Abstract
Introduction
Understanding how smokers perceive reduced nicotine content cigarettes will be important if the FDA and global regulatory agencies implement reduced nicotine product standards for cigarettes. Prior research has shown that some smokers incorrectly believe “light” cigarettes are less harmful than regular cigarettes. Similar misunderstandings of health risk could also apply to reduced nicotine cigarettes. To date, most studies of reduced nicotine cigarettes have blinded subjects to the nicotine content. Therefore, little is known about how smokers experience reduced nicotine content cigarettes when they are aware of the reduced content, and how use may be impacted.
Methods
The present study was a within-subjects experiment with 68 adult daily smokers who smoked two identical very low nicotine content Quest 3 (0.05 mg nicotine yield) cigarettes. Subjects were told that one cigarette contained “average” nicotine content, and the other contained “very low” nicotine content. After smoking each cigarette, subjects completed subjective measures about their smoking experience.
Results
Subjects rated the “very low” nicotine cigarette as less harmful to their health overall compared to the “average” nicotine cigarette; this effect held true for specific smoking-related diseases. Additionally, they rated the “very low” nicotine cigarette as having less desirable subjective effects than the “average” nicotine cigarette and predicted having greater interest in quitting smoking in the future if only the “very low” nicotine cigarette was available.
Conclusions
Explicit knowledge of very low nicotine content changes smokers’ perceptions of very low nicotine content cigarettes, resulting in reduced predicted harm, subjective ratings and predicted future use.
Implications
Before a reduced nicotine product standard for cigarettes can be implemented, it is important to understand how product information impacts how smokers think about and experience very low nicotine content cigarettes. Prior research has shown that smokers incorrectly believed light cigarettes were less harmful products. As such, smokers may also misunderstand the health risks associated with smoking very low nicotine content cigarettes. This study highlights the importance of smokers’ perceptions of nicotine content in cigarettes on the perceived health risks and the subjective effects of smoking very low nicotine content cigarettes.
Introduction
One possible regulatory strategy for reducing the harm caused by cigarettes is to implement a product standard requiring the reduction of nicotine content to make cigarettes less addictive.1–3 Theoretically, a reduced nicotine product standard could improve public health through various pathways, including: (1) decreasing youth uptake if fewer adolescents progress from experimentation to addiction; (2) increasing smoking cessation in current smokers; and (3) reducing smoke exposure in current smokers who are unable to quit.2 Several previous studies have explored the effect of extended use of very low nicotine content (VLNC) cigarettes in current smokers, and their results generally support the notion that a nicotine reduction policy might decrease smoking. 4–10
Despite the aforementioned possible positive outcomes of a reduced nicotine product standard, unintended negative consequences might arise, which should be closely evaluated. For example, nicotine reduction could change smokers’ beliefs about the health risks associated with smoking. Previous double-blind studies have shown that smokers rate cigarettes with greatly reduced nicotine content as having fewer health risks than cigarettes with relatively normal nicotine content,11 indicating that “actual” nicotine content influences risk perception (perhaps through some sensory experience, though this is not known for sure). Less is known about how knowledge of the reduced nicotine content might affect risk perception. One study investigating the effect of advertisement features on risk beliefs found that when smokers naïve to Quest brand cigarettes (previously commercially available in three reduced nicotine levels: 0.6 mg, 0.3 mg and 0.05 mg nicotine yield) viewed a Quest cigarette advertisement that emphasized nicotine-free smoking, respondents incorrectly believed the product would be less harmful than their own cigarettes.12 Other studies have shown that some smokers have an inaccurate understanding about nicotine’s impact on smoking-related diseases (eg, the belief that nicotine causes lung cancer).12–15 If a new product standard was to be implemented, smokers would likely be aware of the nicotine reduction and some may incorrectly reason that VLNC cigarettes have significantly fewer direct health consequences, which could negatively impact use. Indeed, research on “light” cigarettes suggests that smokers perceive them as less harmful.16–18 However, since both nicotine and tar yields are lower in “light” cigarettes, it is unclear which factor is responsible for these misconceptions.
Previous research has established that nicotine content expectancies influence numerous subjective effects of smoking. For example, nicotine content expectancies significantly influence craving reduction, mood, wakefulness, calmness, concentration, satisfaction from smoking, and hunger reduction.19–23 Other studies have found significant influences of nicotine content expectancies for other nicotine delivery products such as sprays and inhalers on satisfaction and craving relief.24–26 These studies suggest the amount of nicotine that smokers believe is in a product can supersede their experience of receiving a different nicotine amount even at relatively high doses. These beliefs about nicotine content may influence the subjective experience of smoking and the perception of risk, which likely influences smoking behavior.27
The present study investigated the effect of a very low nicotine content expectancy on health risk perceptions and subjective effects of smoking cigarettes with actual low nicotine content. It was predicted that the “very low” nicotine cigarette would be perceived as less risky for contributing to various smoking-related diseases and rated as less subjectively desirable than the “average” nicotine cigarette (though both cigarettes were actually identical in nicotine content). Additionally, we explored whether the expectations of low nicotine content were associated with interest in quitting in the future.
Methods
Participants
Daily smokers aged 18 and older who smoked at least 10 cigarettes per day for the past year were recruited from the Pittsburgh community. An expired carbon monoxide level of at least 8 ppm (or urine cotinine level > 100 ng/m) was required for study enrollment. Exclusion criteria included significant medical changes in the previous week, currently seeking treatment to quit smoking, alcohol intoxication at the time of the visit, and pregnancy/breastfeeding.
Procedures
Following an initial phone screen, all eligible volunteers providing informed consent and completed a battery of baseline questionnaires. To standardize time since last cigarette, participants smoked four puffs of their usual brand cigarette through a smoking topography device and subsequently answered questions about their usual brand smoking experience.
Next, participants smoked two identical study cigarettes that differed only in the description of nicotine content (as described below). Order of nicotine content expectancy (ie, “average” nicotine cigarette or “very low” nicotine cigarette) was randomly assigned and stratified by gender. The cigarettes were separated by 45 minutes to avoid satiation. To assess possible changes in smoking behavior, the cigarettes were smoked through handheld smoking topography devices (CReSS Micro, Borgwaldt, KC). Both study cigarettes were 0.05 mg nicotine yield (Quest 3) and were matched to the participant’s menthol preference. The Quest logo on the cigarette was obscured by permanent marker, so that participants remained blind to the brand. Staff was blind to the order of the expectancy conditions for each subject. After the research assistant left the room, the cigarette description for each condition appeared on the computer screen for 30 seconds and a recorded voiceover read the text aloud to ensure attention to the information. The text was as follows:
“The next cigarette that you will be smoking contains a very low/average nicotine level, compared to most cigarettes available in the United States. First, you will smoke as much or as little of this cigarette as you would like to smoke. Then, you will be asked to answer some questions about your opinions of the product.”
The next slide instructed the participant to take the corresponding study cigarette from a large manila envelope (inside were two smaller envelopes labeled “very low nicotine cigarette” and “average nicotine cigarette”). Single cigarettes, rather than whole cigarette packs, were in the smaller envelopes to maintain the product blind. Participants were instructed not to discuss the nicotine content of each cigarette with the research assistant and to put the materials away before the staff returned. Next, participants were told to place the cigarette into the puff topography device, light it, and smoke as much as desired. Cigarette rating measures were completed in reference to the study cigarette just smoked. This process was repeated for both study cigarettes.
Assessments
Demographic and smoking variables included age, gender, race, cigarettes per day, years of daily smoking, dependence (Fagerstrom Test for Nicotine Dependence, “FTND”28), and menthol preference.
The Perceived Health Risk Scale (“PHRS”6,29) assessed smokers’ perceived risk for developing smoking-related health problems associated with each cigarette. Participants were instructed to assume that they would maintain their current rate of smoking while rating their health risk perceptions of each cigarette. The measure includes eight items (lung cancer, emphysema, chronic bronchitis, other cancers, heart disease, stroke, overall health risk, and risk of addiction) for which participants responded on a 1–100 visual analog scale (“very low risk” to “very high risk”).
A modified version of the Cigarette Evaluation Scale (“mCES”30,31) was used to measure subjective cigarette effects. The “mCES” includes 15 items for which participants responded on a 7-point Likert scale to report how much they agree with each statement (not at all to extremely). Cigarette effects measured include satisfaction, taste, enjoyment of sensations in throat/chest, harshness, strength, flavor, calming, awakening, less irritable, help concentrating, hunger reduction, dizzying, nauseating, craving reduction, and enjoyment. Five reliable factors can be derived including satisfaction, psychological reward, aversion, craving reduction, and enjoyment of respiratory tract sensations.31 An additional item was included to assess perceived level of nicotine in each cigarette. Smokers were asked, “How does the nicotine content of the study cigarette differ from your usual brand? Please answer on the following scale from 1–100, and imagine that your usual brand is 50.” The visual analog scale was anchored on the ends by “much less nicotine” and “much more nicotine.”
The Future Smoking Survey is a novel questionnaire created to assess how hypothetical exclusive availability of each type of cigarette would influence predicted smoking rate and interest in quitting smoking in the future. This assessment was designed to capture what participants believe they would do in a regulated marketplace (ie, if the FDA were to enact a low nicotine product standard). Participants were asked to rate on a 1–100 visual analog scale how interested they would be in quitting (not at all interested to definitely interested) at four future time points (1 month, 6 months, 1 year, and 5 years) if the cigarette they had just smoked was the only type of cigarette available to purchase. Participants were also asked to predict how many cigarettes per day they would be smoking at each of those time points.
Statistical Methods
Descriptive statistics were used to characterize the sample’s demographic and smoking history variables as well as ratings of the usual brand cigarette. No statistical comparisons were made between the usual brand cigarette responses and study cigarette responses because usual brand cigarettes were always smoked first. Paired-samples t tests were used to compare normally distributed dependent variables across the two experimental conditions. Repeated measures ANOVA was used to test interactions between nicotine content expectancy and between-subjects variables including order of study conditions, gender, and menthol preference when appropriate. There were no significant interactions between nicotine content expectancy and order of study conditions for any of the measures. Non-normally distributed dependent variables were analyzed using the Wilcoxon matched pairs test. The McNemar’s test was used to assess differences in proportions.
In addition, some participants may have misunderstood the wording of the questions on the Future Smoking Survey assessing the predicted number of cigarettes smoked per day at four future time points. Responses in the thousands suggested that participants may have mistakenly thought the question was asking the total number of cigarettes smoked over the time period assessed rather than estimating the average number of cigarettes per day they would smoke. To rectify this issue, any response over 100 cigarettes per day was excluded from analyses. Furthermore, many participants predicted smoking zero cigarettes per day in the future (at all four time points) if only these study cigarettes were available. Thus, two processes were explored with regard to the predicted number of cigarettes smoked per day data. First, the percentage of zero cigarettes per day responses was compared across study cigarette types at each future time point using a McNemar’s Test. This captured the percentage of participants that predicted being abstinent. Secondly, the zero responses were removed and the remaining responses (which were adequately normally distributed) were tested using a paired-samples t test to explore differences in predicted cigarettes per day among participants who did not predict being abstinent.
Results
Seventy-one participants completed the study. However, three participants were excluded from analyses because they were unblinded, smoked both cigarettes during the same session or indicated smoking two “very low” nicotine cigarettes. The final sample consisted of 68 individuals (38 males, 30 females) between the ages of 19–65 years (M = 40.37, SD = 13.05). The mean number of cigarettes smoked per day was 16.53 (SD = 4.76) and years of daily smoking was 21.95 years (SD = 12.7). Sixty-six percent of the sample smoked mentholated cigarettes. The mean total score on the “FTND” was 5.85 (SD = 1.60, range = 2–9). There were no significant differences between expectancy conditions in smoking topography including total puff count, total puff volume, and interpuff interval.
The “very low” nicotine cigarette was rated as having significantly lower nicotine content (M = 23.56, SD = 23.54) than the “average” nicotine cigarette (M = 40.19, SD = 23.91), t(67) = −5.09, p < .001. However, 12 participants rated the “average” nicotine cigarette as having lower nicotine content than the “very low” nicotine cigarette, and nine participants rated the study cigarettes equally. All analyses presented below included the full sample (ie, regardless of nicotine content estimates); secondary analyses, not reported here, that focused on just those individuals (N = 47) who reported nicotine content in the expected direction (“very low” nicotine cigarette less than “average” nicotine cigarette) confirmed the reported findings.
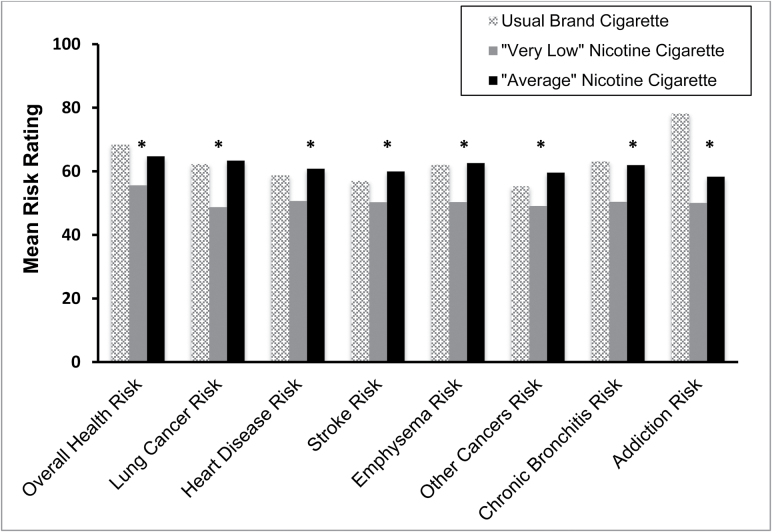
Smokers rated the “very low” nicotine cigarette as less risky to their health overall compared to the “average” nicotine cigarette [t(67) = −3.318, p = .001]. This effect held true for all individual disease risks assessed including lung cancer [t(67) = −4.635, p ≤ .001], heart disease [t(67) = −3.953, p < .001], emphysema [t(67) = −4.521, p < .001], stroke [t(67) = −3.738, p < .001], chronic bronchitis [t(67) = −4.001, p < .001], and other cancers [t(67) = −3.870, p < .001] (Figure 1). Participants also rated the “very low” nicotine cigarette as having a lower addiction risk than the “average” nicotine cigarette [t(67) = −2.647, p = .01]. There were no significant interactions between nicotine content expectancy and gender or menthol preference for any of the perceived health risks.
Figure 1.
Ratings for all perceived health risks for usual brand cigarette and both study cigarettes. *p ≤ .01 for “very low nicotine” versus “average” nicotine comparison. Usual brand cigarette ratings are included for reference but were not included in the analyses.
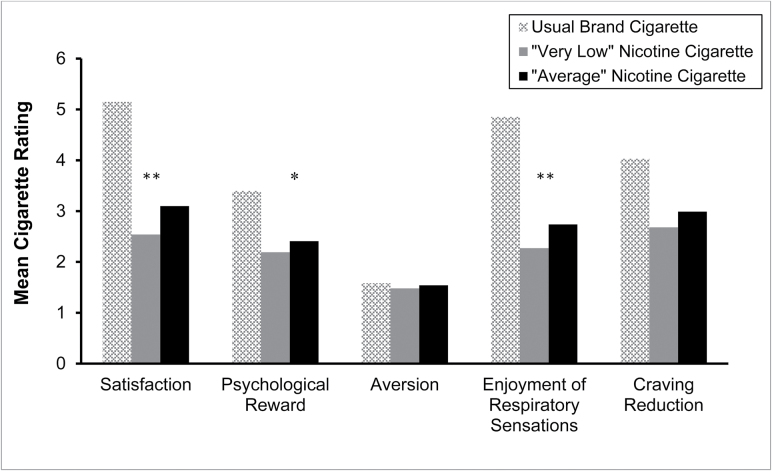
Smokers also rated the “very low” nicotine cigarette as having reduced enjoyment satisfaction [t(67) = −3.481, p = .001], psychological reward [t(67) = −2.330, p = .023], and enjoyment from respiratory sensations [t(67) = −2.913, p = .005] compared to the “average” nicotine cigarette (Figure 2). The nicotine content expectancy did not have a significant effect on the aversion or craving reduction factors. There was a significant interaction between nicotine content expectancy and gender on enjoyment of respiratory tract sensations [F(1, 66) = 4.361, p = .041], such that when males were told the study cigarette contained “average” nicotine they reported greater enjoyment of respiratory tract sensations compared to when they were told the study cigarette contained “very low” nicotine and compared to females in both study conditions. A similar expectancy by gender interaction effect was marginal for psychological reward [F(1, 66) = 3.234, p = .08] and craving reduction [F(1, 66) = 1.492, p = .07]. There were no significant interactions between nicotine content expectancy and menthol preference for any of the subjective effects factors.
Figure 2.
Results from the five factors of the cigarette evaluation scale for usual brand cigarette and both study cigarettes. *p < .05, **p < .01 for “very low nicotine” versus “average nicotine” comparison. Usual brand cigarette ratings are included for reference but were not included in the analyses.
Participants predicted greater interest in quitting smoking when considering a future in which they could only purchase the “very low” nicotine cigarette compared to the “average” nicotine cigarette at 1 month (Z = −2.496, p = .013), 6 months (Z = −2.442, p = .015), and 1 year (Z = −2.636, p = .008) (Table 1). This effect was marginal at 5 years (Z = −1.794, p = .073). Significantly more participants predicted being abstinent in 1 month and 5 years when considering exclusive availability of the “very low” nicotine cigarette compared to the “average” nicotine cigarette (Table 1). Nicotine content expectancy did not significantly impact the predicted number of cigarettes smoked per day among those who indicated they would continue to smoke.
Table 1.
Results From the Future Smoking Survey for Usual Brand Cigarette and Both Study Cigarettes
| Usual brand cigarette | “Very low nicotine” cigarette | “Average nicotine” cigarette | |
|---|---|---|---|
| Predicted quit interest | Median (range) | Median (range) | Median (range) |
| 1 mon | 14 (1–77) | 53.5 (1–100)* | 50 (1–100) |
| 6 mon | 20.5 (1–87) | 65 (1–100)* | 52 (1–100) |
| 1 y | 27.5 (1–100) | 70 (1–100)** | 60 (1–100) |
| 5 y | 41 (1–100) | 83 (1–100) | 64 (1–100) |
| Predicted abstinence | % Predicting zero CPD | % Predicting zero CPD | % Predicting zero CPD |
| 1 mon | 0% | 14.7%* | 4.4% |
| 6 mon | 0% | 17.9% | 9.0% |
| 1 y | 0% | 21.9% | 12.5% |
| 5 y | 5.9% | 32.8%* | 20.3% |
Participants rated their predicted quit interest at four time points on a 1–100 visual analog scale. Data were not normally distributed for predicted quit interest, so median and range values are presented. Percentage of participants that predicted abstinence (smoking zero cigarettes per day) at four time points. Usual brand cigarette values are displayed for reference but were not included in analyses.
*p < .05; **p < .01.
Discussion
Independent of true nicotine content, when smokers were told a cigarette had low nicotine levels, they reported it was less harmful than a cigarette with an average amount of nicotine. Because this finding was consistent across a range of disease types and participants were instructed to assume a constant rate of smoking, it suggests this is a general misconception about how nicotine contributes to the development of smoking-related diseases. Similar to how people viewed “light” cigarettes as a healthier alternative to full flavor cigarettes,18 smokers could misinterpret information about nicotine content and perceive VLNC cigarettes as a safer option less likely to cause harm. In light of the potential for a reduced nicotine product standard, policymakers should consider methods to mitigate this health risk misperception. Health communication campaigns reminding the public that all cigarettes, including VLNC cigarettes, are detrimental to their health should be conducted in conjunction with any policy change. Explaining that reducing nicotine could decrease the addictiveness of cigarettes but that the health risks remain similar to normal nicotine content cigarettes should be clearly conveyed to the public.
When smokers were informed that the study cigarette contained “very low” nicotine they also reported significantly fewer desirable subjective effects than when they were told it contained “average” nicotine. These findings are consistent with prior nicotine expectancy research20,24 and are important for determining how best to frame a reduced nicotine product standard. Because smokers will receive information about potential changes in nicotine content from various sources (packaging, advertisements, word of mouth, etc.), health communication experts will need to consider how to appropriately describe VLNC cigarettes to the public. In this study, simply changing the instructions from “average” nicotine to “very low” nicotine influenced how satisfying subjects found the cigarettes and their predicted likelihood of using the product in the future. If “very low” nicotine cigarettes are perceived by smokers to be less satisfying, then this type of product description could promote greater interest in quitting. However, the perception that VLNC cigarettes are less desirable could also encourage smokers to seek alternative products. If smokers persist in using high nicotine, combusted tobacco products, for example by accessing black market cigarettes or adding nicotine to VLNC cigarettes, the potential positive impact on smoking behavior could be attenuated. A study examining the use of VLNC cigarettes for 6 weeks prior to a quit attempt found that as biomarkers of nicotine exposure increased, likely due to concurrent use of non-study (ie, normal nicotine) and VLNC cigarettes, abstinence rates decreased.32 Conversely, if current smokers who do not become abstinent switch to using non-combusted tobacco products instead of smoking, they would likely dramatically reduce their toxicant exposure.33 Developing language to describe VLNC cigarettes that maximizes abstinence from all combusted tobacco will be an important goal for health communication experts.
Despite being perceived as less harmful, participants predicted a greater interest in quitting smoking in the future if only “very low” nicotine content cigarettes were available to purchase. Because they rated the cigarettes as less satisfactory prior to predicting their future behavior, the subjective effects rather than the perceived harmfulness of the cigarette may have had a greater impact in predicting their long term smoking behavior.
This study has several limitations. First, participants sampled the study cigarettes through smoking topography devices, which could have influenced ratings of the cigarettes. Second, the effects of a very low nicotine content expectancy on perceptions could be short-lived; future studies should measure the dependent variables repeatedly as perceptions may change with repeated exposure to the products. Third, it is possible that unmeasured characteristics of the participants’ usual brand cigarettes (eg, degree of ventilation) could have moderated the impact of the expectancies. Fourth, participants were asked to predict their future behavior over the next 5 years; smokers are unlikely to be very accurate with their predictions, hence this measure only captures intentions, not actual behavior. Fifth, individual cigarettes were provided to participants with no access to cigarette packs. Therefore, this study did not address how packaging and labeling affect perceptions related to reduced nicotine cigarettes. Future research studies assessing the impact of VLNC cigarette packaging and labeling will be necessary to address this issue. Finally, future studies should utilize factorial designs to examine interactions between “actual” nicotine content and “perceived” nicotine content on smokers’ perceptions of reduced nicotine cigarettes, which the present study did not capture.
In conclusion, the present study illustrates how important smokers’ perceptions of nicotine content in cigarettes are on their subjective smoking ratings as well as their comprehension of the health risks associated with the product. Previous literature suggests that a very low nicotine product standard for cigarettes could have a large beneficial public health impact.2,34 Understanding how smokers perceive very low nicotine content cigarettes is important for maximizing the public health impact of regulated reductions in the nicotine content of combusted tobacco products while minimizing unintended consequences.
Funding
This work was supported by research development funds from the University of Pittsburgh and a grant from the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659). The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration.
Declaration of Interests
None declared.
Acknowledgments
The authors would also like to thank Erin Goldstein, Lee Bennett, Cathy Scott, and Kristin Yahner for their assistance in data collection as well Sarah Dermody for her input on study design.
References
- 1. Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(suppl 1):i14–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hatsukami DK, Perkins KA, Lesage MG, et al. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19(5):e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 5. Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–2485. [DOI] [PubMed] [Google Scholar]
- 6. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond D, O’Connor RJ. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomarkers Prev. 2014;23(10):2032–2040. [DOI] [PubMed] [Google Scholar]
- 9. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mercincavage M, Souprountchouk V, Tang KZ, et al. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strasser AA, Tang KZ, Tuller MD, Cappella JN. PREP advertisement features affect smokers’ beliefs regarding potential harm. Tob Control. 2008;17(suppl 1):i32–i38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings KM, Hyland A, Bansal MA, Giovino GA. What do Marlboro Lights smokers know about low-tar cigarettes? Nicotine Tob Res. 2004;6(suppl 3):S323–S332. [DOI] [PubMed] [Google Scholar]
- 14. Shadel WG, Lerman C, Cappella J, Strasser AA, Pinto A, Hornik R. Evaluating smokers’ reactions to advertising for new lower nicotine quest cigarettes. Psychol Addict Behav. 2006;20(1):80–84. [DOI] [PubMed] [Google Scholar]
- 15. Bansal-Travers M, Cummings KM, Hyland A, Brown A, Celestino P. Educating smokers about their cigarettes and nicotine medications. Health Educ Res. 2010;25(4):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Etter JF, Kozlowski LT, Perneger TV. What smokers believe about light and ultralight cigarettes. Prev Med. 2003;36(1):92–98. [DOI] [PubMed] [Google Scholar]
- 17. Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers’ beliefs about light and ultra light cigarettes. Tob Control. 2001;10(suppl 1):i17–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kozlowski LT, Goldberg ME, Yost BA, White EL, Sweeney CT, Pillitteri JL. Smokers’ misperceptions of light and ultra-light cigarettes may keep them smoking. Am J Prev Med. 1998;15(1):9–16. [DOI] [PubMed] [Google Scholar]
- 19. Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol. 2002;111(1):88–97. [DOI] [PubMed] [Google Scholar]
- 20. Juliano LM, Fucito LM, Harrell PT. The influence of nicotine dose and nicotine dose expectancy on the cognitive and subjective effects of cigarette smoking. Exp Clin Psychopharmacol. 2011;19(2):105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelemen WL, Kaighobadi F. Expectancy and pharmacology influence the subjective effects of nicotine in a balanced-placebo design. Exp Clin Psychopharmacol. 2007;15(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perkins KA, Jacobs L, Clark L, Conklin CA, Sayette M, Wilson A. Instructions about nicotine dose influence acute responses to nasal spray. Nicotine Tob Res. 2004;6(6):1051–1060. [DOI] [PubMed] [Google Scholar]
- 23. Mercincavage M, Smyth JM, Strasser AA, Branstetter SA. Reduced nicotine content expectancies affect initial responses to smoking. Tob Regul Sci. 2016;2(4):309–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkins KA, Jacobs L, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. The influence of instructions and nicotine dose on the subjective and reinforcing effects of smoking. Exp Clin Psychopharmacol. 2004;12(2):91–101. [DOI] [PubMed] [Google Scholar]
- 25. Perkins KA, Grottenthaler A, Ciccocioppo MM, Conklin CA, Sayette MA, Wilson AS. Mood, nicotine, and dose expectancy effects on acute responses to nicotine spray. Nicotine Tob Res. 2009;11(5): 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darredeau C, Barrett SP. The role of nicotine content information in smokers’ subjective responses to nicotine and placebo inhalers. Hum Psychopharmacol. 2010;25(7–8):577–581. [DOI] [PubMed] [Google Scholar]
- 27. Van der Pligt J. Perceived risk and vulnerability as predictors of precautionary behaviour. British J Health Psychol. 1998;3(1):1–14. [Google Scholar]
- 28. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 29. Hatsukami DK, Vogel RI, Severson HH, Jensen JA, O’Connor RJ. Perceived health risks of snus and medicinal nicotine products. Nicotine Tob Res. 2015;5(18):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: is smoking still satisfying. Clin Res. 1992;40(4):A871. [Google Scholar]
- 31. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 32. Dermody SS, Donny EC, Hertsgaard LA, Hatsukami DK. Greater reductions in nicotine exposure while smoking very low nicotine content cigarettes predict smoking cessation. Tob Control. 2015;24(6): 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game [published online ahead of print July 1, 2016]. Addiction. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tengs TO, Ahmad S, Savage JM, Moore R, Gage E. The AMA proposal to mandate nicotine reduction in cigarettes: a simulation of the population health impacts. Prev Med. 2005;40(2):170–180. [DOI] [PubMed] [Google Scholar]