Abstract
In an era of Precision Medicine, it is vital to collect biological data within clinical trials and to integrate their analysis within the outcomes of the trial. The identification of genomic biomarkers that affect treatment response to smoking cessation treatment, both pharmacological and behavioral, or susceptibility to medication-related adverse reactions, holds real promise to improve treatment efficacy and to tailor the treatment approach to the individual. However, a clear challenge in identifying reliable biomarkers is in obtaining adequate sample sizes. Consortium-based approaches will likely be necessary to yield real successes. Thus, meta-analyses of data from individual smoking cessation trials will become crucial and will be facilitated by standardized trial designs, assessments, and outcomes and harmonizable measures. To foster increased collection of high-quality genetics data in clinical trials, we discuss (1) genetically informed trial design, (2) biological samples (collection requirements, storage, and analysis with a focus on genomic data) and genetics consortia, (3) participant consent and data sharing requirements for Institutional Review Board (IRB) approvals, and (4) information on phenotype characterization and meta-analysis. This work aligns with the objectives of the Precision Medicine Initiative and offers guidance for integrating treatment research and genetics/genomics within the nicotine and tobacco research community. It is intended to promote the collection and genotyping of biosamples in existing subject samples as well as the integration of genetic research elements into future study designs. This article cross-references a companion paper in this issue that reviews current evidence on genetic and epigenetic markers in cessation trials.
Implications
This article outlines a framework for the consistent integration of biological data/samples into smoking cessation pharmacotherapy trials, aligned with the objectives of the recently unveiled Precision Medicine Initiative. Our goal is to encourage and provide support for treatment researchers to consider biosample collection and genotyping their existing samples as well as integrating genetic analyses into their study design in order to realize precision medicine in treatment of nicotine dependence.
Introduction
Smoking is a major risk for preventable death and disability,1–4 and smoking cessation reduces the risk of mortality.5 However, cessation failure is common, despite clinical practice guidelines6 and available cessation medications, which are associated with different efficacies, side effects, adherence, use constraints, and costs.7 Cessation treatment, pharmacological or behavioral, may be improved via precision medicine: that is, optimizing treatments to maximize efficacy and minimize side effects.8,9 Smokers vary greatly in the benefit they derive from particular pharmacotherapies, and biomarkers can predict a smoker’s response to a specific pharmacotherapy.10–14 Because the health cost of cessation failure is high, there is a need to identify treatments that are most likely to be effective for smokers who want to quit and to maintain long-term abstinence.
An important initiative of the National Institutes of Health (NIH) is to develop precision medicine to improve care.15 The initiative will increase our ability to characterize smokers and predict their responses to cessation pharmacotherapies. Such studies will help optimize treatments for enhanced efficacy and medication adherence and to reduce side effects. In recent years, scientists have gained extensive knowledge on how personal factors (including genetics) can be used to predict important health outcomes.16 The concept of precision medicine is not new; clinical history of allergic reactions to medication, for instance, has been used to guide medication choice for more than a century.17 However, the assessment of individual variation has been dramatically improved by the recent development of large-scale biologic databases (i.e., the human genome sequence) and powerful methods for characterizing patients (i.e., proteomics, transcriptomics, epigenomics, metabolomics, and genomics). What is needed now is to leverage biological samples, test them rigorously, and ultimately use them to build the evidence-base needed to guide clinical practice. This will enable more accurate diagnoses, more rational disease prevention strategies, better treatment selection, and the development of novel therapies, including ones for nicotine dependence and the multitude of tobacco-related diseases.
The promise of precision medicine has already been fulfilled in some areas of medicine. For example, underlying causal genotypes are used to personalize cancer treatment.18 The application of genetic discoveries to clinical decision making and treatment decisions is occurring in a variety of medical specialties. For example, genetic screening has identified a specific molecular subset of non-small cell lung cancer patients, where patients positive for a specific oncogene were more likely to be young never smokers or light smokers, compared to older, heavier smokers.19 In addition, guidelines from the College of American Pathologists and the International Association for the Study of Lung Cancer, recommend testing for two well-characterized genetic biomarkers in patients newly diagnosed with non-small cell lung cancer: epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) for treatment guidance.20 The current clinical approach of using molecular and genetic phenotyping to guide clinical care of patients with lung cancer is a welcome addition to traditional therapy that has markedly limited effectiveness. In the field of addiction, genetic variants in the nicotinic receptor subunit gene CHRNA5, variants in the nicotine metabolism gene CYP2A6, and the nicotine metabolite ratio genotypes, showed promise as a marker for smoking cessation pharmacotherapy selection.21–25
We are beginning to understand how to optimize therapies for other diseases based on different genetic polymorphisms.26,27 Thus, genetic variables are used to optimize drug selection and dosing;28 for example, individual genetic profiles are used to avoid medications likely to cause serious adverse effects such as abacavir, carbamazepine, and thiopurine.29–31 Finally, with individual whole-genome or exome sequencing, we can now not only better classify diseases but also diagnose patients with previously undiagnosed genetic diseases.32,33 The Evaluation of Genomic Applications in Practice and Prevention Initiative,34 established by the National Office of Public Health Genomics at the Centers for Disease Control and Prevention, supports the development and implementation of a rigorous, evidence-based process for evaluating genetic tests and other genomic applications for clinical and public health practice in the United States.34
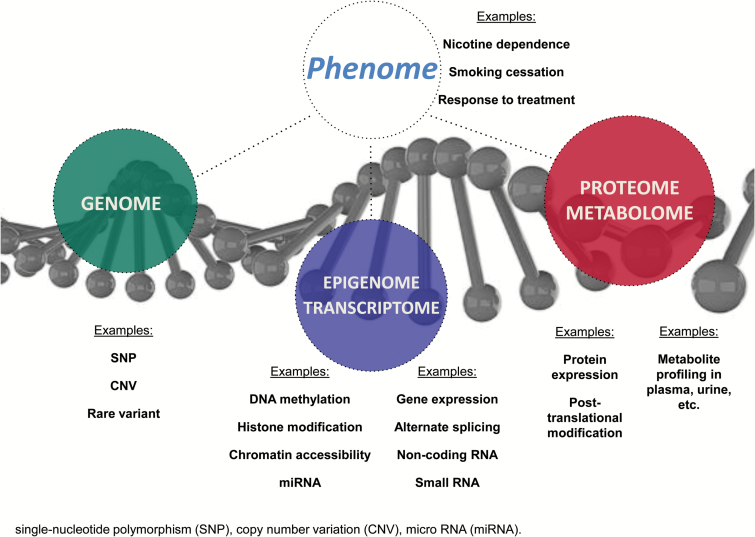
Based on the utility of individual biological variability for clinical care, we propose that, ideally, examination of biological samples should be integrated into clinical trials to address issues that are central to study aims, rather than as a data collection procedure merely affixed to the study. We encourage the next generation of scientists to develop creative new approaches for detecting, measuring, and analyzing a wide range of biomedical information including molecular, genomic, cellular, clinical, behavioral, physiological, and environmental parameters. The NIH’s Precision Medicine Initiative plans to recruit a longitudinal cohort of 1 million or more Americans to give consent for extensive characterization of biologic specimens (cell populations, proteins, metabolites, RNA, and DNA—including whole-genome sequencing when costs permit) and behavioral data, all linked to their electronic health records as summarized in Figure 1.15 Blood specimens will be collected and processed using a standard Clinical Laboratory Improvement Amendments (CLIA)-compliant procedure, ensuring quality control and comparability, and sent to a local or central biorepository that will support collection, processing, storage, retrieval, and biochemical analysis and/or shipment to analytic laboratories, in addition to a wide range of phenotypic data including mobile health measures. Understanding the biological basis of complex traits will be informed by these technological advances in data generation from multiple levels of biological systems—including DNA sequencing,35 RNA expression,36,37 methylation patterns,38 other epigenetic markers,39 proteomics,40 and metabolomics41 (see Figure 1). Corresponding actions are being undertaken in many other countries as part of their national genome strategies.
Figure 1.
Biological systems multi-omics from the genome, epigenome, transcriptome, proteome and metabolome to the phenome. SNP, single-nucleotide polymorphism; CNV, copy number variation; miRNA, micro RNA.
Today, much treatment research is designed to develop and evaluate treatments that are expected to benefit the population as a whole based on the expected response of a “typical” patient. However, individual patients can have markedly variable responses to therapy, ranging from highly efficacious outcome, to no effect, to deleterious outcome (see Figure 2). The roots of this variability likely include unrecognized differences in disease pathophysiology, environmental exposures, social and behavioral factors, and genetic factors. Prior research has, of course, used moderator variables to uncover person by treatment interactions. However, the new era will advance this effort via a much more precise and comprehensive assessment of individual biological characteristics. Thus, an important overarching goal is to determine whether and how state-of-the-art genomic biomarkers can be used to optimize smoking cessation pharmacotherapy to enhance efficacy and medication adherence and to reduce side effects.
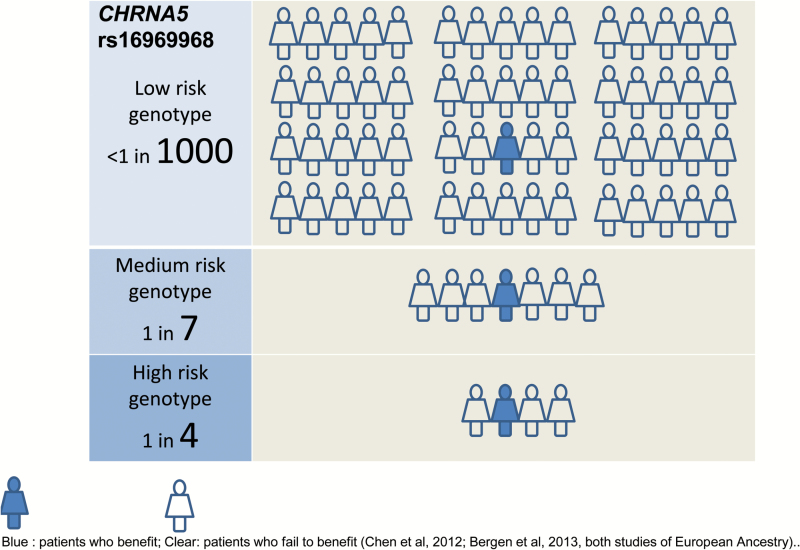
Figure 2.
Example: Benefits of nicotine replacement therapy may vary by genetic marker. Blue: patients who benefit; Clear: patients who fail to benefit,21,95 both studies of European Ancestry.
To promote incorporation of genetics data into smoking cessation treatment research, in this review we discuss (1) study design considerations (e.g., genetically informed trials), (2) practical considerations of biological sample collection and participant consent for genetic data-sharing requirements, (3) development of genetic consortia and meta-analyses to obtain adequate sample sizes for robust pharmacogenetic analyses, and (4) information on phenotype characterization and outcome harmonization for cross-study comparisons.
Key Concepts and Glossary
This review seeks to inform a broad medical readership about the current status of how biological samples can be used in smoking cessation trials and to highlight the rationale, study design, practical considerations, and opportunities for nicotine and tobacco researchers. Views are still evolving about several issues such as the clinical validity of potential genomic biomarkers. Current findings on biological markers for smoking cessation and a glossary for key genetic and “omic” terms are presented in a companion paper by Saccone et al.
Study Design Considerations and Examples
Here, we present examples on how researchers can consider biosample collection and genotyping their existing samples, as well as integrating genetic analyses into their study design. Genetic data collection is easier compared to collection of therapeutic drug level or proteins, which may be more sensitive to temperature or light with more restrictive collection and storage procedures.
Collecting Biomarkers
Biomarkers relevant to nicotine and tobacco cessation research generally fall into three categories42,43: (1) diagnostic biomarkers for patient selection; (2) pharmacodynamic biomarkers for optimal dosing; and (3) predictive biomarkers for therapeutic efficacy, which may include pharmacodynamic (e.g., genotypes at specific genetic variants, electroencephalogram or functional connectivity) and pharmacokinetic (e.g., nicotine metabolite ratio) biomarkers. Additional details on definitions of biomarkers and the Institute of Medicine’s proposed three-part framework for biomarker development (analytical validation, qualification/context of use, and utilization)44 are described in more detail elsewhere.43
Most biomarkers for omic research may be collected from whole blood or saliva. The timing of sample collection needs to be determined by the type and context of the research questions being addressed. For example, for germline DNA analyses, biosamples can be collected at any time prior to, during, or after the study. However, gene expression and epigenetic studies are often timed in relation to an exposure such as before and after drug administration or before and after smoking cessation, requiring careful attention to the timing of a study’s primary end points in relevant tissue types. For example, some epigenetic markers respond to smoking cessation by reverting toward unexposed levels, although the time frame tends to be gradual, with some markers taking months or years to return to levels similar to those seen in never smokers.45–47
Consent forms should be thorough and specific enough to include whatever type of biomarkers one intends to collect. Furthermore, informed consent documents would need to be modified any time a novel omic test is added to an extant research plan. Trained phlebotomists, who could be research assistants, who receive special training and meet CLIA requirements, should collect whole blood samples. Saliva sample collection can be easily performed by study participants according to manufacturer’s protocols and clear participant instructions.
For DNA collection, saliva sample collection can be performed feasibly by mail using pre-addressed, return envelopes and collection kits available from multiple manufacturers.48,49 In contrast, collection of other omics data may be more restrictive such that all samples should be frozen in −80°C or colder freezers as rapidly as possible after collection in appropriate containers to maintain the specific omic features. Investigators designing a biomarker study without previous experience can often contact their institutions’ IRB for standard language required for informed consent documents and contact experienced investigators for sample collection, storage, and processing protocols. We have also provided template language in Supplementary Table 1.
Biomarker-Based Randomization
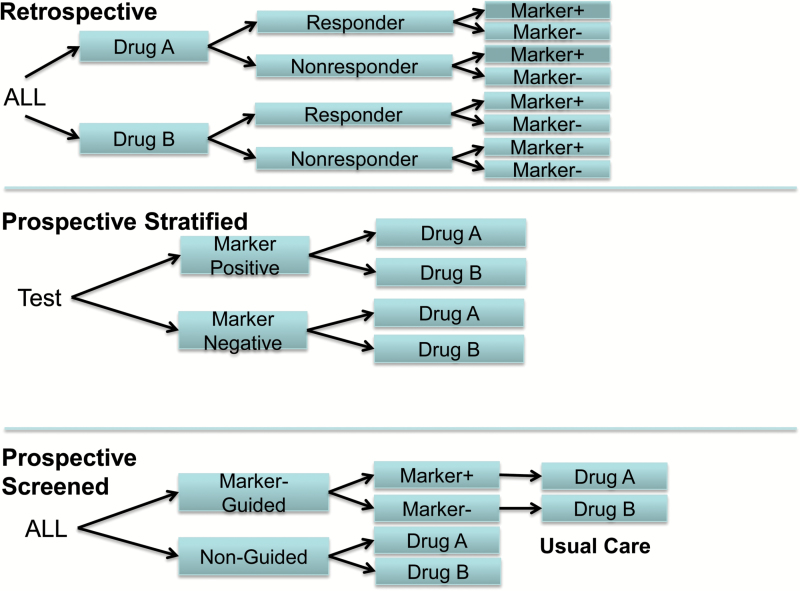
There are at least three general types of randomized controlled trial (RCT) designs for pharmacogenomic investigations of smoking cessation, as illustrated in Figure 3—according to a presentation by Dr. Caryn Lerman at an Institute of Medicine Roundtable on Translating Genomic-Based Research meeting on evidence generation for genomic diagnostic test development.50 Most pharmacogenetics studies of smoking cessation are analyses of genetic data from existing treatment studies (e.g., a single polymorphism, multiple polymorphisms, additive genetic risk scores, or metabolite proxies for polymorphisms) and are called retrospective designs. That is, analyses are conducted after completion of the RCT that relate patient biological variables such as genotype or metabolite status (e.g., normal vs. slow nicotine metabolizers) to targeted clinical outcomes, for example, efficacy of the drug for smoking cessation or the reduction in nicotine withdrawal symptoms, smoking urges, drug dosing, or side effects (Figure 3). Retrospective trials are useful when the clinical utility of such markers is unknown or not well established at the time of trial initiation and can inform hypothesis generation, replication, and independent validation. Retrospective designs have several limitations, such as unbalanced groups (status on a biomarker might be unevenly distributed across groups), reduced power resulting from either unbalanced groups or highly skewed biomarker distributions due to base rates of the biomarker status, and missing data because not all patients consented to provide biosamples.
Figure 3.
Pharmacogenomic trial designs, including retrospective, prospective stratified, and prospective screened. Source: Adapted from Lerman, IOM workshop presentation on November 17, 2010.
Prospective pharmacogenomic trials can be divided into two types: prospective stratified and prospective screened.50Prospective stratified trials conduct testing of a biomarker prior to trial entry and define a biomarker as “positive” or “negative.” An advantage of this design is that the trial is hypothesis-driven, taking into account prior knowledge about a biomarker and members of the test population. Another advantage of this design is that it permits enrichment of the less common genotype or biomarker by oversampling during the screening process—in order to achieve balanced groups. For example, Lerman and colleagues tested smokers for the nicotine metabolite ratio (NMR; 3ʹ-hydroxycotinine/cotinine) and set an initial cut-off based upon prior knowledge51 to define “normal metabolizers” and “slow metabolizers.”25 Marker positive and marker negative smokers were then independently randomized to either nicotine replacement patch or placebo patch or varenicline, resulting in balanced groups by biomarker status and drug. Roughly, 20% of patients, depending on ancestry, were slow metabolizers. Thus, oversampling of slow metabolizers was required in this prospective stratified study and resulted in excluding many patients who tested positive for normal metabolizer status. In another ongoing study by Chen and colleagues, participants receive prospectively stratified treatment randomization by the individual’s cessation-relevant genotypes such as the CHRNA5 D398N gene variant21,23 in order to yield balanced groups for testing the relation of genotype status with medication efficacy and adverse effects.22
A third type of pharmacogenomic trial is the prospective screened design in which patients are randomized to receive biomarker-guided treatment or usual care. In the biomarker-guided treatment group, patients are tested for genotype or metabolite status and assigned to a treatment based on a hypothesized association of the marker with the efficacy of a particular drug (Figure 3). Assume for example that genotype AA (marker positive) for a given polymorphism predicts enhanced efficacy of nicotine replacement therapy (NRT) but not varenicline efficacy. Genotype GG (marker negative), on the other hand, predicts enhanced efficacy of varenicline but not NRT efficacy. Therefore, in the genotype-guided group, patients with genotype AA would receive NRT and patients with genotype GG would receive varenicline. Patients in the usual care group would receive either a predefined standard medication, or they might be randomized to either of the drugs, or their physician might prescribe their medication based on usual practice. The results of the genotype-guided group would then be compared to the usual care (non-guided) group. An advantage of the prospective screened design is its potential for high ecological validity, offering evidence of whether or not a genotype- or metabolite-driven therapy provides improved effectiveness over nonguided therapy in nonresearch settings.50
In addition to these three general designs for pharmacogenomics RCTs, other designs are more fitting for enabling clinical implementation and patient-centered effectiveness, such as pragmatic trial designs that evaluate metrics germane to real-world clinical practice such as cost-effectiveness, patient satisfaction, clinical outcomes, and feasibility.50,52,53 In addition, it may be advantageous for researchers to use factorial designs to explore pharmacogenetic relations. This is, in part, because of the efficiency of such designs, as they permit experimental analysis of multiple, discrete intervention components.54,55 Thus, the researcher might investigate the efficacies of both multiple pharmacotherapies and at different levels of counseling intensities. Another advantage of such designs is that they provide relatively good power; when each factor comprises two levels, all subjects in the design contribute to estimation of the effects of each factor. Also, they uniquely permit estimation of interaction effects. For instance, they might reveal that genetically determined differential response to a medication might be neutralized by more intense counseling or by the conjoint use of two medications. For more details about trial design, we refer readers to these reports.43,50,54,55
Practical Considerations
The benefits and challenges of various biosampling options regarding biospecimen requirements, storage, and analysis are summarized in Table 1.
Table 1.
Pros and Cons of Biological Samples
| Type of biosample | Primary use | Storage | Pros | Cons |
|---|---|---|---|---|
| Whole blood | Generate subfractions (plasma, serum and cells [for extraction, viable storage, or transformation]), and isolate nucleic acids, proteins, and metabolites | Ultra-low temperature with some alternative storage approaches | Wide variety of fractions and analytes. Costs proportional to the number/diversity of tubes drawn and subsequent processing steps |
Requires access to −80°C freezer. Need access to phlebotomist |
| Saliva | Isolate nucleic acids and proteins from host and from the meta-genome | Room temperature for saliva possible; ultra-low temperature for analytes | Ease of collection. Can be done remotely and mailed in | Lack of clinical observation during collection results in minor rate of biospecimen substitution. Quality/quantity of DNA lower than for blood. Contamination of DNA from food etc |
| Urine | Isolate metabolites. | Ultra-low temperature | 24-hour urine collection is standard but processing urine volumes can be challenging | Requires access to −80°C freezer. No DNA. |
| Buccal Cells | Isolate nucleic acids | Ultra-low temperature. | One tissue type exposed to the environment highly relevant to smoking/vaping behaviors | Care in selecting buccal sampling protocol for comparability |
Collection of Appropriate Participant Consent
Collection of informed written consent from participants to use their biological samples for the purposes of genetics testing is critical to the ethical conduct of genetics research. Research participants need to know what data will be studied, who will have access to their genetics data, what protection will be in place to ensure the anonymity of their genetic information is maintained, and any other study-specific information.
Blood sample collection to obtain genetic data is the foundation for the US Precision Medicine Initiative and UK Biobank that involve large-scale diverse populations in order to set up the infrastructure for research toward precision medicine. Similarly, for some clinical trials it may be necessary for the genetic testing to be a mandatory component of participation in the research. If this is the case, then consent for genetic testing should be included as part of the informed consent for the study as a whole. However, for some studies, participating in the genetics part is not essential. In this case, the genetics part of the research can be presented to the participant as an optional “sub-study,” and a separate informed written consent specifically for genetic or other omics participation should be completed. In our experience, the majority of participants consent to give biological samples (e.g., blood or saliva) for genetic studies56; whether these results can be generalized to individuals who decline biological samples needs to be examined in future research.
Participants must be advised of the potential privacy risks associated with donating a DNA sample for research. The consent form should provide instruction to participants regarding the importance of actively protecting their own privacy. Participants should be informed about the Genetic Information Nondiscrimination Act (GINA), which makes it illegal for health insurance companies, group health plans, and most employers to discriminate against people based on their genetic information. The consent form should also describe the protections taken by the study to protect the privacy of participants. These may include assignment of unique numerical identifiers that are used to label all samples and genotypic data, procedures for securely storing hard-copy records and electronic data, and attainment of a Certificate of Confidentiality from the Department of Health and Human Services. Supplementary Table 1 provides research elements and example consent languages for study purpose, risks/benefits, confidentiality, sample, and information on storage and destruction. We acknowledge that there are clinical trial situations in which collection of biosamples may not be feasible due to specific concerns (e.g., certain vulnerable population and costs).
National Institute of Drug Abuse (NIDA) Genetics Consortium (NGC), NIDA Genetics Study Center (Biorepository), and NIH Resource Sharing Guidelines
As described earlier, consortium-based biorepositories are important to enable evidence needed for translation. The NIDA Genetics Consortium was created in 1999 to identify human genes for drug addiction, create a repository of data, generate a database on genetics of drug use and related phenotypes, and establish a consortium of collaborating scientists.57 In particular, the NIDA Center for Genetics Studies (NCGS Biorepository) is a NIDA-funded scientific resource for informing the human molecular genetics of addiction. The Biorepository will produce, store, and distribute clinical data and biomaterials (DNA samples and cell lines) available in the NIDA Genetics Initiative.57 The Biorepository collects high-quality DNA, plasma (if consented), and cryopreserved lymphocytes on all whole blood samples submitted by NGCS members. The sharing of data in the NCGS is done in strict accordance with the informed consent provided for each research subject. Many genotyping arrays are available including an NIDA-funded custom genotyping array for studying the genetics of addiction and treatment.58
Support Outside United States
Most high-income countries in Europe and elsewhere in the world have equivalent ethical and data protection procedures and legislation as in the United States, but details vary. European data protection regulations are more restrictive than in the United States, and genetic study data are typically deposited at the European Bioinformatics Institute (EMBL-EBI; www.ebi.ac.uk). Clinicians planning to undertake such studies outside the United States should refer to national ethics boards and data protection agencies for guidance as needed.
Meta-Analysis and Harmonization
The research is growing on treatment effect in the context of differences in participants, clinical trial designs and treatments using systematic reviews, traditional meta-analyses, and more recent methods such as Bayesian, multiple treatment, multiple outcome, and network meta-analysis. Clinical and statistical experts have estimated effect sizes of participant, clinician, and treatment factors on prospective abstinence using meta-analyses of randomized clinical trials of smoking cessation.6,59 Meta-analysis goals include utilization of the retrospective evidence base (e.g., based on published literature) to (1) provide guidance to patients and clinicians60; (2) evaluate effect modification, for example, of nicotine dependence61 or genetic variants,21 on outcomes; and (3) develop clinical trial hypotheses for design and analysis.62 In addition, de novo collaborative meta-analyses, which utilize new analyses of existing data, usually with harmonized phenotypes and uniform analytic models, have been highly effective in aggregating evidence for human genetic associations, including for smoking behavioral traits.63–66
Development of databases for pursuing meta-analysis of smoking cessation clinical trials involves identifying participant, treatment, and outcome measures from existing RCT data sets, comparing assessments used to obtain these data, and reviewing coding. Harmonization approaches to render clinical trials suitable for data analysis include expert opinion, regression analyses, and multiple imputation methods. The degree of harmonization between phenotypes in two clinical trials can be assessed if at least one of those trials contains all the information required to estimate the phenotype in the other trial. The degree of agreement between those phenotype estimates is a measure of harmonization. Multiple imputation is applicable to target phenotypes that are missing by design, that is, they can be considered to be missing completely at random; when missing values are not missing at random, the resulting imputation of the target phenotype might be biased. This situation may arise for abstinence when the subject fails to report abstinence because they have relapsed. The usual approach is to assume that all nonreporting individuals have relapsed. However, this approach ignores the accessible factors that are related to missing data and the outcome. In such cases, it may be better to apply a principled method to account for the effects of the accessible mechanism.67,68 Harmonization of prospective abstinence outcome measures has been discussed by clinical experts.69 Interval-censored regression is an indirect method of harmonization applicable if response categories differ for a group of phenotypes that are otherwise consistently measured. On the other hand, pharmacogenomic allele nomenclature standardization (to human reference sequence assemblies) contributes to the ongoing nicotine metabolism biomarker and genotype metric harmonization efforts.70
Multiple treatment comparison meta-analysis is a form of integrated data analysis that includes the more familiar meta- and mega-analysis approaches and enables both direct and indirect comparisons of treatment effects.62,71 Direct comparisons of treatment effects take place when individuals are randomized to different treatments; indirect comparisons of treatment effects take place when analyses rely upon multiple direct comparisons to estimate the indirect comparison via a network. Analysis of both direct and indirect treatment effect comparisons increase the total sample size of the treatment comparisons and may, as in conventional meta-analyses, identify heterogeneity between randomization arms or between directly and indirectly estimated effects. Examination of modeling assumptions through simulation, sensitivity analyses, and collaborative standardized approaches will be necessary to integrate multiple related patient and environmental information and extract guidance from analyses of clinical trials.
Information on Phenotype Assessment and Characterization
Phenotype Assessment
The key conceptual and practical considerations for phenotyping overlap considerably with issues surrounding assessment of treatment efficacy. Thus, existing conventions established to promote the rigor and comparability of smoking cessation studies also provide useful guidance for phenotype assessment.69,72
A common primary endpoint for cessation studies is the attainment of an extended period of abstinence from smoking at a distal follow-up after the quit date (typically 6 or 12 months). Individuals who meet these benchmarks remain at risk for relapse73; however, a lengthy period of sustained abstinence is the best available indicator of lifelong abstinence, the typical treatment goal, and the outcome expected to yield the maximal health benefit. The SRNT workgroup on outcomes in clinical trials recommended using a “prolonged abstinence” standard, defined as a period of sustained abstinence following a short (i.e., 2 week) initial grace period,69 with point prevalence of 7-day abstinence as a secondary measure. A proposed alternative, the “Russell Standard Abstinence” definition, requires the conjunction of a self-report of smoking five or fewer cigarettes since the quit date and a negative biochemical test at the follow-up.72 Both definitions incorporate allowances for a limited amount of smoking after the target quit date, recognizing that smoking cessation is a difficult process and temporary setbacks do not necessarily preclude long-term success and that treatment delivery generally continues beyond the quit date. However, the various definitions are differentially sensitive to post-quit lapsing that occurs relatively late in the follow-up period, but that still may be effectively treated by continued treatment.74 In essence, the researcher must try to adopt an outcome definition that is clinically meaningful, mergeable across other studies, and that provides a sensitive signal of targeted treatment effects.
These conventions provide important information about ultimate clinical outcomes, but they offer little insight into the process of cessation or the mechanisms through which treatments exert their effects. One complementary approach is to focus on pivotal clinical milestones in the cessation process, such as the establishment of an initial period of abstinence, the occurrence of the first smoking lapse, and the transition from a lapse to full relapse.75 In treatment evaluation research, a series of survival analyses can be used to test whether treatment condition influences the time to each milestone, providing clues as to how effective treatments work.75,76 An assumption here is that these milestones are differentially sensitive to medication effects and their relations with omic determinants. For instance, there is some evidence that the effects of medication early in the quit attempt (e.g., on initial abstinence) are especially sensitive to medication benefit.54,63,77
Another fruitful approach is to measure presumed mediators of treatment effects.77,78 The most relevant mediators can differ as a function of the treatment being evaluated, but a host of common barriers to cessation have been identified by theory and empirical investigations of lapse antecedents. These include urge/craving, withdrawal symptoms, exposure to tobacco cues, stressors, alcohol use, and reactions to lapse events.79,80 In pharmacotherapy studies, medication compliance and drug side effects may represent important mediators of treatment outcome.81,82 Investigating whether treatment allocation influences these barriers to cessation, and testing whether group differences in long-term outcomes are mediated through effects in these domains, can help to refine our understanding of treatment mechanisms. There is also the prospect that medication effects on sensitive mediators (e.g., craving suppression) provide especially sensitive indices of medication benefit.83
Genetic studies of smoking cessation will benefit from incorporating clinical phenotypes rooted in each of these approaches. Long-term abstinence endpoints seem strongly indicative of the public health benefit of treatment and thus clearly relevant to the development of precision medicine protocols. Investigating how candidate genetic markers influence clinical milestones and treatment mediators in addition to the traditional outcome of end of treatment abstinence may lead to a better understanding of their functional significance (e.g., due to differential contribution of error).77,84 Use of multiple outcomes may be especially valuable for positional candidates discovered via genome-wide scans that are not anticipated by theory and for which knowledge of biological function is lacking. On the other hand, increasing biological knowledge in genomic databases speeds the discovery process to link genes, functions, and clinical outcomes. Of note, is that, the causal gene and variants are not necessarily the ones closest to the genetic marker identified in a genome-wide screen.
Anticipation of genetic analyses may encourage investigators to alter their assessment plans when designing cessation trials. Pooling or meta-analyzing data from many trials represents a powerful method for exploring genetic influences on smoking cessation. This encourages the use of broad and flexible assessment strategies. Ideally, clinical studies would incorporate detailed assessments of smoking behavior with good resolution of timing, amount, antecedents, and consequences of post-cessation cigarette use. Examples include calendar-based methods or intensive longitudinal assessments.85,86 This would allow outcomes to be scored according to multiple criteria (e.g., various grace periods or thresholds for progression to the relapse milestone), facilitating cross-trial harmonization and pooled analyses.
When designing a stand-alone trial, it may make sense to assess a small set of targeted mediators based on a working knowledge of the treatment under study, that is, how the tested treatment is thought to work. However, the possibility of future, pooled genetic analyses should encourage clinical investigators to cast a wider net when it comes to assessing possible mediators. This alternative approach is to assess mediators based on the outcome model—what important factors may influence outcomes. One reason is that there is often uncertainty about which genetic variant(s) may eventually be tested in secondary analyses, and therefore the important mediator(s) may not be knowable in the trial-planning phase. A second consideration is that mediators thought to be irrelevant in an individual trial might be very important in a pooled or aggregated analysis.
Increasing need for interdisciplinary collaboration and data sharing has led to initiatives such as the PhenX Toolkit87 and the PROMIS system88,89 designed to encourage use of consensus measures in health research. Going forward, the smoking cessation field might benefit from development and dissemination of a comparable set of standardized, flexible assessment tools designed to gather information on post-cessation smoking patterns, common barriers to cessation, variables that may mediate of treatment effects, and potentially useful intermediate phenotypes for genetic research.
Conclusion
In the era of “Precision Medicine,” it is becoming increasingly important that investigators collect biological samples within clinical trials and integrate their analysis and interpretation with the goals of the trial. The identification of genomic markers that affect response to smoking cessation pharmacotherapies, or susceptibility to adverse reactions to such drugs, holds real promise to improve smoking cessation treatment efficacy through tailored treatment interventions, pharmacological or behavioral. A major concern for trial design is the timing of genomic assessment. Available genomic data before treatment randomization will allow gene-based stratified randomization or experimental testing of gene-based personalized treatment, while collection of any biosamples at any time in the trial for subsequent genotyping is still beneficial. Another challenge in identifying such genomic biomarkers will be to obtain adequate sample sizes. Consortium-based approaches will likely be necessary to yield real successes, as we have seen from previous genome-wide association studies of complex traits including smoking behavior.63–65,90–92 Thus, for pharmacogenomic studies, meta-analysis of data from individual smoking cessation trials will be crucial and will require comparable trial designs and outcomes.21,93,94 Other related topics such as the genetic effects on smoker response to nonpharmacologic smoking cessation interventions are not included in this review.
In this article, we outline a framework for the consistent integration of biological data/samples into smoking cessation pharmacotherapy trials. This work aligns with the objectives of the recently unveiled Precision Medicine Initiative and addresses a call for practical advice to guide the integration of treatment and genetics research within the nicotine and tobacco research community. Our goal is to encourage treatment researchers to consider biosample collection and genotyping their existing samples as well as integrating genetic analyses into their study design. Of course, identifying an optimal pharmacogenetic strategy is highly complex, as treatment trials vary in study designs, the type and intensity of the counseling treatment provided to all groups including the placebo arm, subject inclusion/exclusion criteria, and other experimental methods. Still, progress is underway, as reviewed in the companion paper by Saccone et al. In summary, this work encourages and provides support for study designs that are needed in order to realize precision medicine in treatment of nicotine dependence.94
Funding
We would like to acknowledge the following funding support for our authors:
From the National Institute on Drug Abuse: R01 DA026911 (NLS), DA030398 (LSC), R01 DA038076 (LSC), HHSN271201300004C (JWB), R43 DA041211 (JWB, AWB), R21DA033813 (AWB), DA017441 & U54MD010724 (SPD), R01DA036583 (LJB), P30CA091842 (LJB), DA 020830 (RFT), 5RO1HL109031(TBB) and KO5CA139871(TBB); Medical Research Council Integrative Epidemiology Unit at the University of Bristol (MC_UU_12013/2) (HRE, CLR), postdoctoral research fellowship award from the Oak Foundation (HRE); U54MD008149 from the NIMHD (MGF); Grants 265240 & 263278 from the Academy of Finland (JK); Sigrid Juselius Foundation (JK) and the support of a Canada Research Chair in Pharmacogenomics (RFT); Global Research Awards in Nicotine Dependence; WI 206396 and WS2391913 (Pfizer, Inc) (LZ); Canadian Cancer Society; 703187 and 703404 (LZ); Canadian Institutes of Health Research; DC0190SR (LZ).
Declaration of Interest
LJB is listed as an inventor on Issued U.S. Patent 8080371 “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction, and served as a consultant for Pfizer in 2008. The spouse of NLS is also listed as an inventor on the above patent. RT has consulted for Apotex. JWB is an owner and employee of BioRealm and AWB is an employee of BioRealm, which offers commercial services related to the Smokescreen Genotyping Array. SPD has consulted for BaseHealth, Inc., which develops predictive platforms for population health management. JK has consulted for Pfizer. RT has consulted for the pharmaceutical company Apotex. The remaining authors declare no conflict of interest.
Supplementary Material
Acknowledgment
This paper and a companion paper were conceived, developed, and written by the Genetics and Treatment Workgroup of the Society for Research on Nicotine and Tobacco (SRNT): Drs. James W. Baurley, Andrew W. Bergen, Li-Shiun Chen, Sean David, Hannah R. Elliott, Marilyn Foreman, Jaakko Kaprio, Thomas Piasecki, Caroline Relton, Nancy Saccone, Laurie Zawertailo. We thank SRNT and especially the SRNT Genetics Network and the SRNT Treatment Network for their support and enthusiasm for this and other collaborative opportunities for genetics and treatment researchers. We thank Dr. Jennifer Ware for her input during early stages of this work, and Dr. Caryn Lerman for her assistance in reviewing an earlier draft. Thank-you also to Mona Johnson for providing administrative and communications support. We wish to thank Nina Smock and Sherri Fisher for editorial and administrative support. We thank SRNT Treatment Network and Genetic Network (Dr. Anne Joseph, Dr. Anu Loukola, and Dr. Marissa Ehringer) for review and comments.
References
- 1. Schroeder SA. New evidence that cigarette smoking remains the most important health hazard. N Engl J Med. 2013;368(4):389–390. [DOI] [PubMed] [Google Scholar]
- 2. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteford HA, Baxter AJ. The Global Burden of Disease 2010 study: what does it tell us about mental disorders in Latin America?Rev Bras Psiquiatr. 2013;35(2):111–112. [DOI] [PubMed] [Google Scholar]
- 4. Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 2012;24(4):1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. [DOI] [PubMed] [Google Scholar]
- 6. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service;2008. [Google Scholar]
- 7. Centers for Disease Control and Prevention. Current cigarette smoking among adults - United States, 2011. Morbidity and Mortality Weekly Report. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 8. Vaidyanathan G. Redefining clinical trials: the age of personalized medicine. Cell. 2012;148(6):1079–1080. [DOI] [PubMed] [Google Scholar]
- 9. McMahon FJ, Insel TR. Pharmacogenomics and personalized medicine in neuropsychiatry. Neuron. 2012;74(5):773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Hum Genet. 2012; 131(6):857–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010;16(7-8):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uhl GR, Drgon T, Johnson C, Ramoni MF, Behm FM, Rose JE. Genome-wide association for smoking cessation success in a trial of precessation nicotine replacement. Mol Med. 2010;16(11-12):513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: preliminary results using an aggregate genetic risk score. Subst Abuse. 2012;6:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. David SP, Strong DR, Leventhal AM, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction. 2013;108(12):2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NIH. Precision Medicine Initiative. 2015; http://www.nih.gov/precisionmedicine/. [Google Scholar]
- 16. Young RP, Hopkins RJ, Gamble GD. Clinical applications of gene-based risk prediction for lung cancer and the central role of chronic obstructive pulmonary disease. Front Genet. 2012;3:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roychowdhury S, Chinnaiyan AM. Translating genomics for precision cancer medicine. Annu Rev Genomics Hum Genet. 2014;15:395–415. [DOI] [PubMed] [Google Scholar]
- 19. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Association for the Study of Lung Cancer (IASLC) Association for Molecular Pathology (AMP). Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors, Summary of recommendations. https://www.iaslc.org/sites/default/files/wysiwyg-assets/cap_iaslc_amp_summary_of_recommendations.pdf Accessed November 22, 2015. [Google Scholar]
- 21. Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen LS, Baker TB, Jorenby D, et al. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug Alcohol Depend. 2015;154:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169(7):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LS, Bloom AJ, Baker TB, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction. 2014;109(1):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lerman C, Schnoll RA, Hawk LW.Jret al. ; PGRN-PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimmel SE, French B, Kasner SE, et al. ; COAG Investigators A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. FDA. Genomics – table of pharmacogenomic biomarkers in drug labeling. 2015. Retrieved from https://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm [Google Scholar]
- 29. Mallal S, Phillips E, Carosi G, et al. ; PREDICT-1 Study Team HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–579. [DOI] [PubMed] [Google Scholar]
- 30. McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Relling MV, Gardner EE, Sandborn WJ, et al. ; Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89(3):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bainbridge MN, Wiszniewski W, Murdock DR, et al. Whole-genome sequencing for optimized patient management. Sci Transl Med. 2011;3(87):87re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Worthey EA, Mayer AN, Syverson GD, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13(3):255–262. [DOI] [PubMed] [Google Scholar]
- 34. Teutsch SM, Bradley LA, Palomaki GE, et al. ; EGAPP Working Group The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: methods of the EGAPP working group. Genet Med. 2009;11(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metzker ML. Sequencing technologies – the next generation. Nat Rev Genet. 2010;11(1):31–46. [DOI] [PubMed] [Google Scholar]
- 36. Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12(2):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang JC, Cruchaga C, Saccone NL, et al. ; COGEND collaborators and GELCC collaborators Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18(16):3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laird PW. Principles and challenges of genomewide DNA methylation analysis. Nat Rev Genet. 2010;11(3):191–203. [DOI] [PubMed] [Google Scholar]
- 39. Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10(10):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altelaar AF, Munoz J, Heck AJ. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet. 2013;14(1):35–48. [DOI] [PubMed] [Google Scholar]
- 41. Shulaev V. Metabolomics technology and bioinformatics. Brief Bioinform. 2006;7(2):128–139. [DOI] [PubMed] [Google Scholar]
- 42. Hurko O. The uses of biomarkers in drug development. Ann N Y Acad Sci. 2009;1180:1–10. [DOI] [PubMed] [Google Scholar]
- 43. Bough KJ, Lerman C, Rose JE, et al. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93(6):526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Institute of Medicine. Perspectives on Biomarker and Surrogate Endpoint Evaluation: Discussion Forum Summary.Washington, DC:National Academies Press; 2011. [PubMed] [Google Scholar]
- 45. Wan ES, Qiu W, Baccarelli A, et al. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Hum Mol Genet. 2012;21(13):3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeilinger S, Kühnel B, Klopp N, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8(5):e63812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Yang R, Burwinkel B, Breitling LP, Brenner H. F2RL3 methylation as a biomarker of current and lifetime smoking exposures. Environ Health Perspect. 2014;122(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nishita DM, Jack LM, McElroy M, et al. Clinical trial participant characteristics and saliva and DNA metrics. BMC Med Res Methodol. 2009;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swan GE, Javitz HS, Jack LM, et al. Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics J. 2012;12(4):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Institute-of-Medicine. Generating Evidence for Genomic Diagnostic Test Development: Workshop Summary.Washington, DC:National Academies Press; ). Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 51. Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brass EP. The gap between clinical trials and clinical practice: the use of pragmatic clinical trials to inform regulatory decision making. Clin Pharmacol Ther. 2010;87(3):351–355. [DOI] [PubMed] [Google Scholar]
- 53. McClure JB, Swan GE, St John J, et al. Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study. Nicotine Tob Res. 2013;15(2):518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baker TB, Collins LM, Mermelstein R, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. 2016;111(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, Mermelstein RJ. Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Transl Behav Med. 2014;4(3):238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hartz SM, Johnson EO, Saccone NL, Hatsukami D, Breslau N, Bierut LJ. Inclusion of African Americans in genetic studies: what is the barrier?Am J Epidemiol. 2011;174(3):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. NIDA. NIDA Genetics Consortium. https://www.drugabuse.gov/about-nida/organization/workgroups-interest-groups-consortia/genetics-workgroup-gwg/nida-genetics-consortium-ngc Accessed May 22, 2016. [Google Scholar]
- 58. Baurley JW, Edlund CK, Pardamean CI, Conti DV, Bergen AW. Smokescreen: a targeted genotyping array for addiction research. BMC Genomics. 2016;17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–597. [DOI] [PubMed] [Google Scholar]
- 60. Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. preventive services task force. Ann Intern Med. 2015;163(8):608–621. [DOI] [PubMed] [Google Scholar]
- 61. Fagerström K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerström test for nicotine dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14(12):1467–1473. [DOI] [PubMed] [Google Scholar]
- 62. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. [DOI] [PubMed] [Google Scholar]
- 63. TAG. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. May 2010;42(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. ; ENGAGE Consortium Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu JZ, Tozzi F, Waterworth DM, et al. ; Wellcome Trust Case Control Consortium Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8) pii: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. [DOI] [PubMed] [Google Scholar]
- 68. Rubin DB. Multiple imputation after 18+ years. J Am Statist Assoc. 1996;91(434):473–489. [Google Scholar]
- 69. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 70. Kalman LV, Agúndez J, Appell ML, et al. Pharmacogenetic allele nomenclature: International workgroup recommendations for test result reporting. Clin Pharmacol Ther. 2016;99(2):172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 72. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. [DOI] [PubMed] [Google Scholar]
- 73. Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33(12):1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction. 2012;107(7):1349–1353. [DOI] [PubMed] [Google Scholar]
- 75. Shiffman S, Scharf DM, Shadel WG, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74(2):276–285. [DOI] [PubMed] [Google Scholar]
- 76. Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41(2):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103(9):1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. [DOI] [PubMed] [Google Scholar]
- 80. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 81. Hays JT, Ebbert JO. Adverse effects and tolerability of medications for the treatment of tobacco use and dependence. Drugs. 2010;70(18):2357–2372. [DOI] [PubMed] [Google Scholar]
- 82. Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive-behavioral therapy for smoking cessation in women. Nicotine Tob Res. 2007;9(6):699–709. [DOI] [PubMed] [Google Scholar]
- 83. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl). 2006;184(3-4):628–636. [DOI] [PubMed] [Google Scholar]
- 85. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- 86. Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, time-line follow-back, and ecological momentary assessment. Health Psychol. 2009;28(5):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hamilton CM, Strader LC, Pratt JG, et al. The PhenX toolkit: get the most from your measures. Am J Epidemiol. 2011;174(3):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Snyder CF, Watson ME, Jackson JD, Cella D, Halyard MY; Mayo/FDA Patient-Reported Outcomes Consensus Meeting Group Patient-reported outcome instrument selection: designing a measurement strategy. Value Health. 2007;10 Suppl 2:S76–S85. [DOI] [PubMed] [Google Scholar]
- 89. Edelen MO. The PROMIS smoking assessment toolkit–background and introduction to supplement. Nicotine Tob Res. 2014;16 Suppl 3:S170–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. David SP, Hamidovic A, Chen GK, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl Psychiatry. 2012;2:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Loukola A, Buchwald J, Gupta R, et al. A genome-wide association study of a biomarker of nicotine metabolism. PLoS Genet. 2015;11(9):e1005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. David SP, Bergen AW, Munafo M, Schuit E, Bennett DA. Genomic analysis to guide choice of treatment for smoking cessation. Cochrane Database Syst Rev. 2015. [Google Scholar]
- 94. Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J. 2006;8(1):E174–E184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen LS, Baker TB, Grucza R, et al. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res. 2012;14(4):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.