start of product use (t0) to the last quantifiable nicotine concentration time point (AUC0-last) and (ii) from t0 to 10 minutes after t0 (AUC0-10’). The pharmacokinetic parameters were derived from plasma nicotine concentrations-versus-time data by means of non-compartmental analysis using Phoenix WinNonlin (version 6.2, Pharsight Corp, Sunnyvale, California) and corrected for baseline following the method described by Kraiczi et al.30 whereby the baseline (C0) was defined as the average concentration of the three time points prior to t0 (45, 30, and 15 minutes prior to t0) of each visit from whose slope the elimination rate constant (k) was estimated and then used for the calculation of the baseline corrected values.
The PK parameters were analyzed for the three P3L variants and the Nicorette inhalator using an analysis of variance (ANOVA) model on logarithmically transformed values (Cmax, AUC0-last, and AUC0-10’) with product exposure as a fixed effect and subjects as a random effect, adjusted for sex.
VAS-craving scores were determined for each product at each measurement time point.
The QSU-brief total score and two subscore values (factors 1 and 2) were measured for each product at each measurement time point. VAS-craving scores and QSU-brief scores were analyzed with adjusted least square (LS) means from repeated ANOVA model, with terms for baseline score, product and sex as fixed effects, and subjects as a random effect.
MCEQ values were calculated for the domain scores for each product at each measurement time point. LS means for each mCEQ domain were obtained from an ANOVA model with product and sex as fixed effects and subject as random effects.
The purpose of the model was not to test hypotheses but to obtain product-specific effect estimates adjusted for sex and subjects (LS means).
Results
Sixteen subjects were enrolled and 14 completed the study. The two non-completers (one after admission and the second one after first P3L product use visit) did not result from AEs. The baseline characteristics of the subjects are summarized in Supplementary Table 1.
Nicotine PK
The geometric mean plasma nicotine concentrations over time curve following single use of the products is shown in Figure 1. The shape of the plasma nicotine concentration–time curves obtained after P3L use at all three nicotine levels was similar, showing rapid rise in plasma nicotine concentration. Analysis of geometric LS means showed a Cmax of 9.7, 11.2, and 9.8 ng/mL for the 50, 80, and 150 µg/puff variants, respectively, that was reached after 7.0 minutes for all three. In comparison, the shape of the nicotine concentration–time curves obtained after use of the Nicorette inhalator was different, with a lower Cmax of 6.1 ng/mL that was reached later (after 30 minutes). Similar AUC0-last values of 9.9, 10.3, and 10.0 h×ng/mL were reached after P3L use (with 50, 80, and 150 µg/puff, respectively), while the Nicorette inhalator produced a slightly higher AUC0-last value (12.3 h×ng/mL). Conversely, during the first 10 minutes, P3L led to higher AUC0-10’ values of 1.0, 1.2, and 1.0 h×ng/mL (50, 80, and 150 µg/puff, respectively) compared to the Nicorette inhalator (0.1 h×ng/mL) (Table 1). The individual PK profiles produced by P3L 50 and 80 µg/puff were more consistent than the 150 µg/puff and the Nicorette inhalator products (Supplementary Figure 2).
Figure 1.
Geometric means and 95% confidence intervals of baseline-corrected nicotine concentrations during single use of the P3L system (50, 80, and 150 µg/puff) and the Nicorette inhalator over 4 hours and expanded view from t0 (start of product use) to 20 minutes.
Table 1.
Pharmacokinetics of Nicotine Following Single use of the P3L System or the Nicorette Inhalator
| Parameter | P3L (50 µg/puff), n = 15 | P3L (80 µg/puff), n = 14 | P3L (150 µg/puff), n = 14 | Nicorette inhalator (15mg), n = 15 |
|---|---|---|---|---|
| C max, ng/mL | ||||
| Geometric LS mean (95% CI)a | 9.7 (6.7, 13.9) | 11.1 (7.7, 16.1) | 9.8 (6.8, 14.2) | 6.1 (4.2, 8.8) |
| Min, Max | 1.7, 18.7 | 1.4, 20.8 | 0.8, 33.3 | 1.7, 17.7 |
| t max, min | ||||
| Median | 7.0 | 7.0 | 7.0 | 30.0 |
| Min, Max | 4.0, 30.0 | 4.0, 20.0 | 2.0, 20.0 | 20.0, 60.0 |
| AUC0–10’, h×ng/mL | ||||
| Geometric LS mean (95% CI)a | 1.0 (0.6 to 1.7) | 1.2 (0.7 to 1.9) | 1.0 (0.6 to 1.7) | 0.1 (0.1 to 0.2) |
| Min, Max | 0.1, 2.3 | 0.1, 2.4 | 0.04, 4.1 | 0.02, 0.4 |
| AUC0–last, h×ng/mL | ||||
| Geometric LS mean (95% CI)a | 9.9 (7.5 to 13.2) | 10.3 (7.6 to 13.8) | 10.0 (7.4 to 13.4) | 12.3 (9.3 to 16.4) |
| Min, Max | 3.4, 19.7 | 2.8, 17.7 | 1.6, 31.7 | 3.3, 33.7 |
C max = baseline-corrected plasma concentration; tmax = time to reach Cmax; AUC = baseline-corrected area under the plasma concentration–time curves
aGeometric means and 95% confidence interval (CI) are the adjusted geometric least squares means and CIs from an ANOVA model conducted on log-transformed data with product as fixed effect and subjects as random effect, adjusted for sex. Values are derived from baseline-corrected nicotine concentrations.
Subjective Effects
VAS Craving
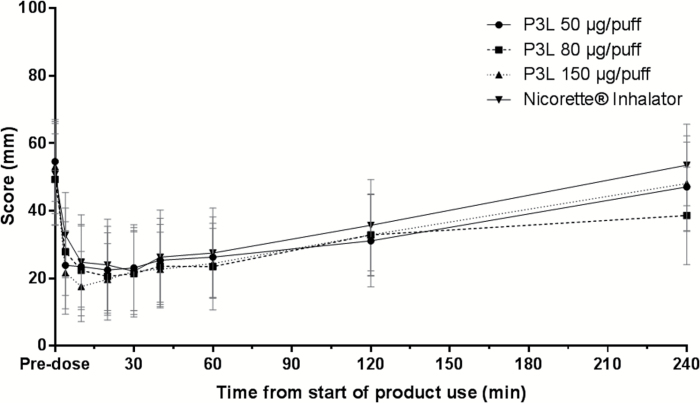
The mean VAS-craving scores rapidly decreased over the first 10–20 minutes following use of P3L with a maximum change from the baseline score of 59% (50 µg/puff), 58% (80 µg/puff), and 67% (150 µg/puff). In comparison, the minimum average score for the Nicorette inhalator was observed 30 minutes after product use start, corresponding to a 56% reduction from the baseline score (Figure 2). The initial early differences in mean VAS-craving scores measured 4, 10, and 20 minutes after product use and the lower mean VAS-craving score values over the 4-hour measurement period for all P3L systems in relation to the Nicorette inhalator suggest a slightly higher craving reduction produced by P3L (Supplementary Table 2).
Figure 2.
Arithmetic mean and 95% confidence intervalof the Visual Analog Scale (VAS)-craving scores over time per product used.
Urge to Smoke
The QSU-Brief total scores over time followed the same pattern for all three P3L variants and the Nicorette inhalator. At baseline, the total mean scores were similar between all products (3.8 for 50 µg/puff, 3.6 for 80 µg/puff, 3.9 for 150 µg/puff, and 4.3 for Nicorette inhalator) which was followed by a similar transient reduction from baseline (−1.1 for 50 µg/puff, −1.0 for 80 µg/puff, −1.5 for 150 µg/puff, and −1.8 for Nicorette inhalator), at 10 minutes, for all products. At 4 hours postproduct use start, the time of last measurement, the mean total scores approached the baseline values (Supplementary Figure 3). The mean QSU-Brief total score and the two subscores were similar between all the products (Supplementary Table 3).
Modified Cigarette Evaluation Questionnaire
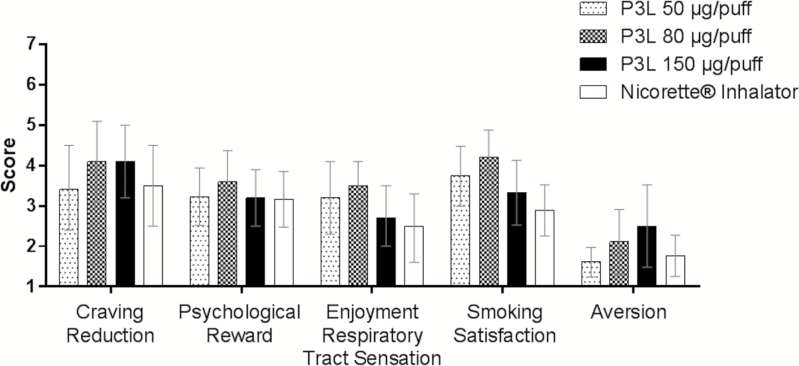
Among the five different subscales of the mCEQ, “Craving Reduction” score appeared to be higher for the P3L 80 and 150 µg/puff variants than the P3L 50 µg/puff and the Nicorette inhalator, while “Psychological Reward” was similar across all products. “Enjoyment, Respiratory Tract Sensation” and “Smoking Satisfaction” scored the lowest for the Nicorette inhalator followed by the 150 µg/puff variant. The recorded “Aversion” subdomain scores were higher with increasing P3L nicotine delivery levels (Figure 3).
Figure 3.
Modified Cigarette Evaluation Questionnaire Domain Scores Profiles (arithmetic mean and 95% confidence intervals) per product used.
Cough Assessment
Three subjects reported a regular need to cough with a very mild to mild scoring in terms of bothering, intensity, frequency, and amount of sputum. This occurred in two subjects only at admission and in one subject at the days of the P3L 80 µg/puff and 150 µg/puff and the Nicorette inhalator product use (Supplementary Table 4).
Safety
There were neither serious AE (SAEs) nor AEs leading to product discontinuation in this study. No specific pattern in AEs related to study procedures or related to the Nicorette inhalator were noticed. In total, 16 AEs related to P3L (8 subjects) were detected and the majority rated mild, the most common one being dizziness. One AE-rated severe (syncope) occurred during P3L 80µg/puff product use and resolved within the course of the visit day without treatment.
Discussion
We present the first in human data from the clinical assessment of a novel nicotine delivery system, P3L. For the P3L prototype assessed in this study, device ventilation channels aimed to test three different nicotine delivery levels. The PK data show a more rapid onset and higher levels of nicotine in the venous blood after delivery by the new P3L system compared to the Nicorette inhalator. The nicotine exposure during the first 10 minutes after product use start (AUC0-10’) was approximately 10-fold higher with P3L than with the Nicorette inhalator and the Cmax almost twice higher. The onset of action with the novel system indicates pulmonary nicotine delivery as expected from P3L’s aerosol characteristics with droplet sizes in the submicron range (mean mass median aerodynamic diameter 0.7–0.9 µm).
The nicotine PK profile obtained with the Nicorette inhalator was in line with literature reported data.12 The PK parameters indicate that P3L is able to deliver nicotine more efficiently than the Nicorette inhalator. The concentration–time profiles and PK parameters produced by the different nicotine delivering P3L variants were similar. The similar PK profiles of P3L variants along with controlled number of puffs and puff intervals suggests that the subjects, who were also aware of P3L’s ascending nicotine levels in this study, possibly adapted their puffing behavior to achieve individual desired levels of nicotine (self-titration). It has been reported, for example, that some smokers alter their puffing behavior when smoking products with different nicotine yields in order to get a relatively constant level of nicotine from different products.31,32 The Cmax and tmax values produced by P3L are in a range which is comparable to published CC data.29,33,34 Future studies to assess the puffing topography could be considered to evaluate if the users adapt their puffing behavior to potentially self-titrate to a given plasma nicotine concentration.
The nicotine PK parameters in female subjects appeared toward lower Cmax and AUC, an observation described previously,35 although the strength of the trend was inconsistent across nicotine delivery levels (Supplementary Table 5).
No direct comparisons can be made with other nicotine delivery systems, other than the one tested. However, the results obtained are promising, with Cmax and tmax values similar to data obtained by a recently described nicotine pMDI36 or apparently higher nicotine levels reached faster than that of a novel nicotine inhaler with 0.67 mg nicotine per dose.25 The rapid availability of nicotine in plasma occurs as fast as with the nicotine–pyruvate system described by Rose et al.,3 upon which technology the concept of P3L is based.
On average, the occurrence of craving reduction obtained with P3L appeared with a faster onset than for the Nicorette inhalator corresponding to P3L’s rapidly achieved maximal plasma nicotine concentrations. Initial differences in craving reduction were already measured 4 minutes after product use start (VAS craving score).
The mCEQ domain scores for P3L, with the exception of “Aversion,” appeared to be on average as good as the Nicorette inhalator, with highest values (better) obtained with the 80 µg/puff, suggesting that 150 µg/puff was less enjoyable than the intermediate level. One possible explanation for the higher “Aversion” scoring with increased P3L nicotine delivery levels might be that the subjects experienced the menthol flavor as too strong, considering that the per puff delivered amounts of menthol and nicotine were proportional and that the product was used by non-menthol smokers in this study.
Coughing was reported by only one subject during the exposure periods (for P3L 80 µg/puff, 150 µg/puff, and Nicorette inhalator) and rated as very mild, suggesting that the aerosol delivered by P3L was well tolerated. In comparison, a recently reported study with a nicotine lactate containing pMDI indicated a high initial coughing rate (174/242 subjects in the active group one week after product use) which declined over the course of the 6-month period (23/128 subjects) as recorded by a standard questionnaire using Likert-type scales.17 An explanation here could be the instant irritation caused by the pressurized aerosol stream (ie, throat impact) from the pMDI device, in contrast to the individually self-controllable nicotine delivery with the P3L device.
The frequency and type of AEs reported for P3L were consistent with the known effects of oral and inhaled nicotine replacement therapies and further substantiate tolerability of the nicotine salt containing aerosol.
Conclusion
At all three nicotine levels tested, the inhalation of the nicotine lactate aerosol delivered with the P3L system provided plasma nicotine concentrations higher and faster compared to the Nicorette inhalator. The plasma nicotine concentration–time profile supports a pulmonary route of absorption for P3L compared to the oromucosal absorption of the Nicorette inhalator as indicated by a later onset of action for the latter. On average, the nicotine concentration–time profiles and PK parameters between the three P3L levels did not indicate a nicotine dose–response relationship. Craving reduction was similar between P3L and Nicorette inhalator, with an earlier onset reached with P3L. With the exception of “Aversion,” the product evaluation appeared to be at least as good for P3L as for the inhalator with an apparent preference for the P3L 80 µg/puff variant. P3L was generally well tolerated.
The combination of nicotine and lactic acid with the P3L device shows potential over existing nicotine delivery systems by delivering nicotine with kinetics close to CC and without exogenous carrier substances as used in current ENDS.
Altogether, the PK profile, subjective effects, and safety profile obtained in this study suggest P3L is an acceptable nicotine delivery product. Further studies should consider evaluation of long-term product use and acceptance.
Supplementary Material
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
The study was supported by Philip Morris Products S.A.
Declaration of Interests
AT, PB, LFM, LS, MF and FL are employees of Philip Morris Products S.A. CW, the study PI, was responsible for the implementation and conduct of the study. He has no declaration of interest to make. ML contributed to the interpretation of the study results and critically revised this article for important intellectual content. He received no funding for this work and declared that no competing interests exist.
Supplementary Material
Acknowledgments
The authors acknowledge with gratitude the work of the staff at Christchurch Clinical Trust Ltd, Christchurch, New Zealand for the preparation of the ethics committee application and conduct of the study protocol, Celerion Laboratories, Lincoln, USA for the bioanalytical services, and Clinical Network Services Pty Ltd, Brisbane, Australia, for the coordination of the study operations.
References
- 1. Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: assessing the science base for tobacco harm reduction. Tob Control. 2001;10:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Royal College of Physicians. Harm Reduction in Nicotine Addiction: Helping People Who Can’t Quit. A report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP; 2007. [Google Scholar]
- 3. Rose JE, Turner JE, Murugesan T, Behm FM, Laugesen M. Pulmonary delivery of nicotine pyruvate: sensory and pharmacokinetic characteristics. Exp Clin Psychopharmacol. 2010;18(5):385–394. doi:10.1037/a0020834. [DOI] [PubMed] [Google Scholar]
- 4. Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27(12):1007–1019. doi:10.1007/s40263-013-0116-4. [DOI] [PubMed] [Google Scholar]
- 5. Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 2008;83(4). doi:10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 6. Mc Neill A, Brose LS, Calder R, Hitchman SC, Hajek P. E-cigarettes: an Evidence Update. A Report Commissioned by Public Health England. 2015; Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf (Accessed on 10 June 2016). [Google Scholar]
- 7. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. doi:10.1016/s2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. St Helen G, Havel C, Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2015. doi:10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farsalinos KE, Spyrou A, Stefopoulos C, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers). Sci Rep. 2015;5:11269. doi:10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khoudigian S, Devji T, Lytvyn L, Campbell K, Hopkins R, O’Reilly D. The efficacy and short-term effects of electronic cigarettes as a method for smoking cessation: a systematic review and a meta-analysis. Int J Public Health. 2016;61(2):257–267. doi:10.1007/s00038-016-0786-z. [DOI] [PubMed] [Google Scholar]
- 11. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi:10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet. 2001;40(9):661–684. [DOI] [PubMed] [Google Scholar]
- 13. Cipolla DC, Gonda I. Inhaled nicotine replacement therapy. Asian J Pharm Sci. 2015;10(6):472–480. doi:10.1016/j.ajps.2015.07.004. [Google Scholar]
- 14. Caldwell B, Sumner W, Crane J. A systematic review of nicotine by inhalation: is there a role for the inhaled route?Nicotine Tob Res. 2012;14(10):1127–1139. doi:10.1093/ntr/nts009. [DOI] [PubMed] [Google Scholar]
- 15. Rose JE, Rose SD, Turner JE, Murugesan T, Inventors; Duke University, Durham, NC (US), assignee Device and method for delivery of a medicament. US patent 2008/0241255 A1. 2008.
- 16. Phillips B, Esposito M, Verbeeck J, et al. Toxicity of aerosols of nicotine and pyruvic acid (separate and combined) in Sprague-Dawley rats in a 28-day OECD 412 inhalation study and assessment of systems toxicology. Inhal Toxicol. 2015;27(9):405–431. doi:10.3109/08958378.2015.1046000. [DOI] [PubMed] [Google Scholar]
- 17. Caldwell BO, Crane J. Combination nicotine metered dose inhaler and nicotine patch for smoking cessation: a randomized controlled trial. Nicotine Tob Res. 2016. doi:10.1093/ntr/ntw093. [DOI] [PubMed] [Google Scholar]
- 18. World Medical Association (WMA). WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.France: WMA; 2013. [DOI] [PubMed] [Google Scholar]
- 19. EMA (European Medicines Agency). Notes for Guidance on Good Clinical Practice (CPMP/ICH/135/95). London: EMA; 1995. [Google Scholar]
- 20. New Zealand Medicines, Medical Devices Safety Authority. Guideline on the Regulation of Therapeutic Products in New Zealand; Part 11: Clinical Trials – Regulatory Approval and Good Clinical Practice. Edition 1.4. 2015; Available from: http://www.medsafe.govt.nz/regulatory/current-guidelines.asp (Accessed on 17 July 2016). [Google Scholar]
- 21. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005;4(4):287–291. doi:10.1002/pst.185. [Google Scholar]
- 22. McNeil Products Limited. Nicorette 15mg Inhalator. 2014; Available from: http://www.medicines.org.uk/emc/medicine/24853 (Accessed on 17 June 2016).
- 23. Health Canada, Tobacco Control Programme. Determination of “Tar,” Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke. T-115. Ontario, Canada: Health Canada; 1999. [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14(12):1467–1473. doi:10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 26. Moyses C, Hearn A, Redfern A. Evaluation of a novel nicotine inhaler device. Part 2: effect on craving and smoking urges. Nicotine Tob Res. 2015;17(1):26–33. doi:10.1093/ntr/ntu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 28. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette. Addict Behav. 2007;32(5):912–923. doi:10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29. Picavet P, Haziza C, Lama N, Weitkunat R, Lüdicke F. Comparison of the pharmacokinetics of nicotine following single and ad libitum use of a Tobacco Heating System or Combustible Cigarettes. Nicotine Tob Res. 2016;18(5):557–563. doi:10.1093/ntr/ntv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraiczi H, Hansson A, Perfekt R. Single-dose pharmacokinetics of nicotine when given with a novel mouth spray for nicotine replacement therapy. Nicotine Tob Res. 2011;13(12):1176–1182. doi:10.1093/ntr/ntr139. [DOI] [PubMed] [Google Scholar]
- 31. Scherer G, Lee PN. Smoking behaviour and compensation: a review of the literature with meta-analysis. Regul Toxicol Pharmacol. 2014. doi:10.1016/j.yrtph.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 32. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi:10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKinney DL, Davies BD, Gogova M, et al. Rapid automated blood sampling system for pharmacokinetics studies of cigarette smoking. Nicotine Tob Res. 2010;12(4):319–325. doi:10.1093/ntr/ntp190. [DOI] [PubMed] [Google Scholar]
- 34. Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009(192):29–60. doi:10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;(3):383–404. [DOI] [PubMed] [Google Scholar]
- 36. Caldwell B, Dickson S, Burgess C, et al. A pilot study of nicotine delivery to smokers from a metered-dose inhaler. Nicotine Tob Res. 2009;11(4):342–347. doi:10.1093/ntr/ntp027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.