Abstract
Introduction:
Electronic cigarette use is rapidly gaining in popularity. However, little is known about correlates and reasons for electronic cigarette use by women of reproductive age, a group for which the safety and efficacy of electronic cigarette use is of particular interest.
Methods:
As part of a clinical trial for smoking cessation, we surveyed pregnant smokers about their lifetime use of electronic cigarettes, previous use of any adjunctive treatments for smoking cessation, and use of electronic cigarettes during pregnancy. We examined associations between electronic cigarette use and participant characteristics.
Results:
Fifty-three percent (55/103) of participants had previously tried electronic cigarettes. Ever users smoked more cigarettes per day before pregnancy (p = .049), had a greater number of previous quit attempts (p = .033), and were more likely to identify as being Hispanic or non-Hispanic white than never users (p = .027). Fifteen percent of participants (15/103) reported previous use of electronic cigarettes for smoking cessation, which was more common than the use of any specific FDA-approved smoking cessation medication. Fourteen percent of participants (14/103) reported electronic cigarette use during pregnancy, most commonly to quit smoking. A history of substance abuse (p = .043) and more previous quit attempts (p = .018) were associated with electronic cigarette use during pregnancy.
Conclusions:
Use of electronic cigarettes to quit smoking may be common in women of reproductive age, including those who are pregnant. More research is needed to determine the risks and benefits of electronic cigarette use in this population of smokers.
Implications:
This study shows that electronic cigarettes are used by women of reproductive age, including pregnant smokers. The implications of this finding are that there is an urgent need to examine the risks and benefits of electronic cigarette use, especially by pregnant women. The study also shows that electronic cigarettes are commonly used as a smoking cessation aid in women of reproductive age. The greater likelihood of electronic cigarette use compared to proven adjunctive smoking treatments suggests that electronic cigarettes should be examined as a potential aid to cessation in this population.
Introduction
Electronic cigarette use is increasing in the United States, especially in younger individuals.1–4 Recent surveys in young adults showed that electronic cigarettes are perceived to be less likely than cigarette smoking to cause lung cancer or have less adverse effects during pregnancy.5,6 Moreover, a recent review and case report showed that pregnant women may be using electronic cigarettes with some frequency.7 Although there is considerable evidence that electronic cigarettes reduce toxicant exposure compared to regular cigarettes,8,9 they may have unknown health risks.
Despite the growing popularity of electronic cigarette use, there is a paucity of data on correlates of and reasons for electronic cigarette use in various populations of smokers. People may use electronic cigarettes for a variety of reasons, including to reduce or to quit smoking.3,10 Female smokers of reproductive age who use electronic cigarettes may have greater nicotine dependence and more quit attempts.10 Unfortunately, it is not yet clear whether electronic cigarette use aids in smoking cessation. Although available evidence suggests that electronic cigarettes could increase long-term cessation rates, more studies are needed to substantiate this effect.11,12
The present study examined electronic cigarette use among women of reproductive age who were entering a trial of nicotine replacement therapy for smoking cessation during pregnancy. We sought to describe the characteristics of pregnant women who have used electronic cigarettes and compare the frequency of electronic cigarette use for smoking cessation to that of medications that are approved by the US Food and Drug Administration (FDA) for that indication. Given the paucity of data on use of electronic cigarettes in pregnant women, and concern that flavorings and additives in electronic cigarettes may pose general and reproductive health risks,13–17 we added questions about electronic cigarette use during pregnancy to the evaluation completed by a portion of the study sample.
Methods
We collected data for the current report as part of screening assessments for a trial of nicotine replacement therapy for smoking cessation in pregnancy. The study was a randomized, double blind, placebo-controlled trial of the safety and efficacy of the nicotine inhaler in combination with behavioral counseling for smoking cessation during pregnancy. The Institutional Review Boards at UConn Health (Farmington, Connecticut) and at both of the enrollment sites−Hartford Hospital (Hartford, Connecticut), and Baystate Medical Center (Springfield, Massachusetts)—approved the study protocol. The study is registered on http://Clinicaltrials.gov (NCT00888979).
We began recruiting study participants in October, 2012 and stopped enrollment in March, 2016. Subjects were primarily recruited from prenatal clinics at Hartford Hospital and Baystate Medical Center. We also accepted referrals from private practitioners. Study staff identified smokers and inquired as to whether they were interested in participating in a research study. We included women who smoked at least 5 cigarettes per day and who were unable to quit smoking on their own during pregnancy. We excluded women who were at greater than 26 weeks of gestation, had unstable medical problems, or were using either medication to quit smoking or electronic cigarettes. Interested individuals were then seen by research personnel.
We obtained written informed consent (available in both English and Spanish) prior to collecting any information at the screening visit. As part of this initial screening visit, we collected demographic information, medical and obstetric histories, and smoking history. The smoking history included questions about the number of cigarettes smoked per day recently and prior to pregnancy, number of years of smoking, smoking status of partner and friends, number of household smokers, perceived support for staying abstinent, and the composition of the household (including age and relationship to the subject). Women were also asked about their current and past use of other tobacco products, including electronic cigarettes, number of past quit attempts, and their previous use of any adjunctive treatments to stop smoking. Because of the bilingual nature of our population and the concern that we obtain complete data, research coordinators collected answers to the questions “Have you ever tried electronic cigarettes?” Another question was “Have you tried electronic cigarettes during pregnancy?” If affirmative, the participant was asked further questions, including her reasons for use during pregnancy.
We also administered several standardized questionnaires at the screening visit. The Fagerstrom Test for Cigarette Dependence18 was administered to measure severity of dependence on nicotine. A score of 6 or higher indicates high nicotine dependence and a score of 5 or less indicates low-to-moderate nicotine dependence. The Patient Health Questionnaire (PHQ-9)19 was used to screen for depression. The Patient Health Questionnaire (PHQ-9) items are scored 0 to 3, providing a total severity score of 0 to 27. The Smoking Cessation Self-Efficacy Questionnaire20 was administered to assess the participant’s level of confidence in resisting smoking across nine different situations. For each situation, subjects rated their level of confidence to resist smoking on a scale of 1 (“Not at all confident”) to 5 (“Extremely confident”). A total score was calculated by summing the scores for each question. Motivation to quit smoking was assessed on a 10-point scale, with 0 being “Not at all motivated,” and 10 being “Extremely motivated.”
After the study started, we added questions about electronic cigarette use during pregnancy. Women who were asked these questions (N = 103) are the subject of this report. Women who reported using electronic cigarettes were asked about the length of use, frequency of use per day, reasons for use (where they were asked to identify all that applied: to quit smoking, reduce smoking, curiosity, availability, health benefits), in which trimester of pregnancy they had used electronic cigarettes, and the type and brand of electronic cigarette used.
Prior to statistical analyses, we classified variables as continuous or categorical. Continuous variables were summarized by sample mean and SD. The conformity of the distributions for electronic cigarette users and non-users to normality were tested by the Shapiro-Wilk test. If both p values were greater than .05, that is, providing no evidence to reject the hypothesis that both were normally distributed, we compared baseline differences between electronic cigarette users and non-users using a two-sample t test. Otherwise, we utilized the Wilcoxon rank sum test.
We summarized each categorical variable by a frequency distribution or a bar plot. We applied the Fisher’s exact test to compare binary categorical variables between electronic cigarette users and non-users and the chi-square test without Yates’s correction for continuity to compare categorical variables with 3 or more outcomes. If the chi-square approximation was inadequate due to sparse cells, a Monte Carlo p value was reported based on 104 simulation replicates. To make our results reproducible, we arbitrarily set the seed number to 987 654. A p value smaller than 5% was considered statistically significant. All of the statistical analyses were performed using R 3.1.2.21
Results
Demographic and other characteristics of the 55 participants who had ever used electronic cigarettes and non-users are shown in Table 1. Ever users smoked more cigarettes prior to pregnancy (p = .049), had a greater number of previous quit attempts (p = .033), and were more likely to identify as being Hispanic or non-Hispanic white than never users (p = .027). Although the differences were not statistically significant, ever users tended to be less educated (<High school: 40% vs. 25%) and more likely to report a history of depression or anxiety (67% vs. 50%) and substance abuse (45% vs. 31%). Other baseline characteristics were similar between the two groups.
Table 1.
Demographic and Other Characteristics of Participantsa
| Variable | Electronic cigarette ever users (n = 55) | Electronic cigarette never users (n = 48) | Total sample (n = 103) | p b |
|---|---|---|---|---|
| Age | 26.8±5.0 | 28.7±6.9 | 27.7±6.0 | .109 |
| Cigarettes per day prior to pregnancy | 19.1±7.8 | 16.1±7 | 17.7±7.6 | .049 |
| Cigarettes per day during pregnancy | 10.5±4.7 | 10.4±4.3 | 10.5±4.5 | .946 |
| Nicotine dependence severityc | 4±2.2 | 4.5±2.0 | 4.2±2.1 | .230 |
| Motivation to quit smoking (0–10) | 8.4±1.6 | 8.3±1.8 | 8.3±1.7 | .978 |
| Self-efficacy to quit smoking | 25.2±6.4 | 25.5±6.1 | 25.3±6.3 | .823 |
| Patient Health Questionnaire score (0–27) | 5.6±4.6 | 5.1±4.7 | 5.3±4.6 | .425 |
| Race/ethnicityd | .027 | |||
| Hispanic or Latina | 24 (48) | 15 (35) | 39 (42) | |
| White, non-Hispanic | 23 (46) | 16 (37) | 39 (42) | |
| Black, non-Hispanic | 3 (6) | 11 (26) | 14 (15) | |
| Other | 0 | 1 (2) | 1 (1) | |
| Education | .142 | |||
| <High school | 22 (40) | 12 (25) | 34 (33) | |
| Smoking quit attempts | .033 | |||
| <2 | 23 (42) | 30 (63) | 53 (51) | |
| 2 | 10 (18) | 11 (23) | 21 (20) | |
| 3 | 10 (18) | 2 (4) | 12 (12) | |
| 4 or greater | 12 (22) | 5 (11) | 17 (17) | |
| History of depression or anxiety | .108 | |||
| % Yes | 37 (67) | 24 (50) | 61 (60) | |
| History of substance abuse | .160 | |||
| % Yes | 25 (45) | 15 (31) | 40 (39) |
aResults are reported as mean ± SD, or # of subjects (percent).
bThe t test was applied for continuous variables that are normally distributed and the Wilcoxon rank-sum test was used for non-normal continuous variables. The Fisher’s exact test was applied for binary categorical variables and the chi-square test was used for categorical variables with three or more outcomes.
cFagerstrom Test for Cigarette Dependence was used to assess nicotine dependence severity.
dTen subjects did not answer questions about race/ethnicity.
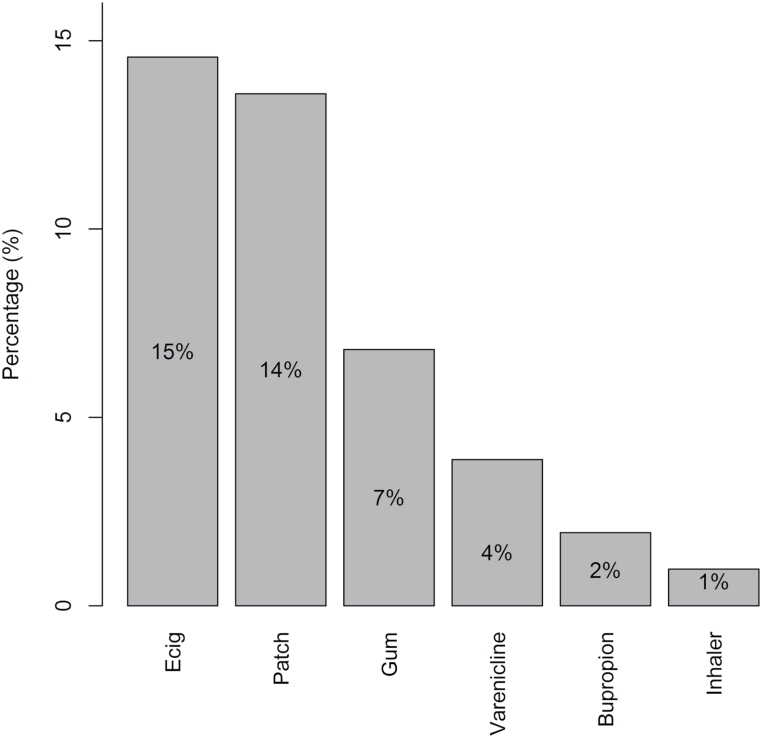
Thirty-five percent (35/103) of participants reported using electronic cigarettes and/or an FDA-approved medication during a previous quit attempt. Participants could check more than one response. As shown in Figure 1, the highest percentage of subjects (ie, 15%) used electronic cigarettes as an aid in smoking cessation, followed by the nicotine patch (14%), nicotine gum (7%), varenicline (4%), bupropion (2%), and nicotine inhaler (1%). None of the subjects reported previous use of the nicotine lozenge or nasal spray for smoking cessation.
Figure 1.
Percentage of subjects that had previously used each product for smoking cessation (n = 103).
Smoking characteristics of users and non-users of electronic cigarettes during pregnancy is shown in Table 2. Fourteen percent of women (14/103) reported using electronic cigarettes during pregnancy and 89 women denied such use during pregnancy. Women reporting electronic cigarette use during pregnancy were more likely to have a history of substance abuse (p = .043), and a greater number of quit attempts than non-users (p = .018). There was a trend for lower self-efficacy in users, but this was not statistically significant (p = .057). Among the 14 women who used electronic cigarettes during pregnancy, 10 had done so in the first trimester. The length of time using electronic cigarettes ranged from 1 to 30 days, and the number of times that electronic cigarettes were used per day ranged from 1 to 25. Women could check more than one reason that they used electronic cigarettes during pregnancy. Eight of the 14 users indicated that they used them to quit smoking. Only five subjects could recall the brand of electronic cigarette that they used (four Blu electronic cigarettes and one Greensmoke).
Table 2.
Smoking Variables and Electronic Cigarette Use During Pregnancya
| Variable | Electronic cigarette users (n = 14) | Non-users (n = 89) | p b |
|---|---|---|---|
| Cigarettes per day before pregnancy | 18.0±5.9 | 17.6±4.7 | .515 |
| Nicotine dependence severityc | 4.3±1.6 | 4.2±2.2 | .941 |
| Motivation to quit smoking (0–10) | 8.1±1.3 | 8.4±1.8 | .364 |
| Self-efficacy to quit smoking | 22.4±5.8 | 25.8±6.2 | .057 |
| Smoking quit attempts | .018 | ||
| <2 | 4 (29) | 49 (55) | |
| 3 | 2 (14) | 19 (21) | |
| 4 | 5 (36) | 7 (8) | |
| 4 or greater | 3 (21) | 14 (16) | |
| History of depression or anxiety | .390 | ||
| % Yes | 10 (71) | 51 (57) | |
| History of substance abuse | .043 | ||
| % Yes | 9 (64) | 31 (35) | |
| Mean days of electronic cigarette use | 7.5±10.3 | NA | |
| Frequency of electronic cigarette use per day | 6.4±7.5 | NA | |
| % Using electronic cigarettes in first trimester | 10 (71) | NA | |
| % Recall electronic cigarette brand | 5 (36) | NA | |
| % Using pre-filled cartridges | 7 (50) | NA | |
| Reasons for electronic cigarette used | NA | ||
| To quit smoking | 8 (57) | ||
| To reduce smoking | 5 (36) | ||
| Curiosity | 5 (36) | ||
| Availability | 3 (21) | ||
| Health benefits | 1 (7) |
NA = not applicable.
aResults are reported as mean ± SD, or # of subjects (percent).
bThe t test was applied for continuous variables that are normally distributed and the Wilcoxon rank-sum test was used for non-normal continuous variables. The Fisher’s exact test was applied for binary categorical variables and the chi-square test was used for categorical variables with three or more outcomes.
cFagerstrom Test for Cigarette Dependence.
dParticipants could endorse more than one reason.
Discussion
We found that approximately 53% of pregnant smokers entering a medication treatment trial for smoking cessation had previously used electronic cigarettes. These women smoked more cigarettes per day prior to pregnancy, had more quit attempts, and were more likely to identify as Hispanic or white non-Hispanic race/ethnicity than never users. Women were more likely to have used electronic cigarettes to quit smoking than any FDA-approved smoking cessation medication. Approximately 14% of our sample used electronic cigarettes during pregnancy, the primary reason for which was to quit smoking. A higher number of previous smoking quit attempts, and a history of substance abuse were also associated with electronic cigarette use during pregnancy.
The association between ever having used electronic cigarettes and smoking more cigarettes per day prior to pregnancy, and more previous quit attempts suggests that those who may find it more difficulty to quit smoking are more likely to try electronic cigarettes. Our findings are consistent with those from a national sample of adult smokers, which also showed that electronic cigarette users had more quit attempts.22 However, in that study, participants also had other characteristics that may have made them less likely to quit smoking (ie, higher nicotine dependence severity, concurrent use of different kinds of tobacco products, more depressive symptoms, and past use of multiple medications to quit) that were not statistically significant in our study. Although the explanation for an association between a history of substance abuse and electronic cigarette use is not clear, people with a history of substance abuse may be more novelty seeking and thereby more open to trying alternative treatments for tobacco use and dependence. Persons with a history of substance abuse also tend to be heavier smokers.23–25 Of note, a recent case report described a pregnant woman in a substance abuse treatment program who, after learning that she was pregnant, started using electronic cigarettes with the intent of quitting or reducing smoking.7 This case report is consistent with the association that we observed between a history of substance abuse and electronic cigarette use during pregnancy, as well as the reason for using them (ie, to quit or reduce smoking).
We did not anticipate that women in our study would be more likely to report using electronic cigarettes to quit smoking than any FDA-approved medication. This is especially true because electronic cigarettes are not FDA regulated, and it is controversial as to whether they are a safe and effective treatment for smoking cessation.26–28 The high rate of use of electronic cigarettes for smoking cessation may reflect the fact that they replace some of the sensory-motor motor aspects of conventional cigarette smoking. They are also readily available, widely marketed, and come in a variety of flavors. In fact, according to a recent policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology, more than 7700 flavors are available on the Internet.29 By contrast, nicotine gum comes in just two flavors. Like electronic cigarettes, the nicotine patch and gum are also available for over-the-counter purchase; however, neither is widely marketed nor are these products the subject of media attention. Some electronic cigarettes may cost less than pharmacotherapies, which could be a factor for low-income individuals, such as those recruited to participate in our study. Flavored electronic cigarettes may be particularly appealing for some individuals because they mimic the sensory aspects of smoking, which may be especially important for female smokers and women who are pregnant.30 Given the acceptability in this population of using electronic cigarettes for smoking cessation and the lack of effective medications for smoking cessation during pregnancy, electronic cigarettes could be useful for cessation in this group.
We also did not anticipate that 14% of women in the study would have used electronic cigarettes during pregnancy. However, this finding is consistent with a recent report suggesting that young adults perceive electronic cigarette use to be less harmful than smoking during pregnancy.6 Suter et al.31 also suggested that there may be less stigma associated with electronic cigarette use than smoking during pregnancy, which could increase electronic cigarette use by pregnant women. A survey of obstetricians found that although 29% believed that electronic cigarettes are likely to have adverse health effects, they viewed them as safer than cigarettes, which could also contribute to their use in pregnancy.32
Indeed, considerable evidence suggests that many electronic cigarette brands reduce exposure to reproductive and developmental toxins such as nicotine, carcinogens, carbonyl compounds, heavy metals, and carbon monoxide compared to tobacco smoke.9 Although available evidence is that electronic cigarettes would likely be safer than conventional cigarette smoking electronic cigarettes may in some cases present risks during pregnancy that either are not present with conventional cigarettes or exceed the risk associated with cigarettes. This is particularly the case given the considerable variability among electronic cigarette brands. A national survey showed that over 75% of electronic cigarette users also concurrently smoke conventional cigarettes,1 which theoretically could produce greater nicotine exposure than that associated with either method alone. Because nicotine has been shown to be toxic in animal studies,33,34 it seems prudent to minimize nicotine exposure during pregnancy. One study showed that electronic cigarette tank systems using a very high voltage could markedly increase formaldehyde exposure compared to conventional cigarette smoking.16 Although it seems unlikely that electronic cigarette users would use voltages comparable to this laboratory study, it is noteworthy that an increased risk of spontaneous abortion and other adverse reproductive outcomes has been observed in pregnant women exposed to formaldehyde in the occupational setting (ie, textile workers, lab and wood workers).35 Propylene glycol, a medium commonly used in electronic cigarettes, may have an irritant effect,15 which could be problematic for pregnant asthmatics. Additionally, flavorings and other additives in electronic cigarettes, although approved for oral consumption, may not be safe when inhaled.14 Studies in human embryonic stem cell lines have shown that some flavor additives are highly toxic, though the clinical significance of these findings is unknown. This is particularly true for cinnamon flavoring, which is an electronic cigarette flavor choice.17,36 There also may be adverse effects of electronic cigarettes that have not yet been identified.
Strengths of this study include its focus on electronic cigarette use in pregnancy, a topic on which there are few published data. The study is limited by the fact that the questions regarding electronic cigarettes were added after the study began, so that not all subjects were asked about their electronic cigarette use. Consequently, the sample size is small, which limited our ability to find significant correlates. A potential bias in recruiting pregnant women who were unable to stop smoking during pregnancy is that they may differ on a number of features from the general population of pregnant smokers. The study is also limited by the fact that we surveyed women entering a nicotine replacement trial for smoking cessation during pregnancy. Consequently, we may have overestimated the use of electronic cigarettes in this population, as these women may be more likely to use electronic cigarettes in pregnancy because of personality or other features. We may also have under-estimated the use of electronic cigarettes in this population, as many women prefer to stop smoking on their own, rather than enter a formal treatment program or study.37 Excluding current electronic cigarette users in our study could have introduced bias by lowering our estimated prevalence rate of electronic cigarette use and days of use during pregnancy. Women who are current electronic cigarette users may use electronic cigarettes for longer than the average 2 weeks observed in our study.
In summary, we found that the use of electronic cigarettes is not uncommon in women of childbearing age, including those who when pregnant are unable to quit smoking on their own. Studies are needed to determine the risks or benefits of electronic cigarette use for smoking cessation in pregnant women and the impact of electronic cigarette use on reproductive and developmental outcomes. Regulation of these products is needed to ensure that toxicant exposure is minimized for people who may use them, including pregnant women.
Funding
This report was supported partly by National Institutes of Health (NIH) grants R01 HD069314, Pfizer pharmaceuticals, and the Clinical Research Center at the University of Connecticut Health Center.
Declaration of Interests
CO received study medication (nicotine inhaler and placebo) from Pfizer pharmaceuticals for an NIH-funded study of a nicotine inhaler for smoking cessation during pregnancy. HRK has been an advisory board member, consultant, or CME speaker for Indivior, Lundbeck, and Otsuka. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported over the last 3 years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, and XenoPort. KAR, C-LK, ED, and HZS report no conflict of interest.
References
- 1. King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010-2013. Nicotine Tob Res. 2015;17(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010-2011. Nicotine Tob Res. 2013;15(9):1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walton KM, Abrams DB, Bailey WC, et al. NIH electronic cigarette workshop: developing a research agenda. Nicotine Tob Res. 2015;17(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. [DOI] [PubMed] [Google Scholar]
- 5. Kahr MK, Padgett S, Shope CD, et al. A qualitative assessment of the perceived risks of electronic cigarette and hookah use in pregnancy. BMC Public Health. 2015;15:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeza-Loya S, Viswanath H, Carter A, et al. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull Menninger Clin. 2014;78(3):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farquhar B, Mark K, Terplan M, Chisolm MS. Demystifying electronic cigarette use in pregnancy. J Addict Med. 2015;9(2):157–158. [DOI] [PubMed] [Google Scholar]
- 8. McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila). 2015;8(9):873–878. [DOI] [PubMed] [Google Scholar]
- 9. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. [DOI] [PubMed] [Google Scholar]
- 10. Chivers LL, Hand DJ, Priest JS, Higgins ST. E-cigarette use among women of reproductive age: impulsivity, cigarette smoking status, and other risk factors. Prev Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McRobbie H, Bullen C, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. 2012. [DOI] [PubMed] [Google Scholar]
- 12. Beard E, Shahab L, Cummings DM, Michie S, West R. New pharmacological agents to aid smoking cessation and tobacco harm reduction: what has been investigated, and what is in the pipeline? [published online ahead of print July 15, 2016]CNS Drugs. [DOI] [PubMed] [Google Scholar]
- 13. Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–537. [DOI] [PubMed] [Google Scholar]
- 14. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. [DOI] [PubMed] [Google Scholar]
- 17. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 19. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 20. Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–283. [DOI] [PubMed] [Google Scholar]
- 21. R: A Language and Environment for Statistical Computing [Computer Program]. Version 3.1.2. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 22. Pulvers K, Hayes RB, Scheuermann TS, et al. Tobacco use, quitting behavior, and health characteristics among current electronic cigarette users in a national tri-ethnic adult stable smoker sample. Nicotine Tob Res. 2015;17(9):1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shu C, Cook BL. Examining the association between substance use disorder treatment and smoking cessation. Addiction. 2015;110(6)1015–1024. [DOI] [PubMed] [Google Scholar]
- 24. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 25. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pearson JL, Stanton CA, Cha S, Niaura RS, Luta G, Graham AL. E-cigarettes and smoking cessation: insights and cautions from a secondary analysis of data from a study of online treatment-seeking smokers. Nicotine Tob Res. 2015;17(10):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 28. Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc. 2014;11(2):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. J Clin Oncol. 2015;33(8):952–963. [DOI] [PubMed] [Google Scholar]
- 30. Perkins KA, Fonte C, Meeker J, White W, Wilson A. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behav Pharmacol. 2001;12(1):35–44. [DOI] [PubMed] [Google Scholar]
- 31. Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res. Part A Clin. Mol. Teratol. 2015;103(3):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. England LJ, Anderson BL, Tong VT, et al. Screening practices and attitudes of obstetricians-gynecologists toward new and emerging tobacco products. Am J Obstet Gynecol. 2014;211(6):695.e1–695.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol. 2011;33(3):431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–945. [PubMed] [Google Scholar]
- 35. Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L. Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat Res. 2011;728(3):118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. [DOI] [PubMed] [Google Scholar]
- 37. Morasco BJ, Dornelas EA, Fischer EH, Oncken C, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: a preliminary investigation. Addict Behav. 2006;31(2):203–210. [DOI] [PubMed] [Google Scholar]