Abstract
Introduction
Individuals with attention deficit/hyperactivity disorder (ADHD) are susceptible to earlier and more severe nicotine addiction. To shed light on the relationship between nicotine and ADHD, we examined nicotine’s effects on functional brain networks in an animal model of ADHD.
Methods
Awake magnetic resonance imaging was used to compare functional connectivity in adolescent (post-natal day 44 ± 2) males of the spontaneously hypertensive rat (SHR) strain and two control strains, Wistar-Kyoto and Sprague-Dawley (n = 16 each). We analyzed functional connectivity immediately before and after nicotine exposure (0.4 mg/kg base) in naïve animals, using a region-of-interest approach focussing on 16 regions previously implicated in reward and addiction.
Results
Relative to the control groups, the SHR strain demonstrated increased functional connectivity between the ventral tegmental area (VTA) and retrosplenial cortex in response to nicotine, suggesting an aberrant response to nicotine. In contrast, increased VTA-substantia nigra connectivity in response to a saline injection in the SHR was absent following a nicotine injection, suggesting that nicotine normalized function in this circuit.
Conclusions
In the SHR, nicotine triggered an atypical response in one VTA circuit while normalizing activity in another. The VTA has been widely implicated in drug reward. Our data suggest that increased susceptibility to nicotine addiction in individuals with ADHD may involve altered responses to nicotine involving VTA circuits.
Implications
Nicotine addiction is more common among individuals with ADHD. We found that two circuits involving the VTA responded differently to nicotine in animals that model ADHD in comparison to two control strains. In one circuit, nicotine normalized activity that was abnormal in the ADHD animals, while in the other circuit nicotine caused an atypical brain response in the ADHD animals. The VTA has been implicated in drug reward. Our results would be consistent with an interpretation that nicotine may normalize abnormal brain activity in ADHD, and that nicotine may be more rewarding for individuals with ADHD.
Introduction
Clinical studies indicate that the earliest symptoms of nicotine addiction can appear after the first cigarette.1 This has focused attention on the potential effects of a single dose of nicotine. In terms of biochemical effects, a single dose of nicotine increases glutamate transmission in various brain regions both in vivo2,3 and in vitro.4–6 In terms of behavioral effects, locomotor sensitization may be apparent with the second dose, suggesting that neurologic alterations associated with sensitization can begin with the first dose.7,8
The first doses of nicotine do not initiate addiction in all tobacco users; in fact, there is significant individual variability in susceptibility to the development of nicotine addiction which is attributed in part to genetic influences.9 At particular, risk of addiction are youths with attention deficit hyperactivity disorder (ADHD). Adolescents diagnosed with ADHD begin smoking at a younger age and are more than eight times more likely to develop nicotine addiction than are healthy controls.10 There is interest in identifying the characteristics of the brains of individuals with ADHD that might facilitate nicotine addiction. Aside from addiction issues, it has been hypothesized that nicotine might help to normalize brain function in youths with ADHD.11 Therefore, we were interested in determining whether the ADHD brain reacts to nicotine in a way that suggests that brain function is normalized or that suggests that nicotine may be more rewarding.
In order to advance our understanding of ADHD, a variety of animal models have been developed. The most extensively studied model is the adolescent spontaneously hypertensive rat (SHR). It is an inbred strain originating from outbred Wistar rats selected for high blood pressure.12 It develops hypertension beyond puberty, prior to which it has been used and validated as a model of ADHD.12,13 The SHR line shows dopaminergic alterations,14,15 and cholinergic alterations16–18 which are of interest given associations between prenatal maternal smoking and ADHD and cholinergic dysregulation.19,20
Although the role of nicotinic receptor up-regulation in addiction is not fully elucidated, nicotine induces receptor up-regulation in rats and humans,21 but not in the SHR.22 The SHR also shows dampened nicotine-induced norepinephrine release.23 The SHR also differs from other strains in relation to resting state functional connectivity (rsFC).24,25 rsFC analysis identifies correlations in activity in different parts of the brain by detecting temporal correlations of low-frequency spontaneous fluctuations of the blood oxygenation level-dependent signal.26–28 We have found marked functional connectivity differences in mesocorticolimbic reward circuits in the SHR.25 To investigate whether ADHD involves altered responses to nicotine, we used rsFC to study brain responses to the initial exposure to nicotine in the SHR and two control strains, the Wistar-Kyoto parent strain and the outbred Sprague-Dawley.
Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Massachusetts Medical School and carried out in accordance with the guidelines published by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. In total, 48 adolescent male rats (post-natal day 25–28) were obtained from Harlan Laboratories, Inc., which included 16 Sprague-Dawley (SD), 16 SHR (SHR/NHsd) and 16 Wistar-Kyoto (WKY/NHsd), each strain later randomly separated into two equal drug groups. The SHR/NHsd line was selected for (1) its repeatedly demonstrated open field locomotor hyperactivity29–32 and atypical attention patterns29,33–35; (2) its genetic polymorphism in neuronal nicotinic receptor α3,36 a gene that has been associated with nicotine use in subjects with ADHD,37 and (3) our data showing rsFC alterations in mesocorticolimbic reward circuits.25 Animals were housed in pairs and the environment was maintained at 22–24°C with a reversed 12 hour light–12 hour dark schedule (lights on at 9:00 PM and off at 09:00 AM). Food and water were provided ad libitum.
Drugs
Nicotine hydrogen tartrate (Sigma) was dissolved in saline, pH adjusted to 7.2 ± 0.2, and delivered subcutaneously at a dose of 0.4 mg nicotine base/kg in a volume of 1 mL/kg. This commonly used dosage reflects human cotinine plasma levels after smoking38 and produces locomotor sensitization. Being a moderate dose, it allows the potential observation of both amplified and dampened strain effects.39
Acclimation for Imaging
Magnetic resonance imaging was conducted with awake animals. In spite of general network similarities between anesthetized and awake imaging,40 there are qualitatively different functional connectivity patterns41,42 and substantial dampening in the regional recruitment by nicotinic agonists.43 We employed our validated procedure for the acclimation of rats to restraint and noise.44 Briefly, rats were anesthetized with isoflurane and EMLA cream (lidocaine 2.5% and prilocaine 2.5%, Hi-Tech Pharmacal Co., Inc.) was applied topically to minimize pain from mechanical restraint. The animals were then secured in Plexiglas stereotaxic head holders using plastic ear bars. Animals were then placed in a black opaque tube “mock scanner” and exposed to recorded scanner noises. Animals were acclimated for 8 days, one session per day. The time of acclimation increased from 15 minutes on the first day to 90 minutes on days 6, 7, and 8, at increments of 15 minutes per day.
Animal Preparation for Imaging
The animals were briefly anesthetized using isoflurane. The head was fitted into a head restrainer with a built-in coil, with the incisors secured over a bite bar. The nose was secured with a nose clamp and ear bars were positioned inside the head-holder with adjustable screws fitted into lateral sleeves. The body of the animal was then placed into a body restrainer, previously demonstrated to successfully minimize head movement.44 Isoflurane was removed after this setup and the restraining system was positioned in the magnet. Imaging sessions started following signal optimization approximately 15 minutes after animals were placed in the magnet.
Magnetic Resonance Imaging
Magnetic resonance imaging experiments were performed on a 4.7 T/40 cm horizontal magnet equipped with a Biospec Bruker console (inner diameter 12 cm). A surface coil (internal diameter 2.3 cm) was used for brain imaging.
For each rat, anatomical images were obtained using rapid acquisition relaxation- enhanced (RARE) sequence with the following parameters: relaxation time (TR) = 3000 ms, RARE factor = 8, echo time (TE) = 12 ms, resolution matrix = 256 × 256, field of view (FOV) = 32 mm × 32 mm, slice number = 18, and slice thickness = 1 mm.
Two functional scans were subsequently performed using echo-planar imaging (EPI) sequence with the following parameters: TR = 1 s, TE = 30 ms, flip angle = 60°, resolution matrix = 64 × 64, FOV = 32 mm × 32 mm, and slice thickness = 1 mm. The first EPI (EPI1) was 20 minutes (1200 repetitions) and the second EPI scan (EPI2) was 30 minutes (1800 repetitions). EPI1 was acquired without drug or saline administration. EPI2 had a 1-minute baseline period, followed by a subcutaneous injection over 3 seconds of saline or nicotine and followed by 29 minutes of continuous data acquisition. EPI1 and the final 20 minutes of EPI2 from all animals were used for the rsFC analyses.
Preprocessing of Imaging Data
The imaging data were preprocessed using Medical Image Visualization and Analysis (MIVA, http://ccni.wpi.edu/), MATLAB (The Mathworks Inc., Natick, MA), and SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Each 20-minute resting-state session was then divided into six short runs (200 repetitions per run) for functional connectivity analysis. All EPI sessions were first aligned and coregistered to a fully segmented rat brain atlas in MIVA.28 After registration, all sessions went through the following pre-processing steps: motion correction, spatial smoothing (full width-half maximum = 1 mm), and 0.002–0.1 Hz band-pass filtering. Eight runs with excessive motion (>0.2 mm in each direction) were discarded (two runs each from two animals and one run from four animals). The time course for each individual voxel was further corrected for head movement by regression on the six motion parameters (translations and rotations) estimated in the procedure of motion correction. Furthermore, white matter and ventricle signals were estimated by averaging the time courses of all voxels inside the white matter and ventricles and then regressed out from time courses for rsFC analysis.
rsFC analysis
rsFC was evaluated using a region-of-interest (ROI)-based correlational analysis on a voxel-by-voxel basis.28 Sixteen ROIs were chosen representing reward, emotion, and executive function areas: the prelimbic area (PL), infralimbic area (ILA), anterior cingulate cortex (ACC), nucleus accumbens (NAcc), ventral tegmental area (VTA), insula (INS), amygdala (AMG), retrosplenial cortex (RSP), hippocampus (HP), caudoputamen (CPu), substantia nigra (SNr), visual cortex (VIS), substantia innominata/ventral pallidum (SI), medial habenula (MH), and the interpeduncular nucleus (IPN). For each animal, regionally averaged time courses from all voxels inside the ROI regions were used as time courses to correlate. Pearson cross-correlation coefficients (CCs) between each two ROI time courses were calculated. CCs were transformed using Fisher’s Z transformation. Z- scores from each of the six short runs were averaged and then transformed back to CCs to produce representative rsFC of the animal.
Group Analysis
For each of the two EPI scans, the strength of rsFC for each ROI couple was compared using mixed-design analyses of variance (ANOVA) with the three strains (SD, SHR, or WKY) and two drug groups (saline or nicotine) as between-subject factors, as well as the imaging time points (pre- and post-drug administration) as a within-subject factor. To identify unique responses to nicotine in the SHR strain, the impact of this substance had to differ between the SHR and the other two strains. Specifically, any significant three-way interactions, adjusted for multiple testing according to false discovery rate procedure (FDR, p < .05, according to the number of regional couplings tests)45 were followed up with simple effects analyses adopting the least significant difference adjustment for multiple comparisons.
Results
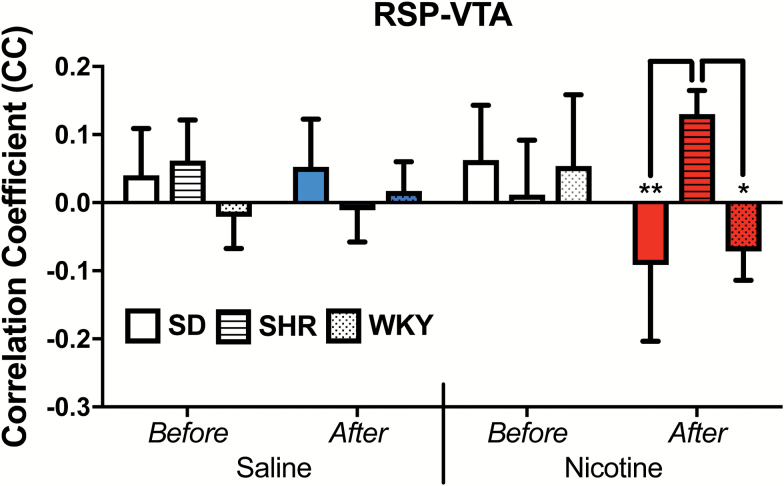
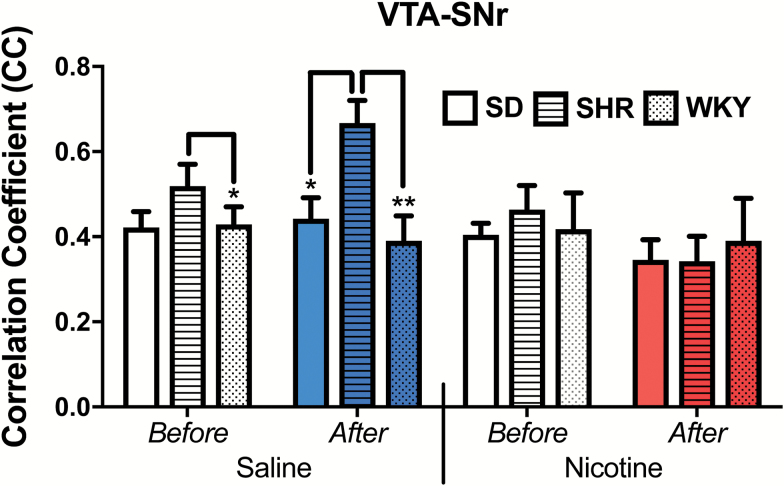
The ANOVAs identified 32 circuits in which rsFC alterations were identified in relation to either differences between strains, differences between saline and nicotine, and/or differences before and after drug administration (Table 1). However, our a priori criterion for results of interest according to a significant three-way interaction was met in only two circuits: the RSP-VTA (F = 3.7, p = .034) and VTA-SNr (F = 3.4, p = .045; FDR-adjusted to 0.047). As shown in Figure 1, RSP-VTA functional coupling was significantly greater after nicotine administration in the SHR, with the SHR showing increased rsFC in this circuit while the opposite effect was seen in the two control strains (F(2,41) = 5.0, p = .012). For VTA-SNr coupling, the SHR showed increased rsFC relative to both control strains in response to a saline injection (p < .05) but no strain differences were seen after a nicotine injection (Figure 2). A small difference between the SHR and one of the control strains, WKY, in the before-saline scan was not observed in the equivalent before-nicotine scan, yet overall strain ranking was maintained across these conditions and no significant within-strain differences between “before” conditions of each drug were observed.
Table 1.
Main Effects, Two-way, and Three-way Interactions for Each Regional Coupling
| Regional coupling | Strain | Time | Drug | Strain × drug | Strain × time | Drug × time | Strain×drug × time | |
|---|---|---|---|---|---|---|---|---|
| VTA-SN | F | / | / | / | / | / | 4.082 | 3.356 |
| p | / | / | / | / | / | .050 | .045 | |
| VTA-IPN | F | / | / | / | / | 3.23 | / | / |
| p | / | / | / | / | .050 | / | / | |
| VTA-CPu | F | / | / | / | 4.998 | / | / | / |
| p | / | / | / | .011 | / | / | / | |
| VTA-MH | F | / | / | 23.872 | / | / | / | / |
| p | / | / | < .001 | / | / | / | / | |
| RSP-VTA | F | / | / | / | / | / | / | 3.667 |
| p | / | / | / | / | / | / | .034 | |
| RSP-INS | F | / | / | / | / | 5.029 | / | / |
| p | / | / | / | / | .011 | / | / | |
| RSP-Acc | F | 4.456 | / | 4.155 | 3.670 | 5.609 | / | / |
| p | .018 | / | .048 | .034 | .007 | / | / | |
| RSP-PL | F | 6.377 | / | / | / | 5.019 | / | / |
| p | .004 | / | / | / | .011 | / | / | |
| RSP-HP | F | / | 7.982 | 7.268 | 5.375 | / | / | / |
| p | / | .007 | .010 | .008 | / | / | / | |
| RSP-CPu | F | / | / | / | / | 4.481 | / | / |
| p | / | / | / | / | .017 | / | / | |
| RSP-MH | F | / | / | / | 3.539 | 3.717 | 10.818 | / |
| p | / | / | / | .038 | .033 | .002 | / | |
| AMG-RSP | F | 3.396 | / | / | / | / | / | / |
| p | .043 | / | / | / | / | / | / | |
| AMG-VTA | F | 5.408 | / | / | / | 6.202 | 7.227 | / |
| p | .008 | / | / | / | .004 | .010 | / | |
| AMG-CPu | F | / | 7.634 | 5.570 | / | 13.212 | 7.431 | / |
| p | / | .009 | .023 | / | < 0.001 | .009 | / | |
| AMG-MH | F | / | 4.565 | / | / | / | / | / |
| p | / | .039 | / | / | / | / | / | |
| INS-Acc | F | / | 5.476 | / | / | / | / | / |
| p | / | .024 | / | / | / | / | / | |
| INS-ILA | F | / | / | 5.212 | / | / | / | / |
| p | / | / | .028 | / | / | / | / | |
| INS-SN | F | / | / | 9.768 | / | 3.279 | / | / |
| p | / | / | .003 | / | .048 | / | / | |
| Acc-ILA | F | / | 4.75 | / | / | / | / | / |
| p | / | .035 | / | / | / | / | / | |
| Acc-NAcc | F | / | 6.012 | / | / | / | / | / |
| P | / | .019 | / | / | / | / | / | |
| Acc-CPu | F | / | 10.824 | / | / | / | / | / |
| p | / | .002 | / | / | / | / | / | |
| ILA-SN | F | / | 4.417 | / | / | / | / | / |
| p | / | .042 | / | / | / | / | / | |
| ILA-NAcc | F | 10.821 | / | / | 4.210 | / | / | / |
| p | < .0010 | / | / | .022 | / | / | / | |
| ILA-PL | F | 5.329 | / | / | 5.510 | / | / | / |
| p | .009 | / | / | .008 | / | / | / | |
| NAcc-VTA | F | / | / | / | 5.057 | / | / | / |
| p | / | / | / | .011 | / | / | / | |
| NAcc-PL | F | 5.323 | 9.22 | / | / | / | / | / |
| p | .009 | .004 | / | / | / | / | / | |
| NAcc-HP | F | / | / | / | / | / | 4.497 | / |
| p | / | / | / | / | / | .040 | / | |
| PL-CPu | F | / | 5.765 | / | / | / | / | / |
| p | / | .021 | / | / | / | / | / | |
| PL-IPN | F | / | 4.956 | / | / | 6.367 | 16.454 | / |
| p | / | .032 | / | / | .004 | < .0010 | / | |
| PL-MH | F | 4.333 | / | / | / | / | / | / |
| p | .020 | / | / | / | / | / | / | |
| SN-MH | F | / | / | 8.899 | / | / | / | / |
| p | / | / | .005 | / | / | / | / | |
| MH-IPN | F | / | / | 5.171 | / | / | / | / |
| p | / | / | .028 | / | / | / | / | |
PL = prelimbic area; ILA = infralimbic area; ACC = anterior cingulate cortex; NAcc, nucleus accumbens; VTA = ventral tegmental area; INS = insula, AMG = amygdala; RSP = retrosplenial cortex; HP = hippocampus; CPu = caudoputamen; SNr = substantia nigra; VIS = visual cortex; SI = substantia innominata/ventral pallidum; MH = medial habenula; IPN = interpeduncular nucleus. F-value of ANOVA; p, statistical probability, unadjusted for multiple testing; /: p > .05.
Figure 1.
Nicotine produced a postive response in RSP-VTA functional connectivity in the SHR, while it had the opposite effect in the SD and WKY. CC = correlation coefficient, RSP-VTA = retrosplenial cortex-ventral tegmental area, SD = Sprague-Dawley, SHR = spontaneously hypertensive rat, WKY = Wistar-Kyoto; *p < .05, **p < .01.
Figure 2.
VTA-SNr functional connectivity enhancement that was present in the SHR following a saline injection was absent after nicotine administration. CC = correlation coefficient, SD = Sprague-Dawley, SHR = spontaneously hypertensive rat, VTA-SNr = ventral tegmental area-substantia nigra, WKY = Wistar-Kyoto; *p < .05, **p < .01.
Discussion
We were interested in determining whether the brain responds to nicotine differently in an animal model of ADHD. As ROIs, we chose brain regions associated with reward, emotion, and executive function.46 Two circuits involving the VTA demonstrated altered rsFC in response to the first dose of nicotine in the SHR as compared to both control strains. Nicotine acts on dopaminergic neurons in the VTA, stimulating the release of dopamine in the NAcc which is hypothesized to contribute to incentive salience.47 In the current study, significant differences in rsFC were identified in 32 circuits (Table 1). Alterations in rsFC in response to nicotine were observed in a number of circuits previously associated with nicotine effects or conditioned responses,18,46,48–52 including VTA-MH, SN-MH, INS-SN, RSC-HP, and RSC-ACC (Table 1, drug main effects). Selective strain differences in the impact of nicotine (pre- vs. post-administration) included an increase in rsFC in the RSP-VTA circuit in the ADHD model, the SHR, while having the opposite effect in the control strains. It is tempting to speculate that this enhancement of VTA functional connectivity may underlie enhanced rewarding properties of nicotine,53,54 or even the dampened aversive properties,55 even though we did not observe rsFC changes in the VTA-NAcc circuit (Table 1).
This absence of altered VTA-NAcc connectivity is notable, even though rewarding properties of nicotine in the VTA have also been associated with engagement of other brain regions.56 Although we observed changes in rsFC in response to nicotine in several circuits, there might be limitations in the sensitivity of rsFC, as examined using a seed-based approach, in relation to NAcc subregional57 or even cell population heterogeneity of response to nicotine.56 Another consideration is that we examined only one dose, 0.4 mg/kg nicotine base. This is the most commonly used dose of nicotine in animal sensitization studies because it produces robust effects and reflects human cotinine plasma levels after smoking,38 while higher doses tend to produce toxicity.58 Nevertheless, a higher dose might have produced more perceptible effects on rsFC for these regions. Another consideration could be the differential impact of repeated exposure. We measured rsFC in response to a single dose of nicotine administered to naïve animals (Table 1). It is known that rats become sensitized to the effects of nicotine such that neural activation is much more widely distributed across brain regions after repeated doses as compared to the first exposure.46 It may be that changes in rsFC in some circuits appear only after repeated exposures to nicotine have triggered neuroplastic changes. Future studies could examine the effects of repeated exposures to test this hypothesis.
Human neuroimaging studies suggest a role for the RSC in craving and smoking-cue responsivity.59,60 In humans, the RSC exhibits the highest nicotinic receptor binding among the cingulate subregions.61 In animals, it responds to nicotinic exposure with increased blood flow.43,46,62,63 Following chronic nicotine exposure, rsFC between the RSC and VTA remains sensitized beyond 12 hours.48 Given the role of the RSC in smoking cue responsivity, our data suggest that ADHD may be associated with increased susceptibility to developing incentive-salience.
In relation to the VTA-SNr functional circuitry, differences between the SHR and control strains were evident in the absence of nicotine and disappeared when nicotine was administered (Figure 2), opposite to previously discussed RSP-VTA connectivity for which differences emerged, rather than disappeared, upon nicotine exposure (Figure 1). Compared to the control strains, the SHR showed increased rsFC in response to a saline injection. This suggests that the SHR is more sensitive to stressful stimuli. However, when the injection delivered nicotine instead of saline, there was no difference in rsFC between the SHR and controls. This suggests that nicotine may have normalized the stress response in the SHR. Smokers commonly report that they smoke to cope with stress.64 Our data suggest that individuals with ADHD might find nicotine to be more effective in this role.
Nicotinic receptor modulators appear to present positive cognitive and attentional effects in ADHD.65,66 The theory that adolescents with ADHD self-medicate with nicotine to improve their symptoms is widely asserted11 but sparsely researched,67 with mixed results.68,69 The data presented in Figure 2 suggest that it is plausible that nicotine might help to normalize some brain functioning in individuals with ADHD.
We found that the SHR differed from both control strains in its initial reaction to nicotine. Humans also differ in their initial reactions to nicotine. While only a minority of individuals experience relaxation with their first exposure to nicotine, those who do are more likely to become addicted smokers.70–72 While socially derived expectations might influence initial reactions to nicotine in humans, expectations cannot explain the differences in initial reactions observed in this experiment.
Genetic factors influence susceptibility to nicotine addiction.9,73 Our data show differences in brain reactivity to the initial dose of nicotine in related, but genetically distinct strains. As the process of becoming addicted can be initiated by the first cigarette,74 differences in the initial reaction to nicotine may be a manifestation of genetic susceptibility. ADHD has been associated with nicotinic receptor subunit single nucleotide polymorphisms.75–77 Gene variants of subunits α3, α6, and β3 predict the level of cigarette consumption with an interaction with ADHD symptoms.37 Furthermore, a genetic variation of the α3 subunit decreased the likelihood of smoking in the general population but increased the risk in individuals with ADHD.78 This is notable as the SHR/NHsd strain used in the current study also carries a variation in the α3 receptor.36 To the degree that the SHR is a useful model of human ADHD, our findings suggest that the brains of adolescents with ADHD may react differently to nicotine than the brains of unaffected adolescents.
Among the limitations of this study is that only one dose and dosage of nicotine were evaluated. Another concern could be the elevated blood pressure that typically appears in SHR animals older than those used in this study.12,13 While elevated blood pressure could affect brain blood flow, such effects should be global and not limited to a few circuits and would not be expected to influence coordination of regional brain activity. Strengths of this study included the use of two control strains and our requirement that the SHR differ from both control strains.
In conclusion, we identified significant differences in brain responses to the initial exposure to nicotine in an adolescent animal model of ADHD as compared to control strains. Brain activity was altered in regions with known involvement in nicotine reward, aversion, and addiction. These data suggest that genetically determined individual differences may alter how individuals respond to nicotine and such differences may contribute to the increased risk of nicotine addiction in adolescents with ADHD.
Funding
Funding for this project was provided by National Institutes of Health (NIH) grants R01 DA 021846, R01 DA 025690, and S10 OD018132-01 to JAK.
Declaration of Interests
None declared.
Acknowledgments
GLP and WH contributed equally to this work.
References
- 1. Scragg R, Wellman RJ, Laugesen M, DiFranza JR. Diminished autonomy over tobacco can appear with the first cigarettes. Addict Behav. 2008;33(5):689–698. [DOI] [PubMed] [Google Scholar]
- 2. Fedele E, Jin Y, Varnier G, Raiteri M. In vivo microdialysis study of a specific inhibitor of soluble guanylyl cyclase on the glutamate receptor/nitric oxide/cyclic GMP pathway. Br J Pharmacol. 1996;119(3):590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lallemand F, Ward RJ, Dravolina O, De Witte P. Nicotine-induced changes of glutamate and arginine in naive and chronically alcoholized rats: an in vivo microdialysis study. Brain Res. 2006;1111(1):48–60. [DOI] [PubMed] [Google Scholar]
- 4. Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383(6602):713–716. [DOI] [PubMed] [Google Scholar]
- 5. Gioanni Y, Rougeot C, Clarke PB, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11(1):18–30. [DOI] [PubMed] [Google Scholar]
- 6. López E, Arce C, Vicente S, Oset-Gasque MJ, González MP. Nicotinic receptors mediate the release of amino acid neurotransmitters in cultured cortical neurons. Cereb Cortex. 2001;11(2):158–163. [DOI] [PubMed] [Google Scholar]
- 7. Bevins RA, Besheer J. Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotinic acetylcholine receptors. Physiol Behav. 2001;72(1–2):237–244. [DOI] [PubMed] [Google Scholar]
- 8. Hahn B, Stolerman IP, Shoaib M. Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology. 2000;39(13):2848–2855. [DOI] [PubMed] [Google Scholar]
- 9. Hong LE, Hodgkinson CA, Yang Y, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107(30):13509–13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groenman AP, Oosterlaan J, Rommelse N, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108(8):1503–1511. [DOI] [PubMed] [Google Scholar]
- 11. Matthies S, Holzner S, Feige B, et al. ADHD as a serious risk factor for early smoking and nicotine dependence in adulthood. J Atten Disord. 2013;17(3):176–186. [DOI] [PubMed] [Google Scholar]
- 12. Sagvolden T, Johansen EB, Rat models of ADHD. In: Stanford C, Tannock R, eds. Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and its Treatment. Berlin: Springer; 2012:301–315. [Google Scholar]
- 13. Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr Protoc Neurosci. 2011;Chapter 9:Unit9.35. [DOI] [PubMed] [Google Scholar]
- 14. Kirouac GJ, Ganguly PK. Up-regulation of dopamine receptors in the brain of the spontaneously hypertensive rat: an autoradiographic analysis. Neuroscience. 1993;52(1):135–141. [DOI] [PubMed] [Google Scholar]
- 15. Papa M, Diewald L, Carey MP, Esposito FJ, Gironi Carnevale UA, Sadile AG. A rostro-caudal dissociation in the dorsal and ventral striatum of the juvenile SHR suggests an anterior hypo- and a posterior hyperfunctioning mesocorticolimbic system. Behav Brain Res. 2002;130(1–2):171–179. [DOI] [PubMed] [Google Scholar]
- 16. Gattu M, Terry AV, Jr, Pauly JR, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors. Part II. Brain Res. 1997;771(1):104–114. [DOI] [PubMed] [Google Scholar]
- 17. Fujita S, Adachi K, Lee J, Uchida T, Koshikawa N, Cools AR. Decreased postsynaptic dopaminergic and cholinergic functions in the ventrolateral striatum of spontaneously hypertensive rat. Eur J Pharmacol. 2004;484(1):75–82. [DOI] [PubMed] [Google Scholar]
- 18. Wigestrand MB, Mineur YS, Heath CJ, Fonnum F, Picciotto MR, Walaas SI. Decreased α4β2 nicotinic receptor number in the absence of mRNA changes suggests post-transcriptional regulation in the spontaneously hypertensive rat model of ADHD. J Neurochem. 2011;119(1):240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, Weitzman M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr Probl Pediatr Adolesc Health Care. 2014;44(8):219–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56 (suppl 1):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32(6):2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hohnadel EJ, Hernandez CM, Gearhart DA, Terry AV.Jr. Effect of repeated nicotine exposure on high-affinity nicotinic acetylcholine receptor density in spontaneously hypertensive rats. Neurosci Lett. 2005;382(1–2):158–163. [DOI] [PubMed] [Google Scholar]
- 23. Sterley TL, Howells FM, Russell VA. Nicotine-stimulated release of [3H]norepinephrine is reduced in the hippocampus of an animal model of attention-deficit/hyperactivity disorder, the spontaneously hypertensive rat. Brain Res. 2014;1572:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Huang SM, Wu YL, Peng SL, et al. Inter-strain differences in default mode network: a resting state fmri study on spontaneously hypertensive rat and Wistar Kyoto rat. Sci Rep. 2016;6:21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poirier GL, Huang W, Tam K, DiFranza JR, King JA. Awake whole-brain functional connectivity alterations in the adolescent spontaneously hypertensive rat feature visual streams and striatal networks. Brain Struct Funct. 2017;222(4):1673–1683. [DOI] [PubMed] [Google Scholar]
- 26. Liang Z, Li T, King J, Zhang N. Mapping thalamocortical networks in rat brain using resting-state functional connectivity. Neuroimage. 2013;83:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011;31(10):3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang N, Rane P, Huang W, et al. Mapping resting-state brain networks in conscious animals. J Neurosci Methods. 2010;189(2):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, Lu G, Antonio GE, et al. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50(6):848–857. [DOI] [PubMed] [Google Scholar]
- 30. Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson AM, Hopkins ME, Bucci DJ. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Dev Psychobiol. 2011;53(4):383–390. [DOI] [PubMed] [Google Scholar]
- 32. van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83(3):380–390. [DOI] [PubMed] [Google Scholar]
- 33. Chess AC, Green JT. Abnormal topography and altered acquisition of conditioned eyeblink responses in a rodent model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2008;122(1):63–74. [DOI] [PubMed] [Google Scholar]
- 34. Green JT, Chess AC, Conquest CJ, Yegla BA. Conditioned inhibition in a rodent model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2011;125(6):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behav Brain Res. 2005;157(2):323–330. [DOI] [PubMed] [Google Scholar]
- 36. Atanur Santosh S, Diaz Ana G, Maratou K, et al. Genome sequencing reveals loci under artificial selection that underlie disease phenotypes in the laboratory rat. Cell. 2013;154(3):691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee CT, Fuemmeler BF, McClernon FJ, Ashley-Koch A, Kollins SH. Nicotinic receptor gene variants interact with attention deficient hyperactive disorder symptoms to predict smoking trajectories from early adolescence to adulthood. Addict Behav. 2013;38(11):2683–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007;190(3):269–319. [DOI] [PubMed] [Google Scholar]
- 39. Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang Z, King J, Zhang N. Intrinsic organization of the anesthetized brain. J Neurosci. 2012;32(30):10183–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang Z, King J, Zhang N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage. 2012;59(2):1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bettinardi RG, Tort-Colet N, Ruiz-Mejias M, Sanchez-Vives MV, Deco G. Gradual emergence of spontaneous correlated brain activity during fading of general anesthesia in rats: evidences from fMRI and local field potentials. Neuroimage. 2015;114:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chin CL, Pauly JR, Surber BW, et al. Pharmacological MRI in awake rats predicts selective binding of α4β2 nicotinic receptors. Synapse. 2008;62(3):159–168. [DOI] [PubMed] [Google Scholar]
- 44. King JA, Garelick TS, Brevard ME, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148(2):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Method). 1995;57(1):289–300. [Google Scholar]
- 46. Li Z, DiFranza JR, Wellman RJ, Kulkarni P, King JA. Imaging brain activation in nicotine-sensitized rats. Brain Res. 2008;1199:91–99. [DOI] [PubMed] [Google Scholar]
- 47. Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens [corrected]. Nicotine Tob Res. 2004;6(6):899–912. [DOI] [PubMed] [Google Scholar]
- 48. Huang W, Tam K, Fernando J, Heffernan M, King J, DiFranza JR. Nicotine and resting-state functional connectivity: effects of intermittent doses. Nicotine Tob Res. 2015;17(11):1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ghatan PH, Ingvar M, Eriksson L, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl). 1998;136(2):179–189. [DOI] [PubMed] [Google Scholar]
- 50. Franklin TR, Wang Z, Wang J, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. [DOI] [PubMed] [Google Scholar]
- 51. Li X, Hartwell KJ, Borckardt J, et al. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2013;18(4):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hong LE, Gu H, Yang Y, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLOS ONE. 2012;7(8):e44234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watterson E, Daniels CW, Watterson LR, et al. Nicotine-induced place conditioning and locomotor activity in an adolescent animal model of attention deficit/hyperactivity disorder (ADHD). Behav Brain Res. 2015;291:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watterson E, Spitzer A, Watterson LR, et al. Nicotine-induced behavioral sensitization in an adult rat model of attention deficit/hyperactivity disorder (ADHD). Behav Brain Res. 2016;312:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun N, Laviolette SR .Dopamine receptor blockade modulates the rewarding and aversive properties of nicotine via dissociable neuronal activity patterns in the nucleus accumbens. Neuropsychopharmacology. 2014;39(12):2799–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull. 2008;76(6):626–639. [DOI] [PubMed] [Google Scholar]
- 58. DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9(1):9–20. [DOI] [PubMed] [Google Scholar]
- 59. Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56(4):2258–2275. [DOI] [PubMed] [Google Scholar]
- 60. Brody AL, Mandelkern MA, Olmstead RE, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62(6):642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palomero-Gallagher N, Zilles K, Transmitter receptor systems in cingulate regions and areas. In: Vogt B, ed. Cingulate Neurobiology and Disease. Oxford: Oxford University Press; 2009:32–63. [Google Scholar]
- 62. Zuo Y, Lu H, Vaupel DB, et al. Acute nicotine-induced tachyphylaxis is differentially manifest in the limbic system. Neuropsychopharmacology. 2011;36(12):2498–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naïve rat. Neuropsychopharmacology. 2006;31(8):1690–1703. [DOI] [PubMed] [Google Scholar]
- 64. DiFranza JR, Wellman RJ, Ursprung WW, Sabiston C. The autonomy over smoking scale. Psychol Addict Behav. 2009;23(4):656–665. [DOI] [PubMed] [Google Scholar]
- 65. Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74(8):1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Potter AS, Schaubhut G, Shipman M. Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date. CNS Drugs. 2014;28(12):1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 68. Wilens TE, Adamson J, Sgambati S, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. Am J Addict. 2007;16(suppl 1):14–21; quiz 22. [DOI] [PubMed] [Google Scholar]
- 69. Liebrenz M, Frei A, Fisher CE, Gamma A, Buadze A, Eich D. Adult attention-deficit/hyperactivity disorder and nicotine use: a qualitative study of patient perceptions. BMC Psychiatry. 2014;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pomerleau CS, Pomerleau OF, Namenek RJ, Marks JL. Initial exposure to nicotine in college-age women smokers and never-smokers: a replication and extension. J Addict Dis. 1999;18(3):13–19. [DOI] [PubMed] [Google Scholar]
- 71. Wellman RJ, DiFranza JR, O’Loughlin J. Recalled first reactions to inhaling nicotine predict the level of physical dependence. Drug Alcohol Depend. 2014;143:167–172. [DOI] [PubMed] [Google Scholar]
- 72. DiFranza JR, Savageau JA, Fletcher K, et al. Susceptibility to nicotine dependence: the Development and Assessment of Nicotine Dependence in Youth 2 study. Pediatrics. 2007;120(4):e974–e983. [DOI] [PubMed] [Google Scholar]
- 73. Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(suppl 2):S51–S57; discussion S69. [DOI] [PubMed] [Google Scholar]
- 74. DiFranza JR. Hooked from the first cigarette. Sci Am. 2008;298(5):82–87. [DOI] [PubMed] [Google Scholar]
- 75. Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol Psychiatry. 2003;8(1):103–108. [DOI] [PubMed] [Google Scholar]
- 76. Stergiakouli E, Hamshere M, Holmans P, et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry. 2012;169(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29(10):2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Polina ER, Rovaris DL, de Azeredo LA, et al. ADHD diagnosis may influence the association between polymorphisms in nicotinic acetylcholine receptor genes and tobacco smoking. Neuromolecular Med. 2014;16(2):389–397. [DOI] [PubMed] [Google Scholar]