Abstract
Introduction
Research suggests a strong association between negative affect (NA) and smoking. However, little is known about the association between NA and smoking among individuals who switch to reduced-nicotine cigarettes. The goal of this study was to examine the extent to which cigarette nicotine content moderates the relationship between NA and smoking over time.
Methods
Seven hundred and seventeen participants, 237 in the normal nicotine content (NNC; 15.8 mg/g and usual brand) cigarette group and 480 in the very low nicotine content (VLNC; 2.4 mg/g nicotine or less) cigarette group, participated in a randomized trial that examined the effects of cigarette nicotine content on smoking behavior over 6 weeks. We used parallel process latent growth curve modeling to estimate the relationship between changes in NA and changes in the numbers of cigarettes smoked per day (CPD), from baseline to 6 weeks, as a function of cigarette nicotine content.
Results
The relationship between NA and investigational CPD reduced over time for those in the VLNC group, but not for those in the NNC group. There was no significant relationship between change in PA and CPD over time for either cigarette group.
Conclusions
Smoking VLNC cigarettes disrupts the relationship between smoking and negative affect, which may help reduce nicotine dependence.
Implications
This study suggests that the association between NA and smoking behavior is reduced over time among those that smoked reduced-nicotine content cigarettes. This provides additional evidence that smoking reduced-nicotine content cigarettes may help reduce nicotine dependence.
Introduction
Negative affect (NA) presents a significant challenge to successful smoking cessation. Many clinical studies of individuals trying to quit smoking have found that NA following a quit attempt is reliably related to treatment failure and relapse across a variety of treatment modalities (eg,1–4). The presence of NA following cessation has been found to characterize over 50% of all smoking lapses, with 19% of all lapses occurring under conditions of extreme negative affect.5 NA during the quitting process can result from nicotine withdrawal or from non-withdrawal sources (eg, stressors). NA from non-withdrawal sources has been linked to smoking relapse,6 and, in terms of withdrawal, NA appears to be the symptom that most profoundly increases the likelihood of relapse.7–9
While NA associated with non-withdrawal processes have not been consistently found to be alleviated by smoking,6 there is substantial evidence that NA associated with nicotine withdrawal can be alleviated by nicotine, which may contribute to its negative reinforcement10,11 When a dependent smoker quits, he or she often experiences a short-term increase in withdrawal, followed by a gradual reduction.12 One approach to mitigate withdrawal symptoms among quitting smokers is to expose them to conditioned stimuli associated with smoking, which have gained the ability to evoke withdrawal relief responses associated with nicotine through learning.13 An example of this type of approach would be exposing smokers to very low nicotine content (VLNC) cigarettes, which have been found to alleviate NA resulting from nicotine withdrawal.14,15 Thus, by smoking VLNC cigarettes, which are largely devoid of nicotine but offer the conditioned stimuli associated with nicotine use, the cycle of withdrawal and withdrawal relief may reduce over time either because withdrawal subsides and/or the conditioned effects extinguish.
However, most of the studies that have evaluated the impact of VLNC cigarettes on NA and other withdrawal symptoms have done so over relatively short time periods, using restricted sample sizes, and have produced equivocal findings. While some short-term laboratory studies using VLNC cigarettes have indicated some beneficial effects on withdrawal, craving,16,17 and reinforcement,18 other studies have shown that VLNC cigarettes are preferred less,19 provide less satisfaction14,20 and reward,21 and alleviate fewer withdrawal symptoms,22 including craving,23 than “normal” cigarettes. It is unknown whether smoking VLNC cigarettes over a longer period would uncouple the relationship between smoking and NA.
Recently, we published a longitudinal randomized trial examining the effects of cigarette nicotine content on smoking behavior, nicotine dependence, and toxicant exposure.24 Participants were provided with one of six types of investigational cigarettes that varied on nicotine content and tar yield, or were given their usual brand, to smoke for 6 weeks. The main finding of the parent study was that smoking VLNC cigarettes reduced the number of cigarettes smoked per day (CPD) and nicotine exposure, without increasing expired carbon monoxide levels, compared to smoking usual brand or the internal control.
The goal of the present study was to conduct secondary data analyses to examine whether cigarette nicotine content moderates the relationship between NA and smoking among daily smokers. We used latent growth curve (LGC) for parallel process modeling25 to examining whether the longitudinal associations between NA and investigational CPD were moderated by cigarette nicotine content. We hypothesized that those smoking VLNC cigarettes would show a weakened relationship between NA and investigational CPD compared to those smoking NNC cigarettes, such that the associations between NA and CPD would be significantly less for the VLNC compared to the NNC group.
Method
Participants
Participants (n = 840) for the current study come from a randomized clinical trial (ClinicalTrials.gov number: NCT01681875) conducted at 10 sites between June 2013 and July 2014 to evaluate the effects of smoking reduced-nicotine cigarettes for 6 weeks on smoking behavior, nicotine dependence, and toxicant exposure.24 In the parent trial, participants were included if they were aged 18 years or older, smoked ≥5 CPD, produced an expired carbon monoxide > 8 ppm or urine cotinine > 100 ng/ml at baseline, and were excluded if they intended to quit smoking in the next 30 days, used other tobacco products besides machine-manufactured cigarettes on more than 9 of the past 30 days, reported significant unstable medical or psychiatric conditions, produced a positive toxicology screen for illicit drugs other than cannabis, were pregnant, trying to become pregnant or breastfeeding, or smoked “roll your own” cigarettes exclusively. The participants were recruited by the individual sites from their communities using print, electronic, and radio advertising. Participants were compensated up to $835 for completing all study requirements. One participant was not included in these analyses due to being determined to be ineligible post-randomization, and 122 participants assigned to the 5.2 mg/g tobacco condition were excluded (see Statistical Methods, below), leaving 717 participants in the analyses.
Procedures
After smoking their own brand during a 2-week baseline period, the participants were randomized to receive 6 weeks of one of six blinded cigarette types, with varying nicotine and tar content, or received their usual brand. The 6-week supply of cigarettes was free of charge to the participants, including those in the usual brand condition. Participants completed in-session questionnaires and provided biospecimens prior to the 2-week baseline period, at randomization (1 day prior to receiving investigational cigarettes), at 2 weeks after receiving investigational cigarettes, and at 6 weeks after receiving investigational cigarettes. An Interactive Voice Response (IVR) system (InterVision Media, Eugene, OR) called the participants every day during these 6 weeks to administer assessments, as described below. A comprehensive description of the study design, the additional measures collected, and the results of the parent study can be found elsewhere.24
Materials
Investigational Cigarettes
The National Institute on Drug Abuse’s Drug Supply Program supplied the investigational cigarettes.26 The six types of Spectrum cigarettes used in this study contained 0.4, 1.3, 2.4, 5.2, or 15.8 mg/g of nicotine, and tar was 7.9–10.4 mg/cigarette, except for one type, which contained 0.4 mg/g of nicotine and 13.2–13.7 mg/cigarette of tar (using the ISO method). Participants received regular- or menthol-flavored cigarettes based on preference. The investigational cigarettes were administered using a double blind random assignment, except for the usual brand group, which was not blinded. Participants received a 14-day supply of the investigational cigarettes at each weekly visit. Once randomized, participants were discouraged from using non-investigational cigarettes and other nicotine products, but were not penalized for doing so.
Cigarettes Per Day (CPD)
CPD was measured daily for 6 weeks using the IVR system, in addition to baseline, at each in person visit. From this, we calculated mean CPD for each week, for a total of 6 weeks, using all days since the last visit. If the participant had missed a visit (~8% were missed), the 7 days following the previous visit was used.
Positive and NA Scale
We measured positive and NA using the Positive and Negative Affect Scale’s (PANAS) Positive (PA) and Negative (NA) scales,27 which was administered onsite using Qualtrics (Qualtrics, Provo, UT) at baseline and at weeks 2 and 6 during investigational cigarette use. The PANAS instructions asked about mood “during the past week” at baseline and “since your last scheduled visit” at weeks 2 and 6. The scales were reliable (both Cronbach’s alpha’s = 0.89) and were negatively correlated (r = −0.15; p < .001).
IVR Measures of Withdrawal Symptoms
In addition to CPD, the IVR captured single-item measures of irritability, craving, restlessness, sadness, concentration, anxiety, appetite, insomnia for 17 days (10 days before randomization and 7 days after). Instructions asked “how you have felt, overall, since yesterday’s phone call,” and used a 0–4 scale (0 = not at all, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe).
Nicotine Exposure
Nicotine exposure was assessed by both total urinary cotinine (urinary free cotinine plus cotinine N-glucuronide) and by urinary total nicotine equivalents (TNE), the sum of nicotine, cotinine, 3’-hydroxycotinine, and their glucuronides.28 The urine specimens were collected onsite at randomization, week 2, and week 6 during investigational cigarette use. The analyses conducted below yielded similar findings for both urinary cotinine and TNE. To simplify the results, and to maximize generalizability, we only present the findings for urinary cotinine (cotinine is the more common biomarker in smoking studies).
Statistical Methods
For purposes of analysis, we classified the seven treatment arms into two groups, based on investigational cigarette nicotine amount. Smokers assigned to the two control conditions used in the parent study, the 15.8 mg/g nicotine content investigational cigarette arm and the usual brand arm, were grouped into the NNC group (n = 237). Participants assigned to one of the three low nicotine content cigarette arms (≤ 2.4 mg/g) were categorized into the VLNC group (n = 480). We excluded the 5.2 mg/g group from these analyses because the effects of this dose on smoking behavior were unclear in the main outcome study, as its effects on CPD and TNE were inconsistent unlike the other six groups, which clearly naturally clustered into two groups (ie, NNC or VLNC).24
We used LGC for parallel process modeling to determine whether the longitudinal associations between PANAS scores and CPD were moderated by nicotine content groups. LGC provides a very flexible framework to capturing individual patterns of change, accounting for both inter- and intra-individual temporal variation and for measurement error of time-specific measurements.29 Our goal was to conduct a multiple group analysis of parallel process between the PANAS NA and CPD and tested specific differences of regression effects between the VLNC and NNC groups. The model building steps are included in the supplement, as are the models evaluating PANAS NA and CPD, due to space constraints. We evaluated the strength of the relationships by standardizing the coefficients, and identified the strength of the coefficients as very weak (between 0 and 0.19), weak (0.20–0.39), moderate (0.40–0.59), strong (0.60–0.79), and very strong (equal to or greater than 0.80).30 The study was adequately powered to detect moderate (beta > 0.4) effect sizes. Additional information on the statistical methods, including model fit parameters and analysis of the IVR withdrawal data, are included in the Supplement.
In terms of IVR measures, we explored the trajectories of CPD and of each IVR withdrawal symptom for 17 consecutive days (10 before randomization), by nicotine content group. We then analyzed each of the eight IVR withdrawal symptoms separately with IVR CPD for the first 7 days after randomization. Specifically, we employed the LGC parallel growth models to examine if change of each IVR symptom was related to change in CPD during the first 7 days after randomization.
All models were estimated using Mplus (version 7.2; Muthén & Muthén, Los Angeles, CA) using full information maximum likelihood (FIML). The goodness of fit indices are described in the supplement. The dropout (N = 59; 7%) due to intermittent missingness was addressed using FIML, which has been shown to provide accurate estimates comparable to multiple imputation methods when data are missing at random.31 In addition, and as a sensitivity analysis, we estimated our models with both maximum likelihood and with Bayesian estimation using a series of different priors.32 The FIML and Bayesian model results were consistent across estimators, and we elected to report the results from the FIML models.
Results
Table 1 presents CPD, PANAS NA, and cotinine (nmols/mg creatinine) means and standard deviations by nicotine level across time. In general, CPD increased slightly over time for the NNC group and decreased slightly for the VLNC group, which reported lower numbers of CPD at all time points. NA remained stable throughout the study, with both groups reporting similar levels of NA in all time points. Specifically, the difference in NA between the two nicotine groups was 0.63 (p = .162) at baseline, 0.85 (p =.071) at week 3, and .17 (p = .733) at week 6. Similar results were found for PANAS PA (see Table S1).
Table 1.
Investigational CPD, PANAS NA, and Cotinine Means and Standard Deviations Across Time, by Cigarette Group
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
|---|---|---|---|---|---|---|---|
| NNC | |||||||
| CPD | 18.7 (10.41) | 19.5 (10.41) | 20.1 (11.52) | 20.9 (11.93) | 20.6 (11.80) | 21.4 (12.68) | |
| NA | 15.4 (5.75) | 15.1 (5.60) | 15.7 (5.85) | ||||
| Cotinine | 15.8 (11.13) | 15.1 (11.33) | 14.7 (11.73) | ||||
| VLNC | |||||||
| CPD | 15.1 (10.02) | 14.7 (10.04) | 14.5 (10.01) | 14.1 (9.81) | 14.2 (9.71) | 14.2 (10.21) | |
| NA | 16.1 (5.62) | 15.9 (5.81) | 15.9 (6.33) | ||||
| Cotinine | 15.6 (11.57) | 8.9 (9.29) | 9.6 (9.13) | ||||
CPD = investigational cigarettes per day; NA = PANAS Negative Affect scale; NNC = normal nicotine content cigarette group; VLNC = very low nicotine content cigarette group. Cotinine = Total cotinine expressed as geometric mean adjusted for creatinine (nmols/mg creatinine).
Univariate LGC Models
Fits for the CPD and PANAS NA univariate LGC models were excellent for the overall, NNC group, and VLNC group models (see Table S2). Table 2 shows the results of the univariate LGC analyses for each nicotine level group. In terms of mean CPD change, significant increases were observed over time for the NNC group (Growth RateCPD = 0.6) and significant declines for the VLNC group (Growth RateCPD = −0.2). In contrast, NA did not change over time, as indicated by the non-significant change estimates in both study groups. The variances of the initial levels and growth rate factors generally paralleled the mean components. In terms of covariance, significant covariation of week 1 CPD with CPD change over time (slope) was observed only for the NNC group (Growth RateCPD = 4.1), indicating that those with greater CPD at week 1 were more likely to have higher increases in CPD over time than those with lower CPD at week 1.
Table 2.
Univariate Linear Growth Curve Model Estimates, by Cigarette Group, for Investigational CPD and PANAS NA
| Growth components | NNC | VLNC |
|---|---|---|
| Means | ||
| CPD at week 1 | 18.8 (0.65)*** | 14.9 (0.45)*** |
| CPD slope | 0.6 (0.08)*** | −0.2 (0.05)** |
| NA at baseline | 15.2 (0.34)*** | 16.1 (0.24)*** |
| NA slope | 0.1 (0.07) | −0.1 (0.06) |
| Variances | ||
| CPD at week 1 | 95.7 (11.71)*** | 85.3 (9.48)*** |
| CPD slope | 0.9 (0.31)*** | 0.9 (0.30)** |
| NA at baseline | 15.2 (3.02)*** | 15.5 (2.14)*** |
| NA slope | 0.01 (0.23) | 0.8 (0.23)*** |
| Covariances | ||
| CPD at week 1 with CPD slope | 4.1 (1.10)*** | 0.2 (0.59) |
| NA at baseline with NA slope | 0.7 (0.60) | 0.1 (0.46) |
CPD = investigational cigarettes per day; NNC = normal nicotine content cigarette group; VLNC = very low nicotine content cigarette group; NA = PANAS Negative Affect scale.
*p < .05; **p < .01; ***p < .001.
In contrast to NA, PANAS PA decreased over time for both groups, as indicated by significant slopes (see Table S3). Similar to NA, there was a significant covariation of week 1 CPD with CPD change over time for the NNC, but not for the VLNC group.
Multivariate LGC Model
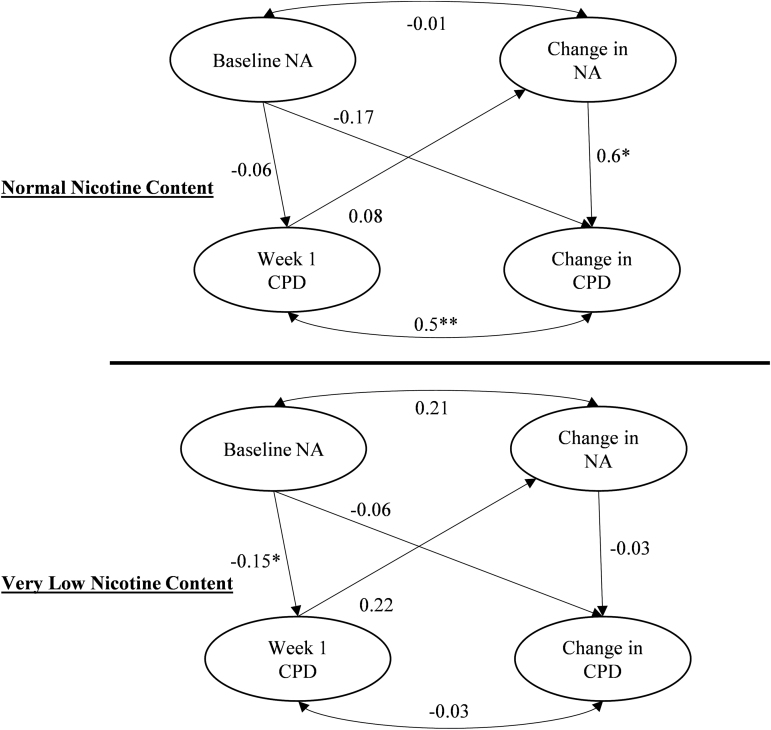
The fit for the final multiple group parallel process model was very good (x2[97] = 216.58, p = .001; CFI = 0.98; TLI = 0.98; RMSEA = 0.059; SRMR = 0.032). In terms of the initial week 1 relationships, greater baseline PANAS NA was weakly associated with lower week 1 CPD for the VLNC (standardized coefficient = −0.15), but not the NNC group (see Figure 1). Moreover, the difference of baseline NA on the week 1 CPD coefficient between the two cigarette groups was not statistically significant (Wald x2[1] = 0.5, p = .476).
Figure 1.
Results of the latent growth curve for parallel process model examining whether the longitudinal associations between PANAS NA and investigational CPD were moderated by nicotine content groups. *p < .05.
In terms of the relationship between PANAS NA and CPD over time, we found a significant and strong effect for the relationship between change in NA over time and change in CPD over time for NNC (β = 0.6, p < .001), but not for VLNC (β = −0.03, p = .816; see Figure 1). The difference of the coefficients between the nicotine groups was significant (Wald x2[1] = 5.59, p = .018). The unstandardized regression coefficients from this multiple group parallel process model are listed in Table 3. The unstandardized coefficient for the relationship of change in NA on change in CPD was significant for the NNC group, indicating an average of a 1.3 weekly increase in CPD for each unit increase in NA, holding baseline level of NA and nicotine exposure constant.
Table 3.
Unstandardized Regression Coefficients (SE) From the Multiple Group Parallel Process Model for the Effect of Change in PANAS NA on Change in Investigational CPD, by Cigarette Group
| Model | NNC | VLNC |
|---|---|---|
| Baseline NA -> Week 1 CPD | −0.13 (0.19) | −0.30 (0.14)* |
| Baseline NA -> CPD slope | −0.04 (0.04) | −0.01 (0.02) |
| NA Slope -> CPD slope | 1.30 (0.42)* | −0.08 (0.35) |
| Week 1 CPD -> NA slope | 0.01 (0.01) | 0.01 (0.01) |
| Baseline NA <-> NA slope | 0.10 (0.38) | 0.43 (0.31) |
| Week 1 CPD <-> CPD slope | 4.30 (1.24)** | −0.15 (0.69) |
CPD = investigational cigarettes per day; NA = PANAS Negative Affect scale; NNC = normal nicotine content cigarette group; VLNC = very low nicotine content cigarette group.
* p < .05; **p < .01; ***p < .001.
To further evaluate the moderating effect of nicotine content on the relationship between NA and CPD, we categorized the estimated change in NA over the 6 weeks into 3 groups: High change in NA included individuals with changes in slope that were > 0.75 SD from the group mean (n = 119; 19%); average change, included individuals whose slope estimates were within 0.75 SD of the slope change mean (n = 407; 64%); and low change in NA included individuals who were < 0.75 SD from the mean slope change (n = 108; 17%). The high and low change categories in NA can be conceptualized as representing positive and negative change in NA, respectively, while the average change category represents stable NA throughout the study. As Figure S1A demonstrates, high slope changes in NA were associated with CPD increases (β = 2.4, p < .001) for NNC but not for VLNC cigarettes (β = −0.14, p = .262). Figure S1B shows that a sharper decline in CPD was observed for NNC group who experienced low NA slope changes (β = −0.81, p < .001) than the VLNC participants (β = −0.29, p < .001). The difference between the NNC and the VLNC group for the slopes in S1B are statistically significant (β = −0.54, p < .001). Finally, Figure S1C shows that stability of NA throughout the study is associated with minimal increases in CPD for the NNC (β = 0.37, p < .001) but not the VLNC group (β = −0.06, p = .160).
For the models with PANAS PA as predictor, greater baseline PANAS PA was associated with greater week 1 CPD for the NNC, but not the VLNC group (see Figure S2). The difference of baseline PA on the week 1 CPD coefficient between the two cigarette groups was not statistically significant (Wald x2[1] = 2.515, p = .113). No significant relationship between change in PA and CPD over time was found for either cigarette group (see also Table S4).
The Potential Impact of Noncompliance
A potential reason for the non-significant finding between PANAS NA and CPD over time for the VLNC group may be underreporting of use of non-investigational cigarettes. However, all effects involving NA and CPD were conditioned on cotinine levels, which factors out variation of cotinine level (and potentially compliance) as a confounder. In addition, the correlation between NA and cotinine at all three time points were both small and negative for the VLNC group at baseline (r = −0.11, p = .015), week 3 (r = −0.02, p = .580), and week 6 (r = −0.10, p = .028), respectively, suggesting that those in the VLNC with higher levels of NA were more likely to present with lower levels of cotinine, at least at baseline and week 6. This relationship was different for the NNC group, who had no significant relationship between NA and cotinine at all three measurement occasions (r = −0.06, p = .354 for baseline; r = −0.09, p = .172 for week 3; and r = 0.03, p = .602 for week 6). The divergence of patterns in the longitudinal analysis between the two nicotine groups extends the argument of the presence of qualitative differences in smoking-related experiences when switching to VLNC cigarettes.
Daily IVR Measures of CPD and Withdrawal
The observed means of IVR CPD for the first 7 days after randomization and the means of each of the 8 IVR withdrawal symptoms for 10 days prior to baseline and 7 days after baseline are depicted in the supplement. Figures S3 shows that CPD was significantly higher for the NNC group across the first week of use (time-varying unstandardized coefficient of nicotine content group = 2.80; p < .001). Figure S4 and Table S5 show that only irritability differed by nicotine content group over the first week of use (b = 0.11, p = .036). However, unlike with our PANAS NA findings, we did not find significant nicotine group interaction effects in the relationship of change in withdrawal and CPD for any of the IVR symptoms in the LGC analyses. In addition, we did not find significant effects of IVR symptoms and CPD for either of the nicotine groups.
Discussion
The purpose of these secondary analyses was to examine the capacity of cigarette nicotine content to moderate the relationship between NA and smoking. We had hypothesized that those smoking VLNC investigational cigarettes for 6 weeks would show a weakened relationship between NA and CPD compared to those smoking normal nicotine content cigarettes. We employed LGC modeling and multiple group analysis, which allowed us to assess the association between initial levels of NA and CPD, changes between NA and investigational CPD over time, and the association of the parameters between these two processes.
Our multivariate LGC model findings indicated that participants in the NNC group displayed a strong positive association between NA and smoking investigational cigarettes over 6 weeks, but that this association was nonsignificant among the VLNC group. This suggests that smoking reduced-nicotine content cigarettes reduces the association between PANAS NA and smoking over time. Moreover, our findings suggest that the positive association between NA and CPD is strongest among those in the NNC group who experienced the greatest increase in NA over the trial, supporting the notion that as NA increases, smokers smoke more in response, but only when the nicotine content of the cigarette is in the range of conventional cigarettes. This relationship was significantly attenuated among those in the VLNC group. Our findings were specific to NA, as they were not replicated for PANAS PA, and emerged over 6 weeks of VLNC use, as they were not present during the initial week of VLNC use as measured by IVR. These findings are novel and resulted from our use of multivariate LGC modeling. Had we only examined these data using univariate LGC models, we might have assumed that there were no relationships between NA and CPD, given that CPD changed over time for the NNC and VLNC groups, but that NA did not change over time for either group.
These findings provide further support that smoking VLNC cigarettes may reduce nicotine dependence and provide insight into a potential mechanism by which VLNC cigarettes reduce smoking behavior. If the FDA chooses to reduce nicotine to these levels, it may be expected that smokers would smoke less and that the relationship between smoking and NA will be disrupted through extinction, which may eventually reduce dependence, and might facilitate a quit attempt, as smoking VLNC cigarettes would have reduced one of its most important psychoactive properties, that is, the management of negative affect. In fact, in the parent study, individuals smoking VLNC cigarettes (< 2.4 mg/g) were more likely to report attempting to quit after completing the study compared to those who smoked the NNC cigarettes (15.8 mg/g).24 Additionally, our data should help alleviate concerns about potential unintended consequences of broadly reducing nicotine, as smokers in the VLNC group neither demonstrated a significant decrease in PA over time nor reported an increase in NA over time when compared to the NNC group, other than an increase in irritability (ie, on the IVR) during the first week of product use.
There are potential limitations that are a result of this study’s design. First, participants had access to alternate sources of nicotine. While dissuaded from doing so, the majority of participants in the parent study smoked their usual brand of cigarettes at least some of the time during the 6 weeks of investigational cigarette use, and smokers of reduced-nicotine content cigarettes were more likely to do so than smokers of higher nicotine content cigarette.24 However, urinary TNE values, a composite measure of nicotine exposure biomarkers,28 was significantly lower for those in the VLNC compared to the NNC groups, suggesting that VLNC smokers still reduced nicotine exposure despite increased use of their usual brand cigarettes than NNC smokers. Second, there may be other substances found in tobacco smoke, including VLNC cigarettes, that influence negative affect, but which were uncontrolled in this study. For example, studies from the animal literature have found that monoamine oxidase inhibitors (MAOIs) influence smoking behavior.33,34 Third, our measures, particularly the PANAS, may have been subject to recall bias. Future studies could reduce this potential bias by using ecological momentary assessment (EMA).35 In addition, we could not explore higher-order growth patterns for PANAS, because we only had measurements at three time points. Fourth, the effects presented may not reflect the actual causal structure between NA and CPD, as the relationship may be bidirectional with reciprocal influences. Finally, our reliance on the PANAS, an instrument that conceptualizes emotional space through the dimensions of pleasantness and arousal,27 precludes us from linking discrete emotions to smoking amount (ie, CPD). Future studies could expand these findings by including discrete measures of emotions.
NA regulation is a major obstacle while quitting smoking. Our results suggest that longer use of VLNC cigarettes disrupts the link between NA and smoking. VLNC cigarettes may be of particular use for those with NA symptoms, who have been found to experience increased NA during initial nicotine withdrawal.36 Thus, our findings support the notion that lowering nicotine content in cigarettes to a level below what is required to maintain addiction might be a promising regulatory/treatment approach for reducing nicotine dependence, which could be considered within a broader framework of other tobacco control regulatory policies.
Supplementary Material
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA) Center for Tobacco Products (CTP; grant number: U54DA031659). The experimental cigarettes were provided by NIDA’s Drug Supply Program (NOT-DA-14-004). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors wish to thank Mustafa al’Absi, Neal Benowitz, Rachel Denlinger, David Drobes, Steve Hecht, Joni Jensen, Joseph Koopmeiners, Tonya Lane, Joseph McClernon, Sharon Murphy, Maxine Stitzer, Andrew Strasser, Jennifer Tidey, Hilary Tindle, Ryan Vandrey, and all the students, fellows, and staff involved with the Center for the Evaluation of Nicotine in Cigarettes. Paul M. Cinciripini and Eric C. Donny Shared senior authorship.
References
- 1. Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on craving and negative affect leading up to lapse episodes. Psychopharmacology (Berl). 2014;231(13):2595–2602. [DOI] [PubMed] [Google Scholar]
- 2. Lam CY, Businelle MS, Aigner CJ, et al. Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res. 2014;16(5):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasilenko SA, Piper ME, Lanza ST, Liu X, Yang J, Li R. Time-varying processes involved in smoking lapse in a randomized trial of smoking cessation therapies. Nicotine Tob Res. 2014;16(suppl 2):S135–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leventhal AM, Greenberg JB, Trujillo MA, et al. Positive and negative affect as predictors of urge to smoke: temporal factors and mediational pathways. Psychol Addict Behav. 2013;27(1):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiffman S, Paty J, Gnys M, Kassel J, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 6. Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. [DOI] [PubMed] [Google Scholar]
- 7. Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70(1):216–227. [PubMed] [Google Scholar]
- 8. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Exp Clin Psychopharmacol. 2003;11(4):276–285. [DOI] [PubMed] [Google Scholar]
- 9. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112(1):14–27. [PubMed] [Google Scholar]
- 10. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 11. Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behav Pharmacol. 1999;10(6–7):639–646. [DOI] [PubMed] [Google Scholar]
- 12. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. [DOI] [PubMed] [Google Scholar]
- 13. O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. [DOI] [PubMed] [Google Scholar]
- 14. Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav. 1995;50(1):91–96. [DOI] [PubMed] [Google Scholar]
- 15. Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biol Psychiatry. 2010;67(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1(4):357–364. [DOI] [PubMed] [Google Scholar]
- 17. Robinson ML, Houtsmuller EJ, Moolchan ET, Pickworth WB. Placebo cigarettes in smoking research. Exp Clin Psychopharmacol. 2000;8(3):326–332. [DOI] [PubMed] [Google Scholar]
- 18. Rusted JM, Mackee A, Williams R, Willner P. Deprivation state but not nicotine content of the cigarette affects responding by smokers on a progressive ratio task. Psychopharmacology (Berl). 1998;140(4):411–417. [DOI] [PubMed] [Google Scholar]
- 19. Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology (Berl). 1999;147(2):210–216. [DOI] [PubMed] [Google Scholar]
- 20. Gross J, Lee J, Stitzer ML. Nicotine-containing versus de-nicotinized cigarettes: effects on craving and withdrawal. Pharmacol Biochem Behav. 1997;57(1–2):159–165. [DOI] [PubMed] [Google Scholar]
- 21. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 22. Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100(4):550–559. [DOI] [PubMed] [Google Scholar]
- 23. Lindsey KP, Bracken BK, Maclean RR, Ryan ET, Lukas SE, Frederick BD. Nicotine content and abstinence state have different effects on subjective ratings of positive versus negative reinforcement from smoking. Pharmacol Biochem Behav. 2013;103(4):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheong J, Mackinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Struct Equ Modeling. 2003;10(2):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institute on Drug Abuse. Nicotine Research Cigarettes Drug Supply Program. www.drugabuse.gov/nicotine-research-cigarette-drug-supply-program Updated January 12, 2016, Accessed March 31, 2016. [Google Scholar]
- 27. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 28. Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–183. [DOI] [PubMed] [Google Scholar]
- 29. Bollen K, Curran JP. Latent Curve Models: A Structural Equation Perspective. New Jersey, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 30. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 31. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 32. Depaoli S. Mixture class recovery in GMM under varying degrees of class separation: frequentist versus Bayesian estimation. Psychol Methods. 2013;18(2):186–219. [DOI] [PubMed] [Google Scholar]
- 33. Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25(38):8593–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith TT, Schaff MB, Rupprecht LE, et al. Effects of MAO inhibition and a combination of minor alkaloids, β-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16(3):199–202. [Google Scholar]
- 36. Gaalema DE, Miller ME, Tidey JW. Predicted impact of nicotine reduction on smokers with affective disorders. Tob Regul Sci. 2015;1(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.