Abstract
Introduction
Routine biochemical assessment of tobacco smoke exposure could lead to more effective interventions to reduce or prevent secondhand smoke (SHS)-related disease in adolescents. Our aim was to determine using urine cotinine (major nicotine metabolite) measurement the prevalence of tobacco smoke exposure among adolescents receiving outpatient care at an urban public hospital.
Methods
Surplus urine was collected in 466 adolescents attending pediatric or urgent care clinics at Zuckerberg San Francisco General Hospital, serving families with lower levels of income and education, in 2013–2014. The majority were Hispanic or African American. Urine cotinine cut points of 0.05 to 0.25 ng/ml, 0.25 to 30 ng/ml, and 30 ng/ml were used to classify subjects as light SHS or thirdhand smoke exposed, SHS or light/intermittent active users, and active tobacco users, respectively.
Results
Among subjects 87% were exposed, including 12% active smoking, 46% SHS and 30% lightly exposed. The SHS exposed group adjusted geometric mean cotinine values were significantly higher in African Americans (1.48 ng/ml) compared to other groups (0.56–1.13 ng/ml).
Conclusions
In a city with a low smoking prevalence (12%), a large majority (87%) of adolescents seen in a public hospital clinic are exposed to tobacco. This is much higher than reported in national epidemiological studies of adolescents, which used a plasma biomarker. Since SHS is associated with significant respiratory diseases and parents and adolescents underreport exposure to SHS, routine biochemical screening should be considered as a tool to reduce SHS exposure. The clinical significance of light exposure needs to be investigated.
Implications
Urine biomarker screening found that a large majority (87%) of adolescents treated in an urban public hospital are exposed to tobacco. Since SHS is associated with significant respiratory diseases and parents and adolescents underreport exposure to SHS, routine biochemical screening should be considered as a tool to reduce SHS exposure.
Introduction
Tobacco smoke exposure in children is a substantial cause of respiratory infection, ear infection, asthma, and an increased risk of smoking initiation.1,2 Many, but not all, pediatricians ask their patients and families about active smoking and secondhand smoke (SHS) exposure. Even so pediatricians rarely intervene on parental smoking3 and patient self-report is often inaccurate.
Prior studies determined it is feasible to test for tobacco smoke exposure using the biomarker cotinine (COT, a major metabolite of nicotine), when built into blood lead testing during well visit check-ups.4,5 Routine biochemical assessment of tobacco smoke exposure is potentially an important tool for identifying and reducing exposure and related disease in children.
In a prior publication we reported plasma cotinine levels in 496 infants and children who attended clinics at San Francisco General Hospital Medical Center, an urban county hospital.4 We found that 55% of infants and children were exposed to tobacco smoke, compared with 13% exposure reported by parents as recorded in the medical records. Additionally, exposure was highest in African American children, suggesting racial differences in exposure and/or cotinine metabolism.
Differences in the extent of tobacco smoke exposure may differ between children and adolescents due to several factors. Young children are exposed to tobacco smoke primarily in their homes and sometimes in motor vehicles. Adolescents have many additional potential sources of exposure outside their homes, including contact with friends who smoke and proximity to SHS at social events. Additionally some adolescents are themselves active cigarette smokers and/or may be exposed by using tobacco products such as cigars, cigarillos, electronic cigarettes and/or blunts (marijuana in a hollowed out cigar). Serum cotinine data from the National Health and Nutrition Survey (NHANES) indicate that nationwide among adolescents 63% were exposed in 1999–2000, and 41% in 2011–2012.6 Adolescents from vulnerable backgrounds may be even more likely to be exposed to SHS. NHANES data demonstrated that African Americans had higher levels of SHS exposure compared to other racial/ethnic groups.7
One aim of the present study was to examine the feasibility of routine biochemical screening for tobacco exposure using urine samples from adolescents in an urban public hospital. Most epidemiological studies reporting cotinine levels have used plasma or saliva samples. Urine collection has advantages of being non-invasive; and because cotinine levels are much higher than blood or saliva, urine provides greater sensitivity.8
Other aims of our study were: (1) to measure cotinine levels in adolescents who attended pediatric clinics and who gave urine specimens as part of routine clinical care; (2) to determine urine cotinine cut points in adolescents that indicate active versus SHS or low level exposure; and (3) to compare prevalence of exposure and urine cotinine levels in adolescents by race/ethnicity.
Methods
Study Procedures
We studied 466 adolescents, aged 13–19, who received pediatric care at the Children’s Health Center (CHC) at Zuckerberg San Francisco General Hospital and who had surplus urine after collection for other clinical indications during a 12-month interval (June, 2013–May, 2014). The CHC is the primary pediatric outpatient clinic at Zuckerberg San Francisco General Hospital, the county hospital serving the economically disadvantaged population of San Francisco. The CHC serves approximately 10 000 unduplicated children and adolescents per year resulting in 34 000 annual visits including primary, urgent, and specialty pediatric and teen care. Ethnically the adolescent population is: Latino 58.1%, African American 19.1%, Asian 11.0%, white 6.5%, and Other 5.3%. A vast majority (91.4%) of the patients have public health insurance and 7.9% are uninsured.
Adolescents presented to the clinic for both well and sick care, and clinical indications for obtaining urine were varied, including but not limited to urinary tract or sexually transmitted infection diagnosis, abdominal pain evaluation, pregnancy diagnosis, and trauma. Samples were collected by clinic nurses and frozen for later analysis.
We retrospectively retrieved the subjects’ race/ethnicity, gender, age, medical diagnosis, self-reported tobacco use history (reported to the care provider), and insurance status from the hospital electronic database. Responses to the tobacco use question were available in 338 (83%) of subjects’ records. In 146 subjects (43%) smoking status was recorded on the same day of urine collection. For other subjects, medical records were reviewed up to 2 years prior to and 1 year after the time of urine collection. This was an un-consented study approved by the Committee on Human Research at the University of California, San Francisco. There was no direct patient contact; and after the chart review was completed, all patient identifiers were deleted from the database and research charts.
Analytical Chemistry
Urine samples were analyzed for cotinine and trans 3’ hydroxycotinine (3HC) by liquid chromatography–tandem mass spectrometry.9 The lower limit of quantitation (LOQ) for this assay was 0.05 ng/ml. The ratio of 3HC/COT, termed the nicotine metabolite ratio (NMR) was measured as a biomarker of the activity of the enzyme CYP2A6, which is primarily responsible for the metabolism of nicotine and cotinine.10
Data Analysis/Statistics
We developed three groupings to describe cotinine positivity in urine. Most prior biomarker studies used plasma or serum cotinine to distinguish active versus passive smoking. To estimate equivalent cotinine levels in urine, we used the observation that urine cotinine levels are approximately five times greater than those in plasma or serum.8 We started first by determining the urine cotinine level that would indicate active smoking. Based on an analysis of NHANES data, the optimal serum cotinine cut point to separate smoking from not smoking in adolescents was determined to be 3 ng/ml, but differed in males (8.8 ng/ml) and females (2.4 ng/ml).11 To refine our estimate of the cut point to separate smoking versus nonsmoking, we performed latent class analysis on log-transformed urine cotinine levels and examined unconditional models with two and three classes, respectively, using MPLUS version 5.2.12 Based on lower Akaike Information Criterion,13 the 3-class model was the best-fitting model and revealed a cut point of about 30 ng/ml for the third class (active smoking). This would correspond to approximately 6 ng/ml in plasma, close to the optimal cotinine cut point found in the NHANES analysis Zuckerberg San Francisco General Hospital. The superior fitting of a 3-class versus 2-class model was consistent with our categorization of subjects into three groups based on cotinine levels.
The second classification question was what urine cotinine concentration indicates significant exposure to SHS. Prior studies conducted by us and analysis of NHANES data have used a serum or plasma cotinine cut off of 0.05 ng/ml to indicate significant exposure to SHS.4,6 Adjusting for higher urine cotinine levels in urine compared to plasma, we used the lower cut point of 0.25 ng/ml for significant SHS exposure, and the range between 0.25 ng/ml and 30 ng/ml to indicate SHS/light active or intermittent smoking. We interpreted cotinine values between the 0.05 ng/ml (LOQ) and 0.25 ng/ml as light SHS and/or thirdhand smoke (THS) exposure.
We used bivariate analyses to test whether cotinine positivity (χ2 test) and absolute measured levels (t test) were different across covariates. We used analysis of variance to test whether cotinine levels and 3HC/COT, respectively, were different across race/ethnicity for the subjects with cotinine levels between 0.05 and 30 ng/ml (ie, those with SHS exposure), and included covariates sex (male and female) and age (13–19 years). Both cotinine and 3HC/COT were log-transformed given their approximate log normal distribution. Multiple pairwise comparisons were controlled by Dunnett’s method and covariate-adjusted geometric means were obtained from back-log transformed least square means. We used logistic regression analysis to assess the relationship between race/ethnicity and prevalence of three outcomes separately: (1) cotinine above versus below LOQ; (2) heavy SHS (cotinine 0.25–30 ng/ml) versus light SHS/THS (0.05–0.25 ng/ml); and, (3) active smoker (>30 ng/ml) versus SHS exposure. Covariates age and sex were included in each model.
Other than latent class analysis, statistical analyses were carried out using SAS v. 9.4 (SAS Institute, Inc. Cary, NC, USA). Statistical tests were considered significant at p < .05.
Results
Demographics
Our 466 subjects averaged 15.7 years of age and 60.7% were females. The majority (52.4%) were Latino, followed by African Americans (21.9%), Asians (10.9%), whites (3.2%), Mixed (4.1%), and Others (7.5%) Table 1. Our subject demographics were similar to the overall teen demographics at the CHC. Medical records indicated that 9.6% reported smoking, 62.8% did not report smoking, and 27.5% had no information on tobacco use. No information on SHS exposure was reported.
Table 1.
Characteristics of Study Population (n = 466)
| Characteristic | No. (%) of participants | No. (%) of participants | |||||
|---|---|---|---|---|---|---|---|
| Unexposed (Cotinine < 0.05 ng/ml) | Exposed (Cotinine ≥ 0.05 ng/ml) | p | Cotinine level 0.05–0.25 ng/ml | Cotinine level 0.25–30 ng/ml | Cotinine Level >30 ng/ml | p | |
| Total | 59 (100) | 407 (100) | 138 (100) | 214 (100) | 55 (100) | ||
| Sex | |||||||
| Male | 24 (40.7) | 159 (39.1) | .81 | 57 (41.3) | 82 (38.3) | 20 (36.4) | .78 |
| Female | 35 (59.3) | 248 (60.9) | 81 (58.7) | 132 (61.7) | 35 (63.6) | ||
| Age (y) | |||||||
| 13 | 10 (17.0) | 30 (7.4) | .01 | 14 (10.1) | 16 (7.5) | 0 | .06 |
| 14 | 11 (18.6) | 50 (12.3) | 19 (13.8) | 26 (12.2) | 5 (9.1) | ||
| 15 | 10 (17.0) | 92 (22.6) | 37 (26.8) | 46 (21.5) | 9 (16.4) | ||
| 16 | 12 (20.3) | 88 (21.6) | 26 (18.8) | 50 (23.4) | 12 (21.8) | ||
| 17 | 8 (13.6) | 116 (28.5) | 31 (22.5) | 64 (29.9) | 21 (38.2) | ||
| 18–19 | 8 (13.6) | 31 (7.6) | 11 (8.0) | 12 (5.6) | 8 (14.6) | ||
| Race | |||||||
| Asian | 9 (15.3) | 42 (10.3) | .002 | 12 (8.7) | 30 (14.0) | 0 | <.001 |
| African American | 5 (8.5) | 97 (23.8) | 11 (8.0) | 54 (25.2) | 32 (58.2) | ||
| Latino | 43 (72.9) | 201 (49.4) | 100 (72.5) | 93 (43.5) | 8 (14.6) | ||
| Mixed | 0 | 19 (4.7) | 8 (5.8) | 11 (5.1) | 0 | ||
| White, Non-Hispanic | 0 | 15 (3.7) | 3 (2.2) | 7 (3.3) | 5 (9.1) | ||
| Other | 2 (3.4) | 33 (8.1) | 4 (2.9) | 19 (8.9) | 10 (18.2) | ||
| Smoking status | |||||||
| Nonsmoker | 43 (72.9) | 250 (61.4) | .06 | 92 (66.7) | 132 (61.7) | 26 (47.3) | <.001 |
| Smoker | 1 (1.7) | 44 (10.8) | 8 (5.8) | 18 (8.4) | 18 (32.7) | ||
| Not reported | 15 (25.4) | 113 (27.8) | 38 (27.5) | 64 (29.9) | 11 (20.0) | ||
Prevalence of Exposure
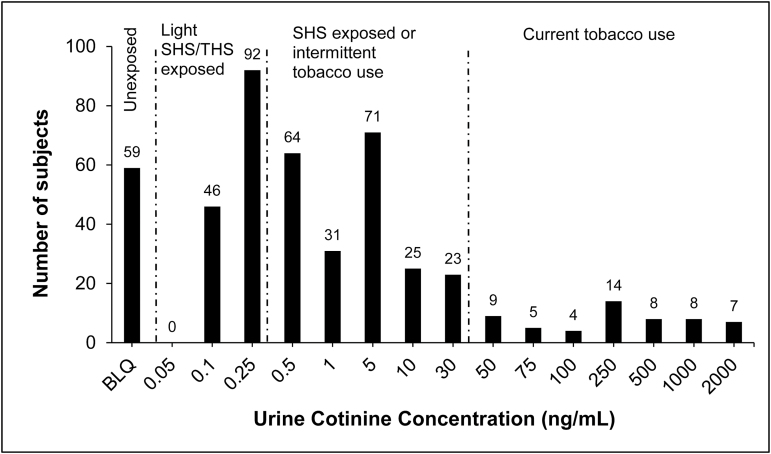
Figure 1 shows a frequency histogram of urine cotinine levels. Overall 87% of teens were exposed to some level of tobacco smoke (Table 1). No sex differences in overall exposure were observed. Prevalence of exposure differed by age (p = .013) and was generally higher among older teens. The prevalence of exposure was 95.0% in African Americans, 100% in whites, 84.3% in Asians, and 82.3% in Latinos. Logistic regression controlling for age and sex found that African Americans were significantly more likely to be exposed than Latinos (p = .013).
Figure 1.
Frequency histogram of urine cotinine concentrations.
Urine cotinine levels consistent with active smoking were observed in 11.8% of subjects, while self-reported smoking was recorded by providers in 9.6% of medical records.14 Of those who self-reported smoking, active smoking was confirmed biochemically in 40%, while another 40% had values consistent with SHS exposure. Of those who self-reported not smoking, 8.9% were biochemically confirmed to be smokers. The prevalence of biochemically-confirmed active smoking was highest in African Americans (31.4%), whites (33.3%), and Others (28.6%), and was quite low in Latinos (3.3%) and Asians (0%).
In 45.7% of adolescents cotinine levels indicated heavy SHS exposure and in 29.5% light SHS or THS exposure. The prevalence of SHS exposure was highest in Asians (71.4%), followed by African Americans (52.9%) and whites (46.6%) and lowest in Latinos (38.1%). The prevalence of tobacco exposure increased with increasing age in all categories. No sex differences were found in prevalence of exposure in any groups. Of those with a cotinine level indicating SHS exposure, 8.4% reported smoking and 61.7% reported not smoking.
Urine Cotinine Levels in Exposed Teens
The unadjusted geometric mean urine cotinine concentration was 192 ng/ml for the active smoker group, 1.52 ng/ml for SHS exposed, and 0.12 ng/ml for light SHS/THS (Table 2). Among smokers, Latinos had lowest cotinine levels. Urine cotinine levels adjusted for sex, age, and race in subjects with SHS exposure are shown in Table 3. Cotinine levels were significantly higher for African Americans (1.48 ng/ml) and others (1.13 ng/ml) compared to Latinos (0.38 ng/ml). No racial differences in concentrations were apparent in subjects in the light SHS/THS group.
Table 2.
Urine Cotinine Levels Within Exposure Category by Sex, Age, and Race
| Characteristic | Cotinine level | Cotinine level | Cotinine level |
|---|---|---|---|
| 0.05–0.25 ng/ml | 0.25–30 ng/ml | >30 ng/ml | |
| Sex | |||
| Male | 0.12 (0.11–0.14) | 1.54 (1.12–2.11) | 182.4 (112.5–295.6) |
| Female | 0.12 (0.11–0.14) | 1.51 (1.21–1.88) | 197.6 (127.1–307.3) |
| Age (y) | |||
| 13 | 0.11 (0.09–0.13) | 1.33 (0.69–2.56) | n/a |
| 14 | 0.13 (0.11–0.16) | 1.20 (0.74–1.94) | 251.2 (37.7–1674.6) |
| 15 | 0.12 (0.11–0.14) | 1.25 (0.86–1.82) | 110.6 (55.1–221.7) |
| 16 | 0.12 (0.10–0.14) | 1.45 (1.00–2.10) | 134.4 (72.4–249.5) |
| 17 | 0.14 (0.12–0.17) | 1.75 (1.24–2.47) | 259.5 (151.9–443.2) |
| 18–19 | 0.11 (0.08–0.15) | 3.86 (1.32–11.27) | 233.5 (67.0–814.4) |
| Race | |||
| Asian | 0.12 (0.10–0.14) | 1.44 (0.87–2.39) | n/a |
| African American | 0.14 (0.10–0.18) | 2.34 (1.65–3.33) | 199.6 (129.1–308.6) |
| Latino | 0.12 (0.11–0.13) | 1.28 (0.98–1.68) | 59.9 (36.4–98.5) |
| Mixed | 0.14 (0.09–0.21) | 0.78 (0.37–1.64) | n/a |
| White, Non-Hispanic | 0.12 (0.02–0.58) | 1.40 (0.55–3.60) | 318.8 (117.1–867.7) |
| Other | 0.14 (0.07–0.29) | 1.72 (0.81–3.65) | 333.7 (152.1–731.9) |
| All subjects | 0.12 (0.11–0.13) | 1.52 (1.27–1.82) | 191.9 (139.2–264.8) |
Numbers are geometric mean and 95% confidence interval.
Table 3.
Urine Cotinine Concentration and 3HC/COT by Sex, Age, and Race of Adolescents With SHS Exposure (SHS Defined as Cotinine Levels Between 0.05 ng/ml and 30 ng/ml)
| Variable | Urine cotinine (ng/ml) | Urine 3HC/COT | ||||
|---|---|---|---|---|---|---|
| N | GM (95% CI) | Ratio (95% CI) | N | GM (95% CI) | Ratio (95% CI) | |
| Sex | ||||||
| Male | 139 | 0.74 (0.53–1.03) | 1 [reference] | 133 | 3.98 (3.35–4.72) | 1 [reference] |
| Female | 213 | 0.69 (0.51–0.93) | 0.93 (0.66–1.31) | 209 | 4.91 (4.21–5.73) | 1.24 (1.04–1.47)* |
| Age (y) | ||||||
| 13 | 30 | 0.55 (0.31–0.99) | 1 [reference] | 28 | 4.45 (3.31–6.00) | 1 [reference] |
| 14 | 45 | 0.62 (0.38–1.02) | 1.13 (0.46–2.79) | 42 | 3.65 (2.84–4.69) | 0.82 (0.52–1.30) |
| 15 | 83 | 0.52 (0.35–0.76) | 0.93 (0.41–2.12) | 83 | 4.73 (3.90–5.75) | 1.06 (0.70–1.61) |
| 16 | 76 | 0.73 (0.49–1.08) | 1.32 (0.58–3.02) | 73 | 4.05 (3.31–4.95) | 0.91 (0.60–1.39) |
| 17 | 95 | 0.91 (0.63–1.32) | 1.64 (0.73–3.67) | 93 | 4.49 (3.73–5.41) | 1.01 (0.67–1.52) |
| 18–19 | 23 | 1.09 (0.55–2.18) | 1.97 (0.68–5.72) | 23 | 5.33 (3.79–7.51) | 1.20 (0.70–2.04) |
| Race | ||||||
| All | 352 | 0.56 (0.47–0.66) | 342 | 4.43 (4.08–4.82) | ||
| Latino | 193 | 0.38 (0.30–0.48) | 1 [reference] | 188 | 4.54 (4.04–5.10) | 1 [reference] |
| Asian | 42 | 0.76 (0.46–1.23) | 1.99 (1.00–3.97) | 40 | 3.60 (2.81–4.62) | 0.79 (0.56–1.12) |
| African American | 65 | 1.48 (0.98–2.24) | 3.89 (2.17–6.99)*** | 65 | 4.46 (3.64–5.48) | 0.98 (0.74–1.31) |
| Mixed | 19 | 0.39 (0.19–0.78) | 1.01 (0.39–2.66) | 17 | 4.80 (3.32–6.94) | 1.06 (0.64–1.74) |
| White | 10 | 0.70 (0.27–1.84) | 1.84 (0.50–6.77) | 9 | 6.42 (3.89–10.62) | 1.42 (0.72–2.78) |
| Other | 23 | 1.13 (0.59–2.17) | 2.98 (1.23–7.26)** | 23 | 3.33 (2.42–4.58) | 0.73 (0.47–1.14) |
ANOVA = analysis of variance; CI = confidence interval; 3HC/COT = Trans 3’-hydroxycotinine to Cotinine Ratio; SHS = secondhand smoke. Values presented are adjusted geometric means obtained from ANOVA.
*p < .5; **p < .01; ***p < .001.
Nicotine Metabolite Ratio
We examined the ratio of 3HC/cotinine in urine in subjects with SHS exposure (urine cotinine 0.25–30 ng/ml) and who had values for both metabolites above LOQ (Table 3). NMR was significantly higher in females versus males (4.91 vs. 3.98, p < .05), but was not significantly different by race/ethnicity or by age.
Discussion
We present novel data on biochemically-determined tobacco smoke exposure in adolescents’ urine samples collected for clinical purposes in pediatric clinics in an urban hospital. We determined urine cut points of 30 ng/ml for active smoking, 0.25–30 ng/ml for significant SHS or light or intermittent active smoking, and 0.05–0.25 ng/ml for light SHS or THS exposure. The cut point of 30 ng/ml cotinine in urine to discriminate smoking from nonsmoking among teens is similar to those estimated for adults in other studies.15,16
We found that 87% of adolescents were exposed to some level of tobacco smoke or other sources of nicotine, including 12% whose cotinine levels indicated active smoking. This is in the context of a 12% overall smoking prevalence in the San Francisco Bay Area.17
Of those not biochemically classified as active smokers, 86% had evidence of nicotine exposure. This is much higher than the 41% based on blood cotinine reported in the most recent NHANES report (years 2011–2012).6 Most likely the reason our subjects’ prevalence was higher is that we measured cotinine in urine, and cotinine levels are known to be on average five times higher in urine than in blood,8 resulting in greater analytical sensitivity in detecting nicotine exposure. We estimated that 46% of our subjects had exposures consistent with significant SHS exposure, which is similar to the prevalence of SHS exposure in adolescents reported by CDC. We found that 30% of our subjects had exposures consistent with either light or intermittent SHS and/or THS exposure, a group not detected by NHANES. A higher overall prevalence of tobacco exposure may also reflect that these adolescents seeking care in an urban public hospital were primarily from families with lower levels of income and education, in whom tobacco use is known to be higher in general.6,17,18 In addition, teens requiring urine testing for sexually transmitted infection or pregnancy may indicate high risk behaviors in general, which are associated with greater tobacco use and smoke exposure.
Among adolescents exposed to SHS, adjusted cotinine levels were substantially higher in African Americans compared to other ethnic/racial groups. This is similar to what we observed in our study among younger children.4 Higher cotinine levels could be due to differences in level of exposure and/or racial differences in the rate of nicotine metabolism. Surveillance data from California indicate that the prevalence of smoking in the home is much higher in African Americans compared to white, Latino, and Asian homes,17 suggesting increased exposure. With respect to metabolism differences, African Americans on average have genetically lower CYP2A6 metabolic activity and slower cotinine metabolism,7,19 and correspondingly would have higher cotinine levels for any given daily intake of nicotine,20 compared to whites and Latinos. However in the present study the NMR was not significantly different in African American subjects, suggesting that reduced CYP2A6 activity is not the explanation for higher cotinine levels in our subjects. African Americans are also more likely to carry genes associated with slower glucuronide conjugation of cotinine, which could also explain higher urine cotinine levels.21 The extent to which the higher cotinine levels in African American adolescents in our study was due to slower metabolism or to greater levels of SHS exposure or disproportionately higher exposure to other nicotine products (such as blunts) or some combination remains to be determined. The finding that NMR was higher in female compared to male adolescents is consistent with data in adults and with the known effects of estrogen to induce CYP2A6 activity.22,23 NMR did not differ by age, which is consistent with data indicating that CYP2A6 activity seems to be fully expressed by early childhood.24
Our study cannot determine subjects’ sources of exposure to nicotine. The highest exposure group most likely represented active cigarette smoking, but could also have represented the use of electronic cigarettes, cigars, smokeless tobacco, water pipe or use of blunts.25 The second highest cotinine exposure group most likely represented SHS exposure, but could also have represented light or intermittent active use of any tobacco or nicotine product. Light and intermittent smoking is relatively common in adolescents who are in early stages of cigarette dependence. The lowest exposure group is of considerable interest. This group has not been identified in other studies because most other studies measured blood cotinine, while cotinine levels are considerably higher in urine. Subjects in this group could have been those with light or intermittent SHS exposure, but also could have been those with THS exposure.
THS is the residual of tobacco smoke that remains on surfaces and fabrics and dust in rooms in which smoking has occurred, but after smoking has ceased.26 Such contamination can persist for months or years after direct smoke contamination. Exposure to THS can result in low levels of cotinine in urine.27–29
The health consequences of active smoking and SHS exposure in adolescents are well established. Of particular concern are respiratory problems and risk of infectious diseases.1,2 The health effects of THS exposure in people have not been established, but in vitro and animal studies suggest that THS is cytotoxic and can affect various body organs.30 Of most concern for THS exposure in adolescents is respiratory disease such as asthma and perhaps other allergic diseases as well.26
In a prior study of young children screened for lead exposure, we found a much higher biochemically-determined prevalence of exposure in children than was reported by parents.4 In the present study we found that adolescents underreported active smoking to their care providers, and there was virtually no information on SHS exposure in the medical records. The high prevalence of tobacco smoke exposure in adolescents highlights the importance of documented tobacco use, both active and passive, at each clinical encounter.
Previously, we suggested that routine cotinine screening in young children could identify children with significant exposures and lead to interventions to reduce or prevent such exposures.4 We now suggest the same approach using urine samples routinely collected from adolescents. Urine samples are noninvasive and potentially easier to collect for routine screening than blood samples. Given the high levels of tobacco exposure we suggest that routine screening should be considered for adolescents as well as young children, particularly children from economically disadvantaged populations and those with chronic respiratory conditions such as asthma.
One potential obstacle to implementing routine screening is that the instrumentation used to detect low levels of cotinine—liquid chromatography–mass spectrometry—is expensive and requires highly trained operators. However, there is increasing use of mass spectrometry for routine clinical application.31 High sensitivity immunoassays are also becoming increasingly available and might be developed for routine cotinine screening.
Limitations of our study include the use of a convenience sample. Adolescents who are asked to give urine samples for medical reasons may have been more likely to be exposed to tobacco smoke. Another limitation is that smoking status was assessed from electronic medical records, and for many subjects the medical record entries and urine collections were temporally disparate. This probably explains why some subjects who reported active smoking were negative by biochemical screening. Also non-daily smoking could result in negative biochemical results.
Funding
This work was supported by the UCSF Bland Lane Center of Excellence on Secondhand Smoke funded by the Flight Attendants Medical Research Institute, and National Institute of Health grants P30 DA102393 and S10 RR026437.
Declaration of Interests
NLB is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to disclose.
Acknowledgments
We thank Faith Allen, for data management, Sandra Tinetti and the nurses in the Children’s Health Center at Zuckerberg San Francisco General Hospital for clinical research assistance, Trisha Mao and Lita Ramos for analytical chemistry and Tyson Douglass for editorial assistance. All were employees of the University of California San Francisco and were paid for their contributions. NLB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. NLB is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to disclose.
References
- 1. Treyster Z, Gitterman B. Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics. Rev Environ Health. 2011;26(3):187–195. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 3. Winickoff JP, Tanski SE, McMillen RC, et al. Acceptability of testing children for tobacco-smoke exposure: a national parent survey. Pediatrics. 2011;127(4):628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dempsey DA, Meyers MJ, Oh SS, et al. Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch Pediatr Adolesc Med. 2012;166(9):851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph A, Murphy S, Thomas J, et al. A pilot study of concurrent lead and cotinine screening for childhood tobacco smoke exposure: effect on parental smoking. Am J Health Promot. 2014;28(5):316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Homa DM, Neff LJ, King BA, et al. ; Centers for Disease Control and Prevention (CDC). Vital signs: disparities in nonsmokers’ exposure to secondhand smoke–United States, 1999-2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- 7. Pérez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. [DOI] [PubMed] [Google Scholar]
- 8. Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., III Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11(8):954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(3–4):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dempsey D, Tutka P, Jacob P, III, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 11. Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. [DOI] [PubMed] [Google Scholar]
- 12. Schwarz G. Estimating the dimension of a model.Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 13. Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 14. SRNT Subcomittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 15. Goniewicz ML, Eisner MD, Lazcano-Ponce E, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13(3):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campo L, Polledri E, Bechtold P, et al. Determinants of active and environmental exposure to tobacco smoke and upper reference value of urinary cotinine in not exposed individuals. Environ Res. 2016;148:154–163. [DOI] [PubMed] [Google Scholar]
- 17. California Department of Public Health. California Tobacco Control Program. California Tobacco Facts and Figures. Sacramento, CA; 2015. [Google Scholar]
- 18. Collaco JM, Aherrera AD, Ryan T, McGrath-Morrow SA. Secondhand smoke exposure in preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 20. Zhu AZ, Renner CC, Hatsukami DK, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev. 2013;22(4):708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wassenaar CA, Conti DV, Das S, et al. UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers. Cancer Epidemiol Biomarkers Prev. 2015;24(1):94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., III Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. [DOI] [PubMed] [Google Scholar]
- 23. Higashi E, Fukami T, Itoh M, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35(10):1935–1941. [DOI] [PubMed] [Google Scholar]
- 24. Dempsey DA, Sambol NC, Jacob P, III, et al. CYP2A6 genotype but not age determines cotinine half-life in infants and children. Clin Pharmacol Ther. 2013;94(3):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Timberlake DS. The changing demographic of blunt smokers across birth cohorts. Drug Alcohol Depend. 2013;130(1–3):129–134. [DOI] [PubMed] [Google Scholar]
- 26. Matt GE, Quintana PJ, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119(9):1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matt GE, Quintana PJ, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control. 2011;20(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matt GE, Quintana PJ, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control. 2014;23(3):264–272. [DOI] [PubMed] [Google Scholar]
- 29. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS One. 2014;9(1):e86391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mass Spectrometry: Applications to the Clinical Laboratory, (MSACL) www.msacl.org. Published February 26, 2008. Updated February 27, 2016. Accessed December 1, 2015.