Abstract
Introduction
Available in hundreds of device designs and thousands of flavors, electronic cigarette (ECIG) may have differing toxicant emission characteristics. This study assesses nicotine and carbonyl yields in the most popular brands in the U.S. market. These products included disposable, prefilled cartridge, and tank-based ECIGs.
Methods
Twenty-seven ECIG products of 10 brands were procured and their power outputs were measured. The e-liquids were characterized for pH, nicotine concentration, propylene glycol/vegetable glycerin (PG/VG) ratio, and water content. Aerosols were generated using a puffing machine and nicotine and carbonyls were, respectively, quantified using gas chromatograph and high-performance liquid chromatography. A multiregression model was used to interpret the data.
Results
Nicotine yields varied from 0.27 to 2.91 mg/15 puffs, a range corresponding to the nicotine yield of less than 1 to more than 3 combustible cigarettes. Nicotine yield was highly correlated with ECIG type and brand, liquid nicotine concentration, and PG/VG ratio, and to a lower significance with electrical power, but not with pH and water content. Carbonyls, including the carcinogen formaldehyde, were detected in all ECIG aerosols, with total carbonyl concentrations ranging from 3.72 to 48.85 µg/15 puffs. Unlike nicotine, carbonyl concentrations were mainly correlated with power.
Conclusion
In 15 puffs, some ECIG devices emit nicotine quantities that exceed those of tobacco cigarettes. Nicotine emissions vary widely across products but carbonyl emissions showed little variations. In spite of that ECIG users are exposed to toxicologically significant levels of carbonyl compounds, especially formaldehyde. Regression analysis showed the importance of design and e-liquid characteristics as determinants of nicotine and carbonyl emissions.
Implications
Periodic surveying of characteristics of ECIG products available in the marketplace is valuable for understanding population-wide changes in ECIG use patterns over time.
Introduction
Electronic cigarettes (ECIG) are gaining popularity around the globe.1–3 Their ever use among U.S. adults has increased from 2.5% in 2010 to 9.6% in 20134,5 and their use among youth has rapidly increased from 4.5% in 2013 to 13.4% in 2014.6,7 The possible role of ECIG in harm reduction7–13 has been contrasted with its possible role as a gateway to nicotine addiction in nicotine-naïve individuals, especially youth.14–16
This debate has been complicated by the continuing evolution of ECIG technology.17–19 For example, contradictory reports on the efficacy of ECIG nicotine delivery have appeared in the literature, likely due to differences between so-called first-generation and second-generation ECIG devices.20–23 Later reports showed that depending on the combination of the device design, power output, and the user puff topography, ECIG can deliver similar or higher levels of nicotine than tobacco cigarettes.22,24–30 In addition to a wide and evolving range of ECIG devices, the liquids continue to evolve, with more than 460 brands of ECIG with 7760 different flavors currently available in the market.31 Despite this evolution, discrepancies between measured nicotine content in the e-liquid and the advertised values have been observed.32–35
Besides nicotine, ECIG vaping produce other toxicants that may come from the thermal breakdown of e-liquid chemical components on the heated coil.36 One such class of toxicants is carbonyl compounds,37–41 that is correlated to pulmonary disease in tobacco smokers.42 Recent studies have found that carbonyl levels are affected by propylene glycol/vegetable glycerin (PG/VG) ratio, battery power output, and device type.43,44
Monitoring the evolution of tobacco cigarette during the last five decades helped scientists and public health agencies build evidence that the concept of “safer cigarette” was a tool to hinder cessation and increase appeal among youth.45,46 Thus, periodically surveying characteristics of ECIG products available in the marketplace is valuable for understanding population-wide changes in ECIG use patterns over time. In this study, we assessed liquid characteristics and aerosol emissions of 10 of the most popular brands of ECIG in the U.S. market. Different flavors and designs of popular commercial brands were selected to form a sample set of 27 products. Characteristics of the battery power, the design specifications and the e-liquid composition (PG/VG ratio, water and nicotine content and pH) were determined. Both the humectant composition (PG and VG) and water are hypothesized to affect the yield of the total particulate matter (TPM) while the nicotine content and the pH of the solution are considered major factors in defining the nicotine concentration and its distribution between free-base and protonated nicotine in the aerosol phase. The battery power output was found to have a direct effect on TPM, nicotine emission and degradation products such as carbonyl yields.44 Statistical analysis and correlations between the defined factors will give regulatory agencies deeper insights on what is in the market in order to implement evidence-based regulations. This approach can provide regulatory agencies with deeper insight on what is in the market in order to implement evidence-based regulations.47,48
Materials and Methods
Materials
The 10 most popular brands were identified using a systematic Internet- and social media-based ranking protocol that is described in another manuscript.49 Starter kits of disposable, prefilled, and tank ECIGs of the Apollo, Blu, Bull Smoke, Green Smoke, V2, Volt, Eversmoke, Halo, Volcano, and South Beach smoke brands were selected from each brand. The selection of ECIG characteristics like nicotine strength, flavor, or battery output were made based on the values recommended by the Web site, or if not available, the choice was guided by the highest number of user reviews for each characteristic. Tobacco and menthol were selected because they were universally available, allowing direct comparison of emissions across brands. The third flavor was selected as the most popular flavor other than tobacco and menthol on each brand’s Web site.
High-performance liquid chromatography grade toluene and ethyl acetate solvents were obtained from Sigma Aldrich. Pure nicotine (CAS registry number 54-11-5) was purchased from Acros Organics. N,O-Bis(trimethylsilyl)trifluoroacetamide (CAS registry number 25561-30-2) was purchased from Sigma Aldrich for PG/VG derivatization. Hexadecane (CAS registry number 544-76-3) and β-Citronellol (CAS registry number 106-22-9) procured from Sigma Aldrich, were used as internal standards in nicotine and PG/VG quantifications, respectively. Glass fiber filters (47 mm diameter) were purchased from Pall Corporation and used for particle phase trapping. High-purity silica adsorbent coated with 2,4-dinitrophenylhydrazine (LpDNPH) H Series Cartridges H10, volume size 3 mL, were purchased from Sigma Aldrich and used for gas phase trapping of carbonyls.
E-liquid Chemical Characterization
PG/VG Quantification
An aliquot of 2 µL of e-liquid was dissolved in 1 mL of ethyl acetate and sonicated for 30 min. Ten µL of 10-folds diluted solution was added to 50 µL of the derivatizing reagent N,O-Bis(trimethylsilyl)trifluoroacetamide in a Gas Chromatograph (GC) vial with insert (total volume was made 100 µL); β-citronellol was used as Internal standard at a concentration of 4 µg/mL. The vial was heated at 70°C for 30 min before being inserted into GC/MS for quantification against a calibration curve (2–25 µg/mL) that was built from lab-prepared solutions with different PG/VG ratios. Recoveries of PG/VG quantification were 90%–100% throughout the calibration curve range. The obtained spectra did not show the silyl ether peak at m/z = 118 in reference to the presence of ethanol.
Water Content Measurements
Water content in the e-liquid was determined using a Karl Fischer Titration. It is reported as percentage by volume of the total e-liquid. Quantification was done on a calibration curve of the range 1 to 22.9 mg of water.
Nicotine Quantification and Partitioning in E-liquid
Both free-base (Nic) and protonated nicotine (NicH+) content in the e-liquid were quantified following a reported extraction method.35 In brief, an aliquot of the e-liquid was immersed in 6 mL of water to form a diluted solution of 600 µg/mL concentration, and then 6 mL of toluene were added to extract free-base nicotine (Nic) from the aqueous phase. This step was repeated to ensure complete extraction of Nic. Two hundred μL of NaOH solution (1N) was later added to the aqueous layer to convert NicH+ in solution into Nic, before extracting twice with toluene as previously described for Nic fraction. In the first step of extraction, toluene showed a 90% extraction efficiency of Nic, in agreement with literature reports.35,50 Using the Hendersen–Haaselbach equation, the calculated dissociation factor of NicH+ in the remaining solutions of 10% Nic and total NicH+ was found to be <0.1%. Considering the initial conditions of nicotine in the sample set, re-equilibration between Nic and NicH+ in the sample extract introduces negligible measurement error.
Toluene extracts were diluted before injection into the Gas Chromatograph coupled to a Mass spectrometer (GC-MS) for analysis. Quantification was done using a calibration curve (50–1000 µg/mL) prepared from standard nicotine solutions.
pH Measurements
Depending on the label, 0.15–0.45 mL of e-liquid was added to deionized water in order to prepare an aqueous extract of 600 µg/mL nicotine concentration in a final volume of 6 mL. The pH of this extract was measured by a Starter 3100 OHAUS pH-meter.
Aerosol Characterization
Aerosol Generation and Sampling
A custom-designed digital puff production machine51 was used to generate ECIG aerosols.44 Puff topography (puff duration, interpuff interval, and flow rate) was selected to represent an experienced ECIG user (4 s puff duration and 10 s interpuff duration) with a puff velocity of 1.5 L/min.44,52,53,26 Each commercial sample was operated as purchased after 1 puff conditioning. TPM generated from a 15-puff session was collected on a glass fiber filter placed at the ECIG mouthpiece outlet. DNPH coated cartridges were placed downstream the filter pad in order to collect gas phase carbonyls. Each sample was tested in triplicates and results are shown as average of three measurements. TPM was determined gravimetrically by weighing the filter pad and the holder before and after each sampling session.
Nicotine Yield Quantification in Aerosols
Each filter was soaked in 6 mL of ethyl acetate and shaken for 30 min. The obtained solution was diluted prior to analysis by GC-MS. Nicotine was quantified against a calibration curve prepared from a range of standard solutions (1–25 µg/mL), which were spiked with an internal standard[hexadecane (5 ppm)]. Recoveries of the extraction from PG/VG matrix were higher than 90%.
Gas Chromatography-Mass Spectroscopy Conditions
The GC-MS analysis was performed on a Thermo-Finnigan Trace GC-Ultra Polaris ITQ 900 equipped with AS 3000 II autosampler. Separation was achieved with RTx-5MS (30 m × 0.25 mm × 0.25 µm film thickness) fused silica capillary column purchased from Restek. A splitless injection mode of 1 µL and He mobile phase of 1 mL/min flow rate were utilized. The injector temperature was set at 250°C.
For nicotine quantification, the oven temperature program was initiated at 70°C for 2 min, and then ramped at 20°C/min until reaching 230°C. The hold time at this high temperature was 1 min. Quantification was completed in the selected ion mode of m/z = 84 for nicotine and m/z = 57 for internal standard.
For PG/VG quantification, the oven temperature program was initiated at 40°C for 1 min, then ramped at 12°C/min until reaching 133°C, hold for 3 min, then ramped at 10°C/min until reaching 140°C, hold for 2 min, then ramped at 30°C/min until reaching 180°C, hold for 1 min, then ramped at 40°C/min until reaching 220°C, hold for 1 min. Quantification was completed in selected ion mode of m/z = 73, 147, and 81 for derivatized PG, VG, and β-citronellol, respectively.
Carbonyl Quantification
Carbonyls from the gas phase were trapped on DNPH cartridges, which were eluted with 5 mL of acetonitrile, filtered, and delivered into amber vials for high-performance liquid chromatography analysis. Recoveries ranged between 90% and 102%.
The analysis method was adopted from the California Air Resources Board (CARB) method (SOP MLD 022) as well as the Environmental Protection Agency method (TO-11A) (EPA, 1999), with some modifications in the time program of elution for better separation as detailed in Rashidi et al.54
The sample set was scanned for the presence of 12 carbonyls in the gas phase including formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, methacrolein, butyraldehyde, valeraldehyde, hexaldehyde, benzaldehyde, and tolualdehyde. A background measurement was obtained by passing air through an empty unactivated ECIG placed upstream the DNPH cartridge for a session of 15 puffs (4s puff duration). The obtained level of C1–C3 carbonyl levels is 0.67 µg/session.
Statistical Methods
Single factor analysis of variance was used to study the significance of the variability of nicotine yield between the different categories of ECIG tested (disposable, prefilled, and tanks). The multiple variable regression in SPSS was used to study the effect of different design and e-liquid characteristics on aerosol constituents. The ECIG type, brand, flavor, power, nicotine concentration, pH, PG/VG ratio, and water content were studied as possible variables affecting TPM and nicotine yield in aerosols as well as carbonyl emissions into the gas phase.
Results
E-liquid Chemical Characterization
Table 1 shows the measured characteristics of the different ECIGs in the selected commercial samples. The power output covered a range of 2.18–6.96 W with the average power of the tank type (6.41 ± 0.59 W) being higher than the respective average powers of disposable and prefilled cartridges (4.54 ± 1.23 and 4.80 ± 0.78 W). The solvent distribution of PG/VG in the e-liquids covered a whole range extending from 0/100 to 80/20. Interestingly, the water content which was found to be minimal in some brands reached as high as 35% by volume of the e-liquid in others. The e-liquid total nicotine concentration (sum of Nic and NicH+) measured a wide variation (7.11–20.90 mg/mL) and an average of 12.16 ± 4.11 mg/mL for the whole sample set. Interbrand and intrabrand variations in chemical and power characteristics were noted. Ethanol, that was reported to be present in e-liquid by other groups, was not detected in the studied sample set.55 Prefilled Halo Tribeca, for example, has higher water (20.41%), lower nicotine (7.37 mg/mL), and power (3.78 W) than the tank (3.19%, 14.02 mg/mL, and 6.50 W, respectively) of the same brand and flavor.
Table 1.
Listed is the Power Output, Total Nicotine Concentration, pH, PG/VG Ratio, and %Water Content in the E-liquid of Selected ECIGs Categorized by Type (Disposable, Prefilled, and Tank), Brand, and Flavor and the Corresponding Nicotine Yield and the Total Amount of C1–C3 Aldehydes in the Aerosol and Gas Phases, Respectively.
| Type | Brand | Flavor | Power (W) | PG/VG ratio (vol/vol) | % Water content | Total nicotine concentration (mg/mL) | pH | Nicotine (mg/session) | Total aldehydes (µg/session) |
|---|---|---|---|---|---|---|---|---|---|
| Disposable | Apollo | Classic tobacco | 5.24 | 58/42 | 28.80 ± 2.54 | 7.11 ± 0.09 | 5.3 ± 0.03 | 0.28 ± 0.02 | 6.37 ± 1.23 |
| Blu | Classic tobacco | 5.42 | 0/100 | 21.00 ± 0.63 | 9.83 ± 0.09 | 7.77 ± 0.15 | 0.56 ± 0.07 | 7.18 ± 0.76 | |
| Bull smoke | American ranger | 3.10 | 78/22 | 27.13 | 13.18 ± 0.15 | 7.81 ± 0.00 | 0.29 ± 0.10 | 3.77 ± 0.28 | |
| Green smoke | Absolute tobacco | 5.16 | 56/44 | 8.87 ± 0.47 | 13.40 ± 0.97 | 8.88 ± 0.07 | 1.00 ± 0.04 | 5.91 ± 0.20 | |
| Green smoke | Menthol ice | 5.16 | 56/44 | 13.44 | 13.04 | 9.06 | 1.02 ± 0.08 | 6.19 ± 0.32 | |
| V2 | Red tobacco | 2.18 | 76/24 | 18.54 ± 0.51 | 11.62 ± 0.26 | 8.80 ± 0.08 | 1.03 ± 0.05 | 3.06 ± 0.06 | |
| Volt | Country tobacco | 5.52 | 70/30 | 13.64 ± 1.10 | 19.48 ± 0.44 | 8.95 ± 0.11 | 0.97 ± 0.06 | 8.57 ± 1.40 | |
| Prefilled | Apollo | Tobacco | 5.06 | 76/24 | 11.66 ± 1.14 | 7.57 ± 0.74 | 8.51 ± 0.19 | 1.56 ± 0.09 | 11.53 ± 3.40 |
| Blu | Classic tobacco | 3.44 | 0/100 | 34.94 ± 3.37 | 8.79 ± 0.46 | 7.89 ± 0.06 | 0.27 ± 0.04 | 7.44 ± 1.48 | |
| Bull smoke | Menthol breeze | 5.27 | 67/33 | 6.04 ± 0.45 | 14.96 ± 0.61 | 9.26 ± 0.01 | 0.73 ± 0.08 | 8.36 ± 2.27 | |
| Bull smoke | Turkish tobacco | 5.58 | 80/20 | 21.58 ± 1.52 | 10.79 ± 0.60 | 8.57 ± 0.10 | 1.28 ± 0.09 | 7.11 ± 0.55 | |
| Eversmoke | Classic tobacco | 4.75 | 77/23 | 14.87 ± 2.33 | 12.62 ± 0.64 | 9.04 ± 0.04 | 1.07 ± 0.07 | 9.94 ± 0.96 | |
| Green smoke | Menthol ice | 4.38 | 56/44 | 5.26 ± 0.05 | 20.09 ± 0.54 | 9.16 ± 0.07 | 1.97 ± 0.09 | 8.27 ± 3.97 | |
| Green smoke | Red label tobacco | 4.52 | 57/43 | 6.28 ± 1.24 | 19.55 ± 1.36 | 9.05 ± 0.07 | 1.56 ± 0.03 | 9.38 ± 2.76 | |
| Halo | Tribeca | 3.78 | 68/32 | 20.41 ± 0.45 | 7.37 ± 0.22 | 8.18 ± 0.03 | 0.84 ± 0.10 | 3.90 ± 2.81 | |
| South Beach smoke | Classic tobacco | 4.44 | 78/22 | 9.99 ± 0.47 | 13.07 ± 0.16 | 8.90 ± 0.05 | 1.25 ± 0.08 | 8.21 ± 0.255 | |
| V2 | Green tea menthol | 5.63 | 75/25 | 8.74 ± 0.68 | 8.31 ± 0.24 | 8.78 ± 0.04 | 0.89 ± 0.05 | 45.07 ± 23.70 | |
| V2 | Red tobacco | 5.63 | 77/23 | 10.50 ± 2.66 | 7.45 ± 0.67 | 9.05 ± 0.06 | 1.03 ± 0.07 | 46.91 ± 54.22 | |
| Volcano | Menthol | 6.04 | 71/29 | 25.51 ± 2.51 | 7.20 ± 0.67 | 8.64 ± 0.02 | 0.58 ± 0.08 | 7.91 ± 2.36 | |
| Volt | RY4 | 3.89 | 70/30 | 8.89 ± 0.24 | 11.73 ± 0.56 | 7.99 ± 0.05 | 0.69 ± 0.06 | 5.15 ± 0.97 | |
| Tank | Apollo | Banana cream | 6.64 | 50/50 | 1.93 ± 0.10 | 9.28 ± 0.53 | 8.29 ± 0.02 | 0.96 ± 0.05 | 10.22 ± 1.50 |
| Apollo | Blueberry kona coffee | 6.96 | 58/42 | 7.45 ± 0.12 | 9.67 ± 0.71 | 8.41 ± 0.01 | 1.14 ± 0.17 | 6.68 ± 0.57 | |
| Apollo | Tobacco | 6.39 | 51/49 | 9.03 ± 1.30 | 8.11 ± 0.64 | 8.29 ± 0.01 | 0.86 ± 0.14 | 16.81 ± 17.71 | |
| Halo | Tribeca | 6.50 | 69/31 | 3.19 ± 0.15 | 14.02 ± 1.13 | 8.41 ± 0.02 | 1.57 ± 0.36 | 8.55 ± 2.7 | |
| South Beach smoke | Classic tobacco | 5.02 | 61/39 | 2.25 ± 0.21 | 14.54 ± 1.08 | 8.97 ± 0.07 | 1.21 ± 0.30 | 9.85 ± 4.24 | |
| Volcano | Blue water punch | 6.69 | 78/22 | 0.76 ± 0.08 | 20.90 ± 0.60 | 8.98 ± 0.02 | 2.91 ± 0.01 | 5.67 ± 0.52 | |
| Volt | 555 | 6.64 | 71/29 | 0.62 ± 0.07 | 14.60 ± 0.11 | 8.88 ± 0.00 | 1.05 ± 0.36 | 5.96 ± 0.04 |
PG = propylene glycol; VG = vegetable glycerin.
The pH of the e-liquid aqueous extract covered a range of 5.35–9.26 with an average of 8.51 ± 0.75. Using Handerson–Haselbach equation, calculated free-base concentrations based on measured pH shows a good correlation (69%) with experimentally quantified free-base nicotine in the e-liquid implicating that pH could be used to determine the extent at which nicotine is partitioned between its two forms (Nic and NicH+).
Aerosol Characterization
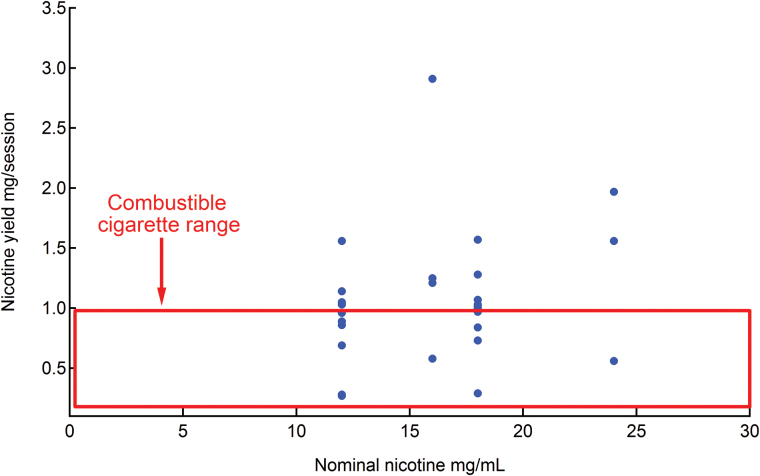
Masses of TPM collected from a vaping session of 15 puffs among the different samples are shown in Supplementary Table S1. A wide variation ranging from 29.37 to 169.00 mg was determined. The aerosol nicotine yield ranged between 0.27 and 2.91 mg/15 puffs with an average of 0.74 ± 0.33, 1.06 ± 0.45, and 1.39 ± 0.7 mg/session for disposable, prefilled, and tanks, respectively (statistically significant difference between the three types, p = 0.0005; Table 1). In general, prefilled and tank types delivered more nicotine to the aerosol than disposable ECIGs when vaped under the same puffing regimen. Plotting nicotine yield versus nominal nicotine concentration (Figure 1) showed that different ECIGs with the same nicotine label can yield different levels of nicotine in the aerosol. In addition, most brands delivered either similar or higher amounts of nicotine as tobacco cigarettes.56 In particular, the Green smoke prefilled with menthol ice flavor and the Volcano tank with blue-water punch flavor, respectively, delivered double (1.97 mg/session), and triple (2.91 mg/session) the maximum yield of conventional cigarette smoked under U.S. Federal Trade Commission protocol on a smoking machine (0.91 mg/cigarette).56,57
Figure 1.
Nicotine yield plotted against nominal nicotine content in e-liquid. The dots represent the average nicotine yield from a 15-puff session of 27 electronic cigarettes (ECIG) brands (n = 3). The rectangle shows the limits of the nicotine range in combustible tobacco cigarettes smoked under Federal Trade Commission (FTC) protocol.56
In order to highlight the efficacy of nicotine delivery from the selected ECIGs, nicotine mass fraction in the aerosol was plotted versus its corresponding fraction in the e-liquid (Supplementary Figure S1). The moderate correlation obtained (28%) highlighted the difference in efficacy between the studied ECIGs and is attributed to a combinatorial effect of all the design and e-liquid parameters (vide infra).
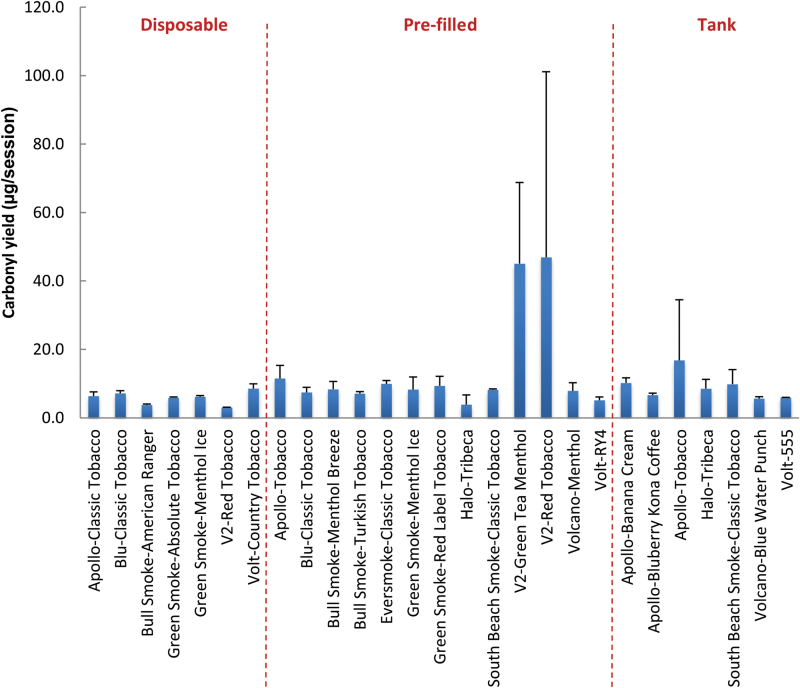
Carbonyl emission into the gas phase was also assessed for the whole sample set. High-molecular weight carbonyls (C4–C7) were not detected in all samples, thus only the sum of low-molecular weight carbonyls (C1–C3) levels collected during a 15-puff vaping session is shown in Figure 2. Individual concentrations of C1–C3 carbonyls (formaldehyde, acetaldehyde, acetone, acrolein, and propionaldehyde) are presented in Supplementary Table S1. The spread in carbonyl concentrations (3.06 and 48.85 µg/15 puffs) is mostly skewed by the high levels determined for two V2 prefilled cartridges. When removed, the average of the 25 sample set drops by 27% (7.68 ± 2.78). The overall average of the whole sample set of 10.52 ± 15.89 in tested ECIGs is much lower than that of conventional tobacco cigarettes.58,59
Figure 2.
Sum of gaseous carbonyl (C1–C3) yield from a 15-puff session of 27 electronic cigarettes (ECIG) brands.
Using multiple variables regression statistical analysis, ECIG design features, including type, brand, flavor, power output, and e-liquid characteristics, listed in Table 1, were scanned for possible contribution to TPM, nicotine, and carbonyl yields. It was found, as shown in Table 2, that power, PG/VG ratio, ECIG brand, type, and flavor are major factors that influence the amount of TPM emitted from the studied e-cigarettes. Similarly, the whole regression model for nicotine yield is highly significant (R2 = 0.89, and p of F value is less than 0.001), meaning that nicotine yield is affected by at least one of the studied parameters. Table 2 shows that nicotine yield is highly correlated with nicotine concentration in the e-liquid, PG/VG ratio, ECIG type, brand, and flavor (p = 0.001). Multiple variable regression also showed that only power and ECIG brand correlate with carbonyl yields (p value is <0.01 and <0.05, respectively). The overall model significance is lower in the case of carbonyls compared to nicotine and TPM.
Table 2.
Multiple Variable Regression Analysis of the Effects of the Different ECIG Design and E-liquid Characteristics on TPM, Nicotine, and Carbonyl Yields
| Variable | TPM (mg/15 puffs) | Nicotine (mg/15 session) | Total aldehydes (µg/session) | |||
|---|---|---|---|---|---|---|
| B | SE B | B | SE B | B | SE B | |
| ECIG type (cat) | *** | *** | ||||
| ECIG brand (cat) | *** | *** | * | |||
| Flavor (cat) | *** | *** | ||||
| Power (W) | 5.81** | 2.05 | 0.04 | 0.03 | 6.95** | 2.27 |
| PG (vol%) | 1.97*** | 0.43 | 0.04*** | 0.01 | −0.14 | 0.48 |
| Nicotine (mg/mL) | −0.26 | 0.78 | 0.06*** | 0.01 | −0.11 | 0.86 |
| pH | −1.12 | 3.78 | 0.10 | 0.06 | −0.19 | 4.18 |
| Water | −0.47 | 0.47 | −0.01 | 0.01 | 0.22 | 0.53 |
| R 2 | 0.86 | 0.89 | 0.40 | |||
| F | 17.86*** | 23.91*** | 1.99* | |||
Cat = categorical variable; ECIG = electronic cigarettes; PG = propylene glycol; TPM = total particulate matter. B: unstandardized regression coefficient, SE B: standard error of regression coefficient. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The current study examined the variability in e-liquid characteristics, power output, and emissions of nicotine and carbonyls among a representative batch of top brands of ECIGs in the U.S. market (total of 27 ECIGs). The results reflected interdifferences and intradifferences in ECIG types and brands. The average power output of the tested samples (5.15 ± 1.16 W) falls within the advertised “ideal range” for best vapor production60 and the nicotine content in e-liquid (7.11–20.90 mg/mL) agrees with the EU parliament regulation of 20 mg/mL as an upper limit of nicotine concentration in the e-liquid.61 Unlike tobacco cigarettes, which mostly show low pH and predominance of NicH+, the selected ECIGs measured a wide range of pH indicating variable Nic/NicH+ ratio in e-liquids. The good correlation (69%) between calculated (based on pH) and measured Nic implied that regulators could use pH of e-liquid aqueous extract as a rough estimation of nicotine distribution. Determining Nic fraction is important since it affects the bioavailability of nicotine in the human body.62
The significant correlation between TPM and ECIG type, brand, flavor, PG/VG ratio, and power is likely related to the effect of these parameters on the temperature and rate of evaporation. In agreement with previous reports,63 several ECIGs produced equal or higher nicotine yields than tobacco cigarettes (Figure 1). The significant correlation observed between aerosol nicotine yield and e-liquid nicotine content, ECIG type, brand, flavor, and PG/VG ratio is reported for the first time. The correlation between nicotine yield and power approached significance (p = 0.0594). As a result, some ECIGs could have relatively low-nicotine content but still emit higher nicotine yield per session than a combustible cigarette. For example, in the disposable category, V2-Red Tobacco and Volt-Country Tobacco have 11.6 and 19.5 mg/mL nicotine concentration, respectively, and their nicotine yield is very similar (~1 mg/session). In contrast, in the prefilled category, Apollo-Tobacco and Volcano-Menthol has similar nicotine concentrations (7.6 and 7.2 mg/mL) but yet very different nicotine yields in the aerosol (1.6 and 0.6 mg/session). Recent studies verified the significant correlation between aerosol nicotine yield from one hand and ECIG type, power output, and nicotine e-liquid concentration from the other.24,44,47 The combination between the discussed parameters (ECIG design and e-liquid characteristics) and user behavior was recently discussed in the context of a regulatory framework that suggests “nicotine flux” as a tool to assess the efficacy of ECIGs in nicotine delivery to the user body.64 Important to note that the question of how much of the inhaled nicotine is actually absorbed by the body is still debatable and understudy.65–68
In addition, regression coefficients showed that carbonyl emission is correlated with ECIG brand and power output. Unlike other reported studies,43 our results did not show a significant correlation between carbonyls in the aerosol and PG/VG ratio in the e-liquid. The formation of low carbonyls, which mainly derive from the degradation of PG and/or VG is expected to have little variability across the different ratios. The case where highest concentrations along with high standard deviations of carbonyl emissions were obtained (46.71 ± 23.74 and 48.85 ± 55.11 µg/15 puffs for Green tea menthol and red tobacco prefilled V2 ECIGs, respectively) is attributed to occasional dry puff coupled with a spike in the temperature.41 Notably, this study shows that while formaldehyde concentration in ECIG aerosols (0.58–5.05 mg/m3) is lower than that reported for cigarette smoke (4.6–148.9 mg/m3),69 the concentration is nonetheless higher than the endogenous formaldehyde concentration measured human breath (<0.5 mg/m3),70 and higher than the recommended short term 15 min exposure limit (REL) of 0.123 mg/m3 set by The National Institute for Occupational Safety and Health (NIOSH).71 Recent evidence also suggests that endogenous formaldehyde concentrations are sufficient to trigger tumor formation when cellular regulatory processes are compromised.70 It should be noted also that formaldehyde in ECIG aerosols is also present in the particle phase and so the exposure to formaldehyde by ECIG user is likely higher than what is reported in this study.72
These observations need to be confirmed by a systematic study approach including physical and chemical simulations in order to verify and better understand the effect of all ECIG designs and e-liquid properties on the vaporization process.
Conclusion
This snapshot in time of the most popular ECIG brands showed wide variability in power (2.18–6.96 W) and e-liquid chemical characteristics including pH (5.35–9.26), nicotine concentration (7.11–20.90 mg/mL), water content (0.6%–35%), and PG/VG ratio (0/100–80/20). Regression analysis shows significant correlation between ECIG type, brand, flavor, e-liquid nicotine content, and PG/VG ratio with aerosol nicotine yield, which was equal or higher than the nicotine yield in tobacco cigarette. Unlike nicotine yield, carbonyl emission showed limited variability across the sample set and was only correlated with ECIG brand and power output. Comparison of carbonyl emissions from ECIGs, especially formaldehyde, to NIOSH exposure limit showed that ECIG users are at considerable risk. This study emphasizes the fact that ECIGs are not a single product but rather a diverse product category that spans a wide range of performance. Accordingly, clinical investigations and observational studies of ECIG use must account for this diversity when assessing such variables as toxicant exposure, subjective effects, and potential utility as a smoking cessation aid.
Supplementary Material
Supplementary Table S1 can be found online at http://www.ntr.oxfordjournals.org
Supplementary Figure S1 can be found online at http://www.ntr.oxfordjournals.org
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declare.
Supplementary Material
References
- 1. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207–215. doi: http://dx.doi.org/10.1016/j.amepre.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pepper JK, Eissenberg T. Waterpipes and electronic cigarettes: increasing prevalence and expanding science. Chem Res Toxicol. 2014;27(8):1336–1343. doi: 10.1021/tx500200j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blake KD, Rutten LFJ, Grana RA, et al. Information exposure about e-cigarettes predicts reduced harm perceptions and e-cigarette use among adult smokers in the US. Tob Regul Sci. 2015;1(3):265–275. doi: 10.18001/TRS.1.3.8 [Google Scholar]
- 4. King B, Patel R, Nguyen K, Dube S. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nic Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nic Tob Res. 2014;17(10):1195–1202. doi: 10.1093/ntr/ntu213 [DOI] [PubMed] [Google Scholar]
- 6. McCarthy M. “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. BMJ. 2015;350:h2083. doi: 10.1136/bmj.h2083 [DOI] [PubMed] [Google Scholar]
- 7. Goniewicz ML, Gawron M, Nadolska J, Balwicki L, Sobczak A. Rise in electronic cigarette use among adolescents in Poland. J Adolesc Health. 2014;55(5):713–715. doi: http://dx.doi.org/10.1016/j.jadohealth.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 8. Mcgraw D. Current and future trends in electronic cigarette use. Int J Psychiatry Med. 2014;48(4):325–332. doi: 10.2190/PM.48.4.g [DOI] [PubMed] [Google Scholar]
- 9. Ramo DE, Young-Wolff KC, Prochaska JJ. Prevalence and correlates of electronic-cigarette use in young adults: findings from three studies over five years. Addict Behav. 2015;41:142–147. doi: http://dx.doi.org/10.1016/j.addbeh.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adriaens K, Gucht DV, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220–11248. doi:10.3390/ijerph111111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shahab L, Goniewicz M. Electronic cigarettes are at least as effective as nicotine patches for smoking cessation. Evid Based Med. 2014;19(4):133. doi: 10.1136/eb-2013–101690 [DOI] [PubMed] [Google Scholar]
- 12. Rüther T, Wissen F, Linhardt A, et al. Electronic cigarettes—attitudes and use in Germany. Nic Tob Res. 2015;18(5):660–669. doi: 10.1093/ntr/ntv188 [DOI] [PubMed] [Google Scholar]
- 13. Richards C, Eischen S, Joseph A, et al. A pilot product preference study of electronic cigarettes and nicotine gum as potential smoking cessation aids for African-American menthol smokers. Tob Regul Sci. 2015;1(3):243–253. doi: 10.18001/TRS.1.3.6 [Google Scholar]
- 14. Cooke A, Fergeson J, Bulkhi A, Casale TB. The electronic cigarette: the good, the bad, and the ugly. J Allergy Clin Immunol Pract. 2015;3(4):498–505. doi: http://dx.doi.org/10.1016/j.jaip.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 15. Fillon M. Electronic cigarettes may lead to nicotine addiction. J Natl Cancer Inst. 2015;107(3). doi: 10.1093/jnci/djv070 [DOI] [PubMed] [Google Scholar]
- 16. Schneider S, Diehl K. Vaping as a catalyst for smoking? An initial model on the initiation of electronic cigarette use and the transition to tobacco smoking among adolescents. Nic Tob Res. 2015;18(5):647–653. doi: 10.1093/ntr/ntv193 [DOI] [PubMed] [Google Scholar]
- 17. Lopez AA, Eissenberg T. Science and the evolving electronic cigarette. Prev Med. 2015;80:101–106. doi: http://dx.doi.org/10.1016/j.ypmed.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119. doi: 10.1186/s12916-015-0355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do?Ann N Y Acad Sci. 2016. doi: 10.1111/nyas.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567 [DOI] [PubMed] [Google Scholar]
- 22. Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nic Tob Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055–9965.epi-10–0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farsalinos KE, Spyrou A, Tsimopoulou K, et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramôa CP, Hiler MM, Spindle TR, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob Control. 2015;25(e1):e6–e9. doi: 10.1136/tobaccocontrol-2015–052447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nic Tob Res. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108(6):1115–1125. doi: 10.1111/add.12150 [DOI] [PubMed] [Google Scholar]
- 28. Farsalinos KE, Spyrou A, Stefopoulos C, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci Rep. 2015;5:11269. doi: 10.1038/srep11269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nic Tob Res. 2015;17(2):175–179. doi: 10.1093/ntr/ntu153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71(1):24–34. doi: http://dx.doi.org/10.1016/j.yrtph.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 31. Zhu S, Sun J, Bonnevie E, Cummins S, Gamst A, Yin L. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014–051670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S, Goniewicz M, Yu S, Kim B, Gupta R. Variations in label information and nicotine levels in electronic cigarette refill liquids in South Korea: regulation challenges. Int J Environ Res Public Health. 2015;12(5):4859–4868. doi: 10.3390/ijerph120504859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nic Tob Res. 2013;15(1):158–166. doi: 10.1093/ntr/nts103 [DOI] [PubMed] [Google Scholar]
- 34. Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the US, Korea, and Poland. Int J Drug Policy. 2015;26(6):583–588. doi: http://dx.doi.org/10.1016/j.drugpo.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El-Hellani A, El-Hage R, Baalbaki R, et al. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem Res Tox. 2015;28(8):1532–1537. doi: 10.1021/acs.chemrestox.5b00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hutzler C, Paschke M, Kruschinski S, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–1308. doi: 10.1007/s00204-014-1294-7 [DOI] [PubMed] [Google Scholar]
- 37. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012–050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bekki K, Uchiyama S, Ohta K, et al. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–11200. doi: 10.3390/ijerph111111192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talih S, Balhas Z, Salman R, Karaoghlanian N, Shihadeh A. “Direct Dripping”: a high-temperature, high-formaldehyde emission electronic cigarette use method. Nic Tob Res. 2016;18(4):453–459. doi: 10.1093/ntr/ntv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. doi:10.1056/NEJMc1413069 [DOI] [PubMed] [Google Scholar]
- 41. Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction. 2015;110(8):1352–1356. doi: 10.1111/add.12942 [DOI] [PubMed] [Google Scholar]
- 42. Talhout R, Schulz T, Florek E, et al. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–628. doi: 10.3390/ijerph8020613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors—effects of nicotine solvent and battery output voltage. Nic Tob Res. 2014;16(10):1319–1326. doi: 10.1093/ntr/ntu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nic Tob Res. 2015;17(2):150–157. doi: 10.1093/ntr/ntu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. The Changing Cigarette. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010. http://www.ncbi.nlm.nih.gov/books/NBK53013/#top. Accessed February 20, 2016. [PubMed] [Google Scholar]
- 46. How The Tobacco Industry Made Cigarettes Much Deadlier Than They Were 50 Years Ago. 2014. https://publichealthwatch.wordpress.com/2014/06/23/how-the-tobacco-industry-made-cigarettes-much-deadlier-than-they-were-50-years-ago/. Accessed January 29, 2016. [Google Scholar]
- 47. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500–507. doi: 10.1111/add.12410 [DOI] [PubMed] [Google Scholar]
- 48. Han S, Chen H, Zhang X, Liu T, Fu Yn. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nic Tob Res. 2015;18 (5):708–714. doi: 10.1093/ntr/ntv189 [DOI] [PubMed] [Google Scholar]
- 49. Malek N, Talih S, Lotfi T, et al. Design features of the most popular e-cigarette products available online identified using a prioritization protocol Submitted to Nic Tob Res 2016. [Google Scholar]
- 50. Badgett CO. Solvents for extracting nicotine from aqueous solutions. Ind Eng Chem. 1950;42(12):2530–2531. doi: 10.1021/ie50492a036 [Google Scholar]
- 51. Shihadeh A, Azar S. A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. J Aerosol Med. 2006;19(2):137–147. doi: 10.1089/jam.2006.19.137 [DOI] [PubMed] [Google Scholar]
- 52. Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011–050226 [DOI] [PubMed] [Google Scholar]
- 53. Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rashidi MA, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem Toxicol. 2008;46(11):3546–3549. doi: 10.1016/j.fct.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter J-F. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796–4815. doi: 10.3390/ijerph120504796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: evidence from a representative population survey. J Natl Cancer Inst. 2001;93(2):134–138. doi: 10.1093/jnci/93.2.134 [DOI] [PubMed] [Google Scholar]
- 57. National Institutes of Health (NIH)/National Cancer Institute (NCI) Monograph No. 7. The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of U.S. cigarettes. Report of the NCI Expert Committee. NIH Publ No. 96–4028. Bethesda, MD: NCI, NIH; 1996. http://cancercontrol.cancer.gov/brp/TCRB/monographs/7/. Accessed February 01, 2016. [Google Scholar]
- 58. Houlgate PR, Dhingra KS, Nash SJ, Evans WH. Determination of formaldehyde and acetaldehyde in mainstream cigarette smoke by high-performance liquid chromatography. Analyst. 1989;114(3):355–360. doi: 10.1039/AN9891400355 [DOI] [PubMed] [Google Scholar]
- 59. Miyake T, Shibamoto T. Quantitative analysis by gas chromatography of volatile carbonyl compounds in cigarette smoke. J Chromatogr A. 1995;693(2):376–381. doi: http://dx.doi.org/10.1016/0021-9673(94)01179-I [Google Scholar]
- 60. Variable Voltage and Vaping Power Chart Significance of Variable Voltage and Vaping Power Chart https://www.misthub.com/blogs/vape-tutorials/76788421-tutorial-variable-voltage-and-vaping-power-chart. Accessed February 22, 2016.
- 61. European Commission. Revision of the Tobacco Products Directive-Press Release. (2013). http://www.europarl.europa.eu/pdfs/news/expert/infopress/20131216IPR31001/20131216IPR31001_en.pdf. Accessed February 22, 2016. [Google Scholar]
- 62. Reininghaus W. Bioavailability of Nicotine, Research Center USA: Philip Morris; 1994. https://industrydocuments.library.ucsf.edu/tobacco/docs/gypk0093. Accessed February 05, 2016. [Google Scholar]
- 63. Etter J-F. Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. Addiction. 2016; 111(4):724–733. doi: 10.1111/add.13240 [DOI] [PubMed] [Google Scholar]
- 64. Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating “nicotine flux”. Nic Tob Res. 2015;17(2):158–162. doi: 10.1093/ntr/ntu175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. BMJ. 1980;280(6219):972–976. doi: 10.1136/bmj.280.6219.972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gori GB, Lynch CJ. Analytical cigarette yields as predictors of smoke bioavailability. Regul Toxicol Pharmacol. 1985;5(3):314–326. doi: http://dx.doi.org/10.1016/0273-2300(85)90045-5 [DOI] [PubMed] [Google Scholar]
- 67. Benowttz NL, Hall SM, Herning RI, Jacob P, 3rd, Jones RT, Osman A-L. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309(3):139–142. doi:10.1056/NEJM198307213090303 [DOI] [PubMed] [Google Scholar]
- 68. St Helen G, Havel C, Dempsey DA, Jacob P, 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–544. doi: 10.1111/add.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227. doi: http://dx.doi.org/10.1016/j.yrtph.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 70. Riess U, Tegtbur U, Fauck C, et al. Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOx in exhaled human breath. Anal Chim Acta. 2010;669(1–2):53–62. doi: http://dx.doi.org/10.1016/j.aca.2010.04.049 [DOI] [PubMed] [Google Scholar]
- 71. NIOSH. Formaldehyde: Evidence of Carcinogenicity http://www.cdc.gov/niosh/docs/81–111/. Accessed February 22, 2016.
- 72. Uchiyama S, Senoo Y, Hayashida H, et al. Determination of chemical compounds generated from second-generation e-cigarettes using a sorbent cartridge followed by a two-step elution method. Anal Sci. 2016;32(5):549–555. doi: 10.2116/analsci.32.549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.