Abstract
A mandated reduction in the nicotine content of cigarettes may improve public health by reducing the prevalence of smoking. Animal self-administration research is an important complement to clinical research on nicotine reduction. It can fill research gaps that may be difficult to address with clinical research, guide clinical researchers about variables that are likely to be important in their own research, and provide policy makers with converging evidence between clinical and preclinical studies about the potential impact of a nicotine reduction policy. Convergence between clinical and preclinical research is important, given the ease with which clinical trial participants can access nonstudy tobacco products in the current marketplace. Herein, we review contributions of preclinical animal research, with a focus on rodent self-administration, to the science of nicotine reduction. Throughout this review, we highlight areas where clinical and preclinical research converge and areas where the two differ. Preclinical research has provided data on many important topics such as the threshold for nicotine reinforcement, the likelihood of compensation, moderators of the impact of nicotine reduction, the impact of environmental stimuli on nicotine reduction, the impact of nonnicotine cigarette smoke constituents on nicotine reduction, and the impact of nicotine reduction on vulnerable populations. Special attention is paid to current research gaps including the dramatic rise in alternative tobacco products, including electronic nicotine delivery systems (ie, e-cigarettes). The evidence reviewed here will be critical for policy makers as well as clinical researchers interested in nicotine reduction.
Implications
This review will provide policy makers and clinical researchers interested in nicotine reduction with an overview of the preclinical animal research conducted on nicotine reduction and the regulatory implications of that research. The review also highlights the utility of preclinical research for research questions related to nicotine reduction.
Nicotine Reduction as a Tobacco Control Strategy
Although the prevalence of smoking has declined since the first US Surgeon General’s report on smoking in 1964, tobacco use continues to be a major public health problem. In the United States, nearly one in six adults reports smoking cigarettes, and approximately 480000 deaths each year are attributed to tobacco use.1,2 Thus, reducing the use of combustible tobacco must be a top priority to improve public health. Nicotine is the primary addictive component of cigarettes, and one possible strategy to reduce the health burden of tobacco use would be to enact a mandated reduction in the nicotine content of cigarettes. In the United States, The Family Smoking Prevention and Tobacco Control Act gives the Food and Drug Administration (FDA) regulatory authority over tobacco, including the ability to regulate the content of cigarettes.3 Worldwide, more than 170 countries have ratified the World Health Organization Framework Convention on Tobacco Control, and Article 9 specifically discusses the regulation of the content and emissions of tobacco products.
Over 20 years ago, Drs Benowitz and Henningfield hypothesized that there is a unit dose of nicotine per cigarette that is required to maintain addiction.4 Based on the nicotine intake of a group of smokers who smoked fewer cigarettes per day and often did not meet the criteria for dependence, they predicted the necessary amount of nicotine per cigarette needed to sustain smoking is between 0.2 and 0.3 mg nicotine/g tobacco. If true, a public health policy requiring all cigarettes to have nicotine levels below this threshold could result in a reduction in nicotine dependence and a dramatic decline in the prevalence of smoking.
Recent clinical data have supported the idea that a mandated reduction in the nicotine content of cigarettes would improve public health. When smokers experience a drastic reduction in cigarette nicotine content, nicotine intake is reduced with no evidence of sustained withdrawal.5 In some trials, use of very low nicotine content (VLNC) cigarettes has resulted in a reduction in the number of cigarettes smoked per day,5–8 a reduction in nicotine dependence,5,7,8 and an increase in quit attempts.5 In a recent multisite clinical trial by Donny and colleagues,5 839 smokers were randomly assigned to smoke cigarettes with one of five different nicotine contents or their usual brand cigarette. After 6 weeks, participants assigned to the lowest nicotine content group (0.4 mg nicotine/g tobacco) smoked fewer cigarettes per day, scored lower on measures of nicotine dependence, and were more likely to make a quit attempt following the study than participants in the control group. Together, these clinical trials provide critical empirical evidence supporting the regulation of nicotine content within cigarettes.
The Role of Rodent Self-Administration Research in Tobacco Regulatory Policy
Animal self-administration research, in which an animal is given the opportunity to respond to elicit drug delivery, is an essential complement to clinical studies on the science of nicotine reduction because of limitations associated with clinical research. The vast majority of this research has been conducted in rodents, most often with rats. Intravenous self-administration is often considered the gold standard test for abuse liability because of its clear face validity and because responding for the drug is a function of drug reinforcement. A nicotine reduction policy targets the reinforcing effects of nicotine, making self-administration an ideal model for understanding how nicotine reduction is likely to impact behavior. Several advantages provided to regulatory science by preclinical research have been previously discussed including addressing factors related to tobacco initiation, assessing the impact of nicotine and other constituents, assessing wider ranges of nicotine doses than may be available for clinical populations, controlling for history of nicotine exposure, and measuring unanticipated consequences of reducing nicotine exposure.9 In recent years, two additional advantages of preclinical research have been emphasized. First, product compliance has proven to be a major challenge in clinical trials, where participants are provided VLNC cigarettes to smoke while still having easy access to nonstudy products (eg, usual brand cigarettes). In the study by Donny et al.,5 78% of participants assigned to smoke cigarettes with the lowest nicotine content had urinary nicotine biomarker levels greater than would be expected if they were compliant with the study product.5 The combined use of VLNC cigarettes and nonstudy cigarettes is problematic because it could lead to the simultaneous underestimation of the public health benefits and unintended consequences of a nicotine reduction policy. In light of this challenge, animal models in which researchers have control over access are extremely useful.
Second, use of electronic nicotine delivery systems (ENDS; ie, e-cigarettes) has risen dramatically in the past 4 years, with adolescent use of ENDS now exceeding use of combustible cigarettes,10 and in 2014, 15.9% of adult smokers in the United States also reported use of ENDS on some days or every day.11 Following the implementation of a mandated reduction in the nicotine content of cigarettes, some smokers are likely to try alternative sources of nicotine. There is evidence to suggest that at the individual level, alternative tobacco products, such as ENDS, may carry a reduced health burden in comparison to combustible tobacco.12,13 However, it is not clear what impact the availability of ENDS is likely to have on use of combustible tobacco. Thus, it is important to understand the factors that might shift use from one product to another. Dual use of VLNC cigarettes and nicotine replacement patches has been shown to be more effective in reducing smoking behavior than use of VLNC cigarettes alone,8,14 suggesting that access to alternative sources of nicotine may be an important moderator of outcomes for a nicotine reduction policy.15 Thus far, ENDS have not been a focus for animal self-administration researchers, likely because in many ways the typical nicotine self-administration paradigm is already a model of ENDS use (nicotine delivery without additional constituents). However, self-administration can provide a useful model of how access to alternative sources of nicotine, such as ENDS, may moderate the effects of reducing nicotine in combusted tobacco products. Preclinical researchers could model the choice that smokers have between VLNC cigarettes and alternative products by providing rats with more than one choice in the self-administration paradigm. This procedure would allow researchers to investigate variables that might moderate relative responding on the two options including experience with the alternative source of nicotine, cues, flavors, cost, product availability, nonnicotine constituents, and whether the method of reduction (gradual vs immediate) impacts use of alternative nicotine.
The goal of the present review is to describe recent progress made by preclinical researchers on the science of nicotine reduction and to identify existing and emerging research gaps.9 Throughout this review, we take care to consider how research questions may have shifted in recent years, with particular focus on the impact of compliance and alternative sources of nicotine. Because of the clear applicability of the rodent self-administration model, and because it has been utilized for the majority of preclinical nicotine reduction research, this model is the focus of our review. However, the self-administration model has some limitations as a test of nicotine reduction. First, it reveals little about actions of nicotine other than reinforcement which may be of importance for why individuals use nicotine-containing products, such as increased cognitive function, avoidance of withdrawal, self-image, and self-medication of psychiatric symptoms. Second, self-administration does not model sensory characteristics of smoking that could impact a nicotine reduction policy. Third, self-administration requires that experimenters choose methodological parameters (ie, session length, cue condition, infusion duration, access to other experimenter-controlled reinforcers, and operanda) that may not best reflect the conditions under which people smoke and furthermore could impact outcomes of interest. Finally, self-administration only allows for changes in drug intake by changing the number of infusions earned, whereas smokers are able to change smoke intake through changes in the number of cigarettes they smoke and changes in the way they smoke cigarettes.
There are a number of other preclinical models that have the potential to contribute to tobacco regulatory policies such as conditioned place preference (CPP), intracranial self-stimulation, and drug discrimination, although there is little existing research using these models in the context of a nicotine reduction policy. In this article, we have included these models when we believe they offer distinct advantages over self-administration or when there are data from those models directly relevant to nicotine reduction. CPP, for example, is a model in which nicotine is paired over multiple trials with a distinct space, and then preference for that space is measured on a test day when no drug is present. This model is an alternative way to assess the rewarding effects of low nicotine doses.
What is the Threshold Nicotine Content for Maintaining Behavior?
Researchers have hypothesized that there may be a threshold nicotine content for maintaining cigarette addiction, and recent evidence supports this notion.4,5 Animal self-administration researchers typically do not conduct research focused on low nicotine doses, and until recently, we did not know how reduction to low nicotine doses impacted self-administration behavior, what the threshold for maintaining self-administration might be, and what factors might impact that threshold. There are difficulties associated with translating a nicotine dose between rats and humans, but a self-administration model of nicotine reduction can provide important information about the impact of nicotine reduction on nicotine reinforcement.
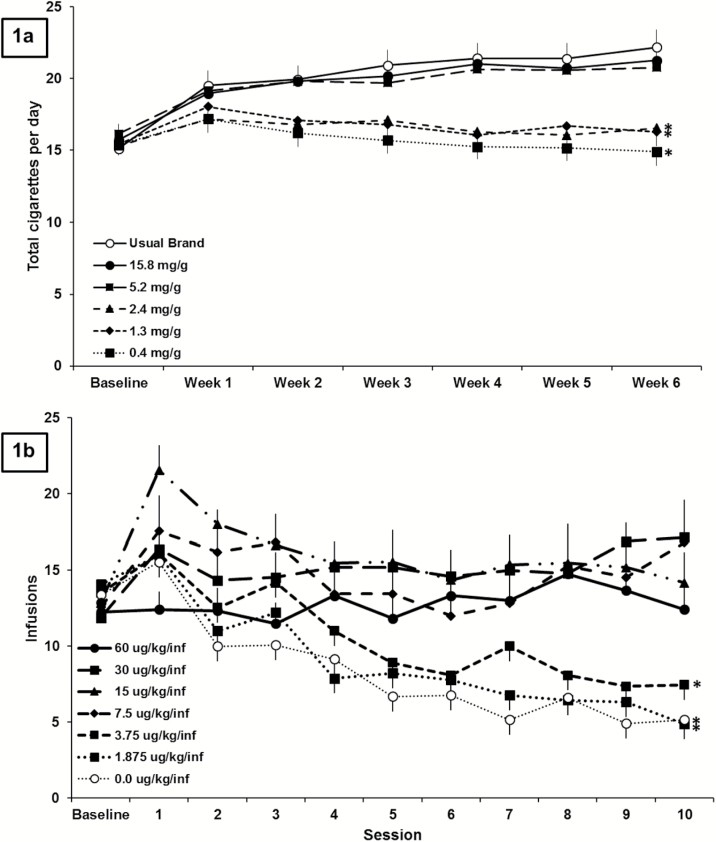
In the recent study by Donny et al.,5 individuals who were not interested in quitting smoking were either provided with their usual brand cigarettes or research cigarettes with one of five nicotine contents free of charge for 6 weeks. The impact on smoking behavior fell into two classes depending on the nicotine content of the cigarettes (Figure 1A). Those individuals assigned to receive cigarettes with 5.2 mg nicotine/g tobacco smoked cigarettes at the same rate as individuals in the control group who smoked cigarettes with a typical level of nicotine. Individuals assigned to receive cigarettes with a further reduction in nicotine content of approximately 50% (to 2.4 mg nicotine/g tobacco) smoked about five fewer cigarettes per day compared to the control group. Thus, the nicotine content required for maintaining smoking reinforcement is between 5.2 and 2.4 mg nicotine/g tobacco. These data are encouraging, but many participants reported using nonstudy cigarettes, and the number of cigarettes smoked per day was self-reported.
Figure 1.
(A) Adapted with permission from Donny and colleagues.5 Total study and nonstudy cigarettes ( ± standard error of the mean [SEM]) smoked when smokers were randomly assigned to either receive their usual brand or research cigarettes with one of five different nicotine contents for 6 weeks (120/group). Significant difference from 15.8 mg/g control group indicated by *. (B) Adapted with permission from Smith and colleagues.16 Earned infusions (± SEM) at baseline (all rats responding for 60 μg/kg/infusion nicotine) and across 10 sessions when rats were assigned to either continue to receive the same dose of nicotine or to experience a reduction to one of five different nicotine doses or saline (9–15/group). Significant difference from 60 ug/kg/infusion control group indicated by *. See primary papers for detailed information on the methods, data analysis, and results from either study.
We and others have used rodent self-administration as a model for nicotine reduction and have shown that changes in earned infusions following nicotine reduction are remarkably similar to the pattern of changes in human smokers described earlier. When the nicotine content available for self-administration is reduced from 60 μg/kg/infusion (a relatively standard dose of nicotine for rodent self-administration) to 7.5 μg/kg/infusion or more, behavior is maintained, with infusions earned at a rate similar to rats in a control group that do not experience a reduction in nicotine dose (Figure 1b).16 When nicotine is reduced to doses 3.75 μg/kg/infusion or lower, there is a reduction in the number of earned infusions across sessions. As in the clinical trial, a 50% reduction in nicotine dose from 7.5 μg/kg/infusion, an above-threshold dose, to 3.75 μg/kg/infusion produces a significant reduction in infusions earned. Behavior following reduction to 3.75 or 1.875 μg/kg/infusion did not differ from behavior following reduction to saline (unpublished analyses). Although only 10 sessions of reduction are shown in Figure 1B, rats in the lowest nicotine dose groups in this experiment continued to respond for their reduced nicotine doses for 40 more sessions, and the number of earned infusions either stayed the same or decreased. The parallel between human and rodent data strengthens the conclusion that there is a threshold dose of nicotine for maintaining self-administration behavior.
One discrepancy between the impact of nicotine reduction in the studies shown in Figures 1, A and B, is that in the clinical study, participants assigned to lower nicotine content cigarettes do not smoke less compared to their own baseline,5 whereas in the rat study, reduction to below-threshold nicotine doses produces a reduction in earned infusions compared to baseline.16 The cigarettes in Donny et al.’s clinical study were provided for free, which likely produced the increase in cigarettes smoked per day in the “above threshold” groups including the usual brand group and is likely the reason that there was no decrease in cigarettes smoked per day from baseline in the “below threshold” groups. In the self-administration study shown in Figure 1B, there was a “cost” associated with infusions of nicotine. Rats were required to make five responses for each infusion of nicotine, and when nicotine was reduced to 3.75 μg/kg/infusion or less, there was a reduction in earned infusions compared to baseline. These data suggest that if the cost of cigarettes were maintained in the marketplace, a nicotine reduction policy would likely produce a reduction in the rate of smoking.
It may also be useful to identify the threshold nicotine content for discriminating between vehicle and nicotine. Some very low nicotine doses may be discriminable from vehicle, increasing acceptability, while still being below a threshold for reinforcement. In the study by Smith et al. (Figure 1B), there is not a significant difference in responding for 1.875 or 3.75 µg/kg/infusion and saline. However, it is impossible to know whether rats can discriminate between these two low doses and saline using self-administration. Rodent models of drug discrimination could be used to assess whether the threshold nicotine dose for discrimination is the same as the one for maintaining self-administration behavior, and whether nicotine doses below the threshold for maintaining behavior are discriminable from saline. Interestingly, recent human behavioral laboratory assessments have begun to assess the minimum nicotine content required for smokers to discriminate from a very low nicotine content cigarette (0.4 mg nicotine/g tobacco).17 Thus far, assessments have shown that the threshold nicotine content for discrimination from a very low nicotine content cigarette (0.4 mg nicotine/g tobacco) varies widely across participants and is typically higher than the threshold for reinforcement as established in a recent clinical trial.5,17
Will Smokers Compensate for the Reduction in Nicotine Content?
One concern regarding nicotine reduction is that smokers might compensate (smoke more cigarettes per day or alter the intensity with which they smoke each cigarette) to increase nicotine intake following a reduction in nicotine content. Clinical researchers have shown that moderate reductions in nicotine content produce an increase in the number of cigarettes smoked per day.7 However, most evidence suggests that after the first few cigarettes, there is either no change or a decrease in smoking behavior, smoking intensity, and biomarkers of nicotine exposure.5,7,18 One exception is a trial in which 51 smokers experienced a gradual reduction in nicotine content, and there was an increase in number of cigarettes smoked per day among more dependent individuals.19 However, this increase in smoking among more dependent individuals was not replicated in a larger recent clinical trial5 (unpublished observations). However, all clinical observations are complicated by noncompliance, and rodent self-administration studies can offer valuable information about the likelihood of compensatory smoking.
Smith et al.16 showed that moderate reductions in nicotine dose (reduction from 60 μg/kg/infusion to doses between 7.5 and 30 μg/kg/infusion) produced a transient increase in earned infusions (ie, compensation; Figure 1b) as is the case for human smokers.7 By days 8–10 of the reduced nicotine dose, the increase in earned infusions was only maintained for rats that experienced the smallest reduction (to 30 μg/kg/infusion). Large reductions in nicotine content produced small (not statistically significant) increases in earned infusions on the first day and produced decreases in earned infusions by days 8–10, suggesting that if reductions in nicotine are large enough, there will not be any sustained compensation. These data are in line with those from Grebenstein et al.,20 which showed that reductions in nicotine content (from 60 to 7 μg/kg/infusion or higher) produced compensation, but larger reductions in nicotine content did not produce any compensation. Taken together, the available clinical and animal research suggest that it will be important for any nicotine reduction policy to utilize a nicotine content that is low enough to minimize the risk of sustained compensation.
How do Individual Differences Impact Nicotine Reduction?
Recent preclinical studies have shown there is individual variability in the reinforcement threshold,16,20 and self-administration is an important tool for identifying which factors might be important determinants of that variability. Studies investigating variables related to nicotine use history have shown no impact of length of exposure or baseline nicotine intake.20,21 There also do not appear to be any sex differences in the threshold nicotine dose for maintaining self-administration, or in the impact of VLNC use on smoking behavior.5,22,23 The impact of the rate of nicotine metabolism on nicotine reduction outcomes is less clear. Some clinical data have shown that nicotine metabolite ratio, which has been shown to be related to smoking behavior,24,25 did not impact the level of compensation following nicotine reduction.26 However, rats that metabolize nicotine more quickly have been shown to have lower reinforcement thresholds.20 The discrepancy between clinical and preclinical data in regard to the impact of nicotine metabolism make it an important target for further investigation.
Does the Method of Nicotine Reduction (Gradual Vs Immediate) Impact Smoking Behavior?
If a nicotine reduction policy was enacted, it is unclear whether policy makers would require this reduction to take place immediately or institute a gradual reduction in nicotine content over a period of months or years. Because a gradual reduction would expose smokers to intermediate nicotine contents, the method of nicotine reduction may impact the rate and prevalence of smoking behavior following a nicotine reduction policy. A post hoc analysis of clinical trials that used different approaches showed no differences in compensation between gradual and immediate reductions,27 and one ongoing clinical trial will directly address this issue.28 A recent rat self-administration study showed that the method of nicotine reduction does not impact the threshold for maintaining self-administration or the rate of self-administration at below-threshold doses.16 These data suggest that if a nicotine reduction policy is implemented, the method of reduction is unlikely to impact the rate at which smokers use VLNC cigarettes, with the primary difference being that a gradual reduction will take longer and prolong smoking at above-threshold nicotine contents, which will likely delay any health gains. The ongoing clinical trial will be critical in addressing whether the two methods of reduction might differ on other outcomes that we are unable to address in rodents (eg, withdrawal and psychiatric symptoms).
Will Nicotine Reduction Impact Current Smokers Differently From Individuals who Try Smoking for the First Time?
One of the most critical questions for preclinical researchers is whether there is likely to be a difference between the threshold nicotine dose for maintaining behavior following nicotine reduction and the threshold nicotine dose for acquisition of self-administration. This research question is especially important for preclinical researchers because questions related to smoking initiation cannot be experimentally addressed in human smokers. In a recent study, the threshold nicotine dose for acquiring self-administration in adult male rats was shown to be similar to or even higher than the threshold nicotine dose for maintaining self-administration following reduction.21,29 These preclinical data suggest that a nicotine reduction policy using clinical data from current smokers is likely to be sufficient in preventing smoking initiation in naive individuals. However, most smokers began smoking as adolescents, making it critically important to understand how period of development impacts the relationship between naive individuals and individuals with a history of nicotine use. We address this variable below in the section titled “How will vulnerable populations be impacted by nicotine reduction.”
How Will Environmental Stimuli Impact Behavior Following Reduction?
Smoking occurs in combination with many environmental stimuli, or cues, which may function to support high levels of behavior through associative and nonassociative processes. Any initially neutral environmental cue that is consistently predictive of nicotine delivery may serve to function as a conditioned stimulus and elicit a conditioned response.30 These responses can be similar to the psychological (eg, craving) or physiological (eg, increased heart rate) responses produced by nicotine itself. Additionally, through frequent pairing with the reinforcing effects of nicotine, environmental cues can become conditioned reinforcers that can act to reinforce behavior in the absence of nicotine.31 The impact of cues on nicotine reduction is an important area for preclinical researchers because of the tight control preclinical research can provide over cues.
A critical question is how long nicotine-paired cues will continue to maintain smoking behavior following a reduction in nicotine content. Most clinical trials have reported reductions in the number of cigarettes smoked per day among participants smoking VLNC cigarettes, but participants generally do not quit smoking.5,6 Some smoking in these groups may have been supported by smoking-related cues, especially given the high levels of noncompliance observed in these trials. Time to extinction of the behavior is likely to be extended by the need for extinction learning to take place in all of the contexts in which smokers usually smoke. One question is whether the long-lasting value of conditioned reinforcers following nicotine reduction will mitigate a shift in preference between VLNC cigarettes and alternative tobacco products. Preclinical researchers could use a model of product switching to understand how cues will impact choices between low nicotine doses and an alternative source of nicotine.
In addition to the associative mechanisms supporting nicotine-seeking behaviors, nicotine can also act nonassociatively to enhance responding for environmental stimuli with reinforcing value, and this action of nicotine may be involved in maintaining smoking behavior.32,33 We have previously established that nicotine acts to robustly enhance responding for a mildly reinforcing visual stimulus (VS, one second cue light presentation and 1 minute extinction of the houselight), and this property of nicotine does not require any temporal or predictive relationship between nicotine and the reinforcing stimulus.34 Smoking has been shown to enhance the value of other reinforcers in humans.35 To date, researchers have largely neglected the potential impact of the reinforcement enhancement effect on a nicotine reduction policy, but this is an area in which preclinical researchers could contribute because of the experimental control provided by preclinical research. A reduction in nicotine content below the threshold for reinforcement enhancement may produce a temporary anhedonia. The threshold nicotine content for the reinforcement-enhancing effects of nicotine may also differ from the threshold for the primary reinforcing effects of nicotine, causing low doses of nicotine that are below the threshold for primary reinforcement to enhance responding for reinforcing stimuli.33 In this case, rates of smoking may continue to be increased by low doses of nicotine if a primary or conditioned reinforcer is paired with smoking.
The reinforcement enhancement effect of nicotine is also likely to impact use of alternative tobacco products that contain characterizing flavors (eg, hookah and ENDS). Flavorants are perceived favorably36 and increase use of ENDS by young adults.37 A recent report showed that nicotine enhances the value of nonnutritive sweeteners,38 which suggests that nicotine is likely to increase the value of characterizing flavors delivered in alternative tobacco products. As nicotine is reduced in cigarettes and the value of conditioned reinforcers declines, we are likely to see increased use of alternative products, driven in part by the value of the primary reinforcers that are delivered along with nicotine.
Will Nonnicotine Cigarette Smoke Constituents Impact the Reinforcing Efficacy of Very Low Nicotine Contents?
A critical area for rodent self-administration research is the role of nonnicotine cigarette smoke constituents in supporting behavior. There are over 8000 nonnicotine cigarette constituents in cigarette smoke, and some of them have psychoactive properties.39 The levels of cigarette smoke constituents vary across cigarette brands,39 and variation in these constituents could moderate the impact of nicotine reduction. This area is especially important for preclinical researchers because in many cases, human researchers lack the tools to manipulate nonnicotine constituents and assess the impact of such a manipulation on behavior. Preclinical researchers have undertaken a variety of methods for assessing whether these nonnicotine constituents are important in reinforcing behavior. Researchers have tested self-administration of individual constituents alone or with nicotine,40–42 self-administration of a combination of constituents,43,44 and self-administration of extract created from cigarette smoke or smokeless tobacco.45–48 Regardless of the method, a review of the existing literature suggests that the impact of cigarette smoke constituents is likely dependent on the mixture of constituents, nicotine dose, constituent dose, schedule of reinforcement, and cue condition.
Some evidence suggests that nonnicotine constituents may increase nicotine self-administration or support self-administration on their own. Nornicotine, norharmane and acetaldehyde have been shown to support self-administration on their own, but the doses required for self-administration are much higher than what is relevant for cigarette smoke.40,41,49,50 One approach taken by several laboratories has been to index the doses of nonnicotine constituents to their relative concentrations in cigarette smoke, given a standard dose of nicotine. One study using this approach showed that a combination of minor alkaloids, including nornicotine, myosmine, cotinine, anatabine, and anabasine, increased nicotine self-administration, although the effect was relatively small and only present at select schedules of reinforcement.44 Another study using this strategy to test the impact of acetaldehyde on nicotine self-administration and showed that it can increase nicotine self-administration in young adolescents but not in adults.42 However, these reports are yet to be reproduced in the literature. More recent work tested the impact of eight constituents in combination, including five minor alkaloids (nornicotine, myosmine, cotinine, anatabine, and anabasine), two β-carbolines (harmane, norharmane), and acetaldehyde. This study tested these constituents in smoking-relevant doses and in doses 10 times higher, across two different nicotine doses, using several different schedules of reinforcement.43 When tested in combination, these constituents failed to increase self-administration for nicotine, casting doubt on whether they alter the reinforcing effects of nicotine in cigarettes.
Some researchers have used cigarette smoke or tobacco extract instead of individual constituents. This line of research is useful because it likely includes many more constituents than other approaches but has some methodological challenges. Researchers do not know which constituents dissolve in the extract or at what doses they dissolve. Additionally, this approach does not allow researchers to identify the source(s) contributing to any significant effects. These types of studies have yielded conflicting results about whether nonnicotine constituents may contribute to the reinforcing efficacy of cigarettes. One study showed increased rates of self-administration of an extract made from roll-your-own tobacco compared to an extract made from factory-made cigarettes,51 suggesting that roll-your-own tobacco may contain additional nonnicotine constituents that increase the reinforcing efficacy of smoking. Costello et al. reported that nonnicotine constituents present in smoke extract may decrease the threshold nicotine dose required for self-administration46 compared to nicotine alone. However, in another study from that laboratory, constituents in cigarette smoke extract were shown to inhibit self-administration of a threshold nicotine dose (7.5 μg/kg/infusion).52 Harris and colleagues have shown that low to moderate doses of an extract made from smokeless tobacco produce similar reinforcement-enhancing effects as nicotine alone using intracranial self-stimulation.47,48 The data on high doses of extract are less clear, with one study showing reduced reinforcement attenuation compared to nicotine alone and one study showing no difference.47,48 Extracts made from alternative tobacco products offer an exciting opportunity for preclinical researchers who could use these extracts in models of product switching to investigate choice following a reduction in nicotine dose.
The class of constituents that has been most reliably shown to increase nicotine self-administration is unidentified monoamine oxidase (MAO) inhibitors. Both subtypes of MAO (MAO-A and MAO-B) have been shown to be inhibited by 30%–40% in chronic smokers.53,54 While β-carbolines may contribute to this inhibition, the constituents contributing to this MAO inhibition have not been adequately characterized.55 Because the constituents that contribute to this inhibition are unclear, known MAO inhibitors that are not present in cigarette smoke have been used to model this inhibition in rat self-administration experiments. Research from several laboratories has shown that MAO inhibition increases self-administration of low nicotine doses.43,56,57 The increase in self-administration appears to be produced by inhibition of the MAO-A enzyme specifically.58,59 Partial inhibition, in the range observed in chronic smokers, also increased self-administration of low nicotine doses.58 These data suggest that constituents which inhibit MAO-A, in the range seen in chronic smokers, are likely to increase the reinforcing value of low nicotine doses. Furthermore, inhibition of MAO decreases the threshold dose required for the reinforcement enhancement effect of nicotine (see above for discussion of reinforcement enhancement), suggesting that these same constituents may cause very low doses of nicotine to increase the reinforcing value of other reinforcers in the environment.58 Clinical researchers should consider measuring MAO activity during nicotine reduction trials to determine whether inhibition of MAO moderates the impact of nicotine reduction.
Menthol is used as a characterizing flavor in many cigarettes, and many researchers have argued that it should be banned from cigarettes.60 Menthol may increase nicotine self-administration when it is paired with nicotine or delivered as a presession injection.61,62 Most research suggests that menthol acts as a reinforcer through its sensory characteristics, but menthol also acts on the central nervous system.63 In the recent clinical trial from Donny and colleagues,5 the impact of nicotine reduction on smoking was dependent on menthol status. At a moderate nicotine content (5.2 mg nicotine/g tobacco), nonmenthol smokers smoked more cigarettes per day than menthol smokers, and there was no impact of menthol on below threshold doses. Similarly, menthol may increase the threshold nicotine content required for discrimination.64 These data suggest that menthol may mask the reinforcing effects of nicotine when nicotine is reduced. However, the current preclinical data on menthol suggest that menthol may increase the reinforcing value of nicotine.61,62 A recent paper from Biswas and colleagues showed that systemic injections of menthol shifted the dose–response curve for nicotine self-administration to the left—increasing the reinforcing efficacy of low nicotine doses—suggesting that menthol could impact the threshold nicotine dose for maintaining behavior.62 One explanation for the discrepant findings on the impact of menthol on nicotine discrimination and reinforcement may be that menthol may reduce nicotine reinforcement through its sensory actions in smokers, an effect not captured by intravenous self-administration. Determining whether menthol might play a role in smoking reinforcement should be a high research priority for animal researchers.
There are a variety of avenues for future self-administration research investigating nonnicotine tobacco constituents. For example, a review of documents made available from the tobacco industry suggested that levulinic acid, a cigarette additive used to improve flavor, may also play a role in reinforcing smoking behavior by increasing nicotine exposure, making it potentially important for a nicotine reduction policy,65 but to our knowledge this constituent has yet to be tested in a self-administration paradigm. The increase in prevalence of the use of alternative tobacco products has added to the long list of potential nonnicotine constituents that might reinforce tobacco use, and animal researchers should test whether some of the more common ingredients (eg, ENDS flavorants) might impact abuse liability.
How Will Vulnerable Populations be Impacted by Nicotine Reduction?
Tobacco use among vulnerable populations has been named as a top research priority by the FDA Center for Tobacco Products.66 Indeed, epidemiological and clinical research have characterized several populations with particular susceptibility to tobacco product initiation and high levels of nicotine dependence, and it is critical to understand whether some populations have increased sensitivity to nicotine at low doses. Populations at potential risk include adolescents, smokers with serious mental illness (eg, schizophrenia and major depressive disorder), other substance users, and obese smokers.9,67–70 Animal studies offer a means of experimentally manipulating factors that may impact vulnerability of use and examining the specific role of nicotine in abuse liability of tobacco products. Although self-administration is a useful model for asking about the impact of nicotine reduction on vulnerable populations, CPP assessments also offer an opportunity to relatively quickly and cost-effectively ask about how vulnerable populations might differ on the rewarding effects of nicotine. Here, we focus on adolescents, individuals with schizophrenia, and obesity as examples of how animal research can contribute to our understanding of nicotine-seeking among vulnerable populations.
Preclinical research on adolescents is critical because ethical restrictions prevent human researchers from investigating the likely impact of nicotine reduction on adolescent initiation of smoking. The vast majority of current smokers start smoking in adolescence.71,72 Previous research utilizing rodents suggests that adolescents may be more sensitive to low doses of nicotine in a CPP test73–75 and may more readily acquire nicotine self-administration and show higher responding for moderate doses of nicotine, although this effect may vary by sex, access period, and prior training.52,76–78 Levin et al.79 reported that female adolescent rats self-administer nicotine at higher rates compared to female adults, across a range of doses (10–90 µg/kg/infusion). A report by Lynch80 supports this finding, demonstrating that female adolescents self-administer nicotine at higher rates than males. These reports suggest there may be an interaction between sex and age, with female adolescents particularly sensitive to nicotine reinforcement. It is important to note, however, that these key differences in self-administration emerged late in the adolescent period (postnatal day (P)54), which many would consider early adulthood.81
In contrast to the previously described findings, two reports have examined age differences in self-administration for the primary reinforcing effects of nicotine, independent of confounds with prior training. In one recent study, the lowest dose of nicotine to support self-administration in male and female adults (10 µg/kg/infusion) was lower than the dose required to support self-administration in male and female adolescents (30 µg/kg/infusion).23 These data confirm and extend the results demonstrating that male adolescent rats without prior operant training responded significantly less than adults for 15 µg/kg infusions of nicotine.82 Determining the underlying mechanisms contributing to the disparate findings across studies is critically important for preclinical researchers because these results have important implications for the impact of a nicotine reduction policy on adolescents. The conflicting results on adolescent nicotine self-administration emphasize the complexity of factors that likely contribute to adolescent vulnerability to tobacco use. Environmental cues have a large impact on nicotine self-administration (see above), and it is possible that the reinforcement-enhancing properties of nicotine, and not the primary reinforcing properties of nicotine, drive adolescent self-administration. The possibility of augmented reinforcement enhancement in adolescence should be evaluated across reinforcers, as reinforcement enhancement may increase the value of many stimuli that are delivered along with combustible (eg, menthol) and noncombustible (eg, ENDS flavoring) tobacco products.
Animal models also provide a valuable opportunity to examine the impact of adolescent exposure to nicotine on later nicotine intake. Such exposure is relevant to smokers and users of other nicotine-containing products, as individuals who begin smoking as adolescents demonstrate a higher daily consumption of cigarettes and a reduced likelihood of quitting as adults.83 In one study, elevated levels of adolescent nicotine intake were shown to persist into adulthood as compared with rats beginning self-administration as adults, but all rats in study were trained to respond for food on the active but not inactive lever, and it is impossible know how this may have differentially impacted adolescents and adults.79 Additionally, one study showed that repeated injections of nicotine in adolescence increase nicotine self-administration in adulthood,84 but other studies have failed to replicate this finding.75,85 A clearer understanding of how exposure to low doses of nicotine in adolescence impacts adult intake would be valuable for understanding whether use of VLNC cigarettes during adolescence may increase likelihood of becoming dependent on other tobacco products as an adult. If so, adolescents may use them for reasons other than the primary reinforcing effects.
Individuals with serious mental illness are more likely to be smokers and have greater difficulty quitting smoking.86 Some researchers have proposed that smokers with schizophrenia smoke as a form of self-medication to alleviate several symptoms of schizophrenia, such as cognitive impairments and anhedonia.87 Thus, the reduction of nicotine content in cigarettes could have a detrimental impact on these symptoms. However, individuals with schizophrenia who are randomized to smoke VLNC or normal nicotine content cigarettes after a period of abstinence report similar improvements in negative symptoms by both cigarettes.88 Studies comparing individuals with and without schizophrenia have found that in an acute setting, VLNC cigarettes reduce withdrawal, reduce craving, and decrease puff volume, and there are no differences between individuals with and without schizophrenia.89,90 Thus, the clinical evidence to date suggests that nicotine reduction may be no more detrimental to individuals with schizophrenia than to individuals without serious mental illness. A clinical trial is currently underway to assess the impact of smoking VLNC cigarettes in people with schizophrenia over a longer course of time,91 but given that individuals with schizophrenia have more difficulty abstaining from smoking,92 they may also have more difficulty remaining compliant to smoking only the study cigarettes. Rodent models of schizophrenia and other serious mental illness could control for some of these important variables and provide converging evidence about the impact of nicotine reduction in these populations. Thus far, there is very little research using rodent models of serious mental illness and nicotine self-administration and no research using these models to investigate the impact of nicotine reduction.
Obese smokers smoke significantly more cigarettes per day than normal weight smokers93 and may have higher levels of nicotine dependence.94 It is possible that obese smokers would be less sensitive to reductions of nicotine content in cigarettes, continuing to smoke.68 Indeed, obese smokers have higher ratings of liking and satisfaction for VLNC cigarettes compared to nonobese smokers.95 Rodent nicotine self-administration experiments have begun to utilize a model of obesity in which a subset of rats maintained on a densely caloric diet become obese.96 The use of a similar model testing nicotine self-administration across a full dose–response curve, including very low doses, would provide information regarding low-dose nicotine reinforcement is affected by obesity. The use of animal models allows for the exclusion of race, ethnicity, and socioeconomic status, factors that influence smoking and obesity, and may confound results in epidemiological or clinical settings.
Direct Effects of Nicotine Reduction on Health
In addition to the behavioral impact of a nicotine reduction policy, it is critical to examine the direct impact of a reduction in nicotine on health. One example is body weight regulation. Smoking cessation produces weight gain,97 but nicotine replacement therapy mitigates this effect,98 suggesting that smoking cessation weight gain is caused by abstinence from nicotine. Thus, nicotine reduction is likely to cause increases in body weight.68 In a recent clinical trial, individuals with low biomarkers of nicotine exposure (indicating compliance with the study product) gained weight over the course of the trial, but individuals with higher biomarkers of nicotine exposure did not, suggesting that nicotine reduction produces weight gain in individuals who are compliant with the study product.99 Indeed, in rats, a reduction in nicotine dose below the threshold for maintaining self-administration behavior produces significant increases in weight gain compared to those maintained on a high standard dose of nicotine.100 However, VLNC cigarettes may still suppress weight gain compared to abstaining from smoking in individuals who try smoking for the first time following implementation of a nicotine reduction policy. Rats given the opportunity to self-administer very low doses of nicotine have self-administration rates similar to rats, given the opportunity to self-administer saline but have suppressed body weight gain.100 These data indicate that if individuals use VLNC cigarettes for reasons other than the primary reinforcing effects of nicotine, the low level of nicotine exposure that accompanies VLNC use may still impact weight and other health related outcomes. These results highlight the importance of animal research, in which nicotine exposure is tightly controlled, in evaluating the health impact of nicotine reduction.
Conclusions and Future Directions
In recent years, preclinical researchers have made significant contributions to the science of nicotine reduction. These findings are likely to be useful in at least three ways. First, these data can guide clinical researchers in deciding which factors warrant focus in their own trials. Second, policy makers can utilize preclinical research to address questions that may be difficult or impossible to study in clinical trials (eg, the impact of nicotine reduction on initiation of smoking). Third, together with clinical data, these data can provide policy makers with converging evidence about the likely impact of a nicotine reduction policy.
The convergence of data from preclinical and clinical studies is critical. Animal self-administration research provides more experimental control than is available in clinical trials. As we have highlighted, one advantage provided by preclinical research is control over nicotine access. Many participants use nonstudy cigarettes in clinical trials, and it is important to show how nicotine reduction impacts self-administration in animal studies when compliance is known. Furthermore, following regulation of nicotine content, there may be some access to normal nicotine content cigarettes (because of black market use, hoarding of normal-nicotine content cigarettes, tampering, etc.), and animal models allow for the manipulation of access to other sources of nicotine and to experimentally investigate the impact of this access on nicotine reduction outcomes. The strong convergence between rodent self-administration and clinical trials validates the self-administration model for use in nicotine reduction research questions and provides strength to studies that use self-administration to address research questions that cannot be addressed in human smokers. For example, rodent self-administration studies using adolescents and testing initiation of nicotine self-administration are likely to be critical for policy makers because of the ethical limitations in gathering experimental evidence relevant to these issues in human smokers prior to regulation.
Use of alternative tobacco products, especially use of ENDS, has risen dramatically in recent years.10,11 It is critical for animal researchers to recognize how this shifting marketplace alters the research questions they should ask and the procedures they should use to answer these questions. It is only with an eye on the changing landscape of tobacco that animal researchers can continue to provide meaningful data for regulatory research questions.
In conclusion, the Family Smoking Prevention and Tobacco Control Act provides the opportunity for a drastic reduction in the nicotine content of cigarettes, if evidence suggests that such a reduction would improve public health. Outside the United States, the Framework Convention on Tobacco Control lists regulation of tobacco product constituents and their emissions as a priority. Animal researchers have much to offer clinical researchers and policy makers regarding the science of nicotine reduction. Indeed, as this review highlights, preclinical researchers have already contributed a great deal to this science in a relatively short time period.
Funding
This work was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Tracy Smith was supported by the National Cancer Institute (T32 CA186783).
Declaration of Interests
None declared.
References
- 1. Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 2. The health consequences of smoking -- 50 years of progress: a report of the Surgeon General: executive summary. Department of Health and Husman Services, (Ed). Washington, D.C: 2014. [Google Scholar]
- 3. US Congress. Family Smoking Prevention and Tobacco Control Act. 2009. [Google Scholar]
- 4. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 5. Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 7. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatsukami DK, Hertsgaard LA, Vogel RI, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donny EC, Taylor TG, LeSage MG, et al. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14(11):1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arrazola RA, Singh T, Corey CG, et al. ; Centers for Disease Control and Prevention (CDC). Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 11. Syamlal G, Jamal A, King BA, Mazurek JM. Electronic Cigarette Use Among Working Adults - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(22):557–561. [DOI] [PubMed] [Google Scholar]
- 12. McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P. E-cigarettes: an evidence update, A report commisioned by Public Health England. Public Health England. 2015. [Google Scholar]
- 13. Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104(1-2):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15(11):1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkins KA, Kunkle N, Karelitz JL, Michael VC, Donny EC. Threshold dose for discrimination of nicotine via cigarette smoking. Psychopharmacology (Berl). 2016;233(12):2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2-3):294–300. [DOI] [PubMed] [Google Scholar]
- 19. Bandiera FC, Ross KC, Taghavi S, Delucchi K, Tyndale RF, Benowitz NL. Nicotine Dependence, Nicotine Metabolism, and the Extent of Compensation in Response to Reduced Nicotine Content Cigarettes. Nicotine Tob Res. 2015;17(9):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 2015;151:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naïve to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014;22(5):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114-115:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schassburger RL, Pitzer EM, Smith TT, et al. Adolescent Rats Self-Administer Less Nicotine Than Adults at Low Doses. Nicotine Tob Res. 2016;18(9):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23(3):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. West O, Hajek P, McRobbie H. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology (Berl). 2011;218(2):313–322. [DOI] [PubMed] [Google Scholar]
- 26. Benowitz NL, Nardone N, Dains KM, et al. Effect of reducing the nicotine content of cigarettes on cigarette smoking behavior and tobacco smoke toxicant exposure: 2-year follow up. Addiction. 2015;110(10):1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24(2):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov. Project 2: Strategies for Reducing Nicotine Content Cigarettes Available: http://clinicaltrials.gov/ct2/show/NCT02139930 Accessed July 12, 2016.
- 29. Schassburger RL, Pitzer EM, Smith TT, et al. Adolescent Rats Self-Administer Less Nicotine Than Adults at Low Doses. Nicotine Tob Res. 2016;18(9):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caggiula AR, Donny EC, White AR, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70(4):515–530. [DOI] [PubMed] [Google Scholar]
- 31. Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl). 2007;195(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci. 2015;24:19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donny EC, Chaudhri N, Caggiula AR, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl). 2003;169(1):68–76. [DOI] [PubMed] [Google Scholar]
- 35. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl). 2013;228(3):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the “e-cigarette”. Am J Public Health. 2010;100(12):2340–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 2016;166:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rupprecht LE, Smith TT, Schassburger RL, Donny EC, Sved AF. Effects of nicotine on rewards varying in palatability and caloric value: Implications for E-cigarette flavoring. Tob Regul Sci. In Press. [Google Scholar]
- 39. Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke, Second Edition Boca Raton, FL: CRC Press, Taylor & Francis Group; 2013. [Google Scholar]
- 40. Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl). 1999;146(3):290–296. [DOI] [PubMed] [Google Scholar]
- 41. Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol. 2014;22(1):9–22. [DOI] [PubMed] [Google Scholar]
- 42. Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. [DOI] [PubMed] [Google Scholar]
- 43. Smith TT, Schaff MB, Rupprecht LE, et al. Effects of MAO inhibition and a combination of minor alkaloids, β-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clemens KJ, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1355–1366. [DOI] [PubMed] [Google Scholar]
- 45. Brennan KA, Putt F, Truman P. Nicotine-, tobacco particulate matter- and methamphetamine-produced locomotor sensitisation in rats. Psychopharmacology (Berl). 2013;228(4):659–672. [DOI] [PubMed] [Google Scholar]
- 46. Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39(8):1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harris AC, Stepanov I, Pentel PR, Lesage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl). 2012;220(3):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris AC, Tally L, Schmidt CE, et al. Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend. 2015;147:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takayama S, Uyeno ET. Intravenous self-administration of ethanol and acetaldehyde by rats. Yakubutsu Seishin Kodo. 1985;5(4):329–334. [PubMed] [Google Scholar]
- 50. Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85:293–304. [DOI] [PubMed] [Google Scholar]
- 51. Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict Biol. 2015;20(2):227–235. [DOI] [PubMed] [Google Scholar]
- 52. Gellner CA, Belluzzi JD, Leslie FM. Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats. Neuropharmacology. 2016;109:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fowler JS, Volkow ND, Wang GJ, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379(6567):733–736. [DOI] [PubMed] [Google Scholar]
- 54. Fowler JS, Volkow ND, Wang GJ, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996;93(24):14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28(1):182–195. [DOI] [PubMed] [Google Scholar]
- 56. Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25(38):8593–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villégier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52(6):1415–1425. [DOI] [PubMed] [Google Scholar]
- 58. Smith TT, Rupprecht LE, Cwalina SN, et al. Effects of Monoamine Oxidase Inhibition on the Reinforcing Properties of Low-Dose Nicotine. Neuropsychopharmacology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24(12):3532–3540. [DOI] [PubMed] [Google Scholar]
- 60. Siegel M. A lost opportunity for public health–the FDA advisory committee report on menthol. N Engl J Med. 2011;364(23):2177–2179. [DOI] [PubMed] [Google Scholar]
- 61. Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Biswas L, Harrison E, Gong Y, et al. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl). 2016;233(18):3417–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wickham RJ. How Menthol Alters Tobacco-Smoking Behavior: A Biological Perspective. Yale J Biol Med. 2015;88(3):279–287. [PMC free article] [PubMed] [Google Scholar]
- 64. Perkins KA, Kunkle N, Karelitz JL. Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs non-menthol smokers. Psychopharmacology (Berl). In Press. [DOI] [PubMed] [Google Scholar]
- 65. Keithly L, Ferris Wayne G, Cullen DM, Connolly GN. Industry research on the use and effects of levulinic acid: a case study in cigarette additives. Nicotine Tob Res. 2005;7(5):761–771. [DOI] [PubMed] [Google Scholar]
- 66. Food and Drug Administration Center for Tobacco Products. Research Priorities; Accessed July 5, 2016. [Google Scholar]
- 67. Hatsukami DK, Perkins KA, Lesage MG, et al. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19(5):e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rupprecht LE, Donny EC, Sved AF. Obese Smokers as a Potential Subpopulation of Risk in Tobacco Reduction Policy. Yale J Biol Med. 2015;88(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- 69. Gaalema DE, Miller ME, Tidey JW. Predicted Impact of Nicotine Reduction on Smokers with Affective Disorders. Tob Regul Sci. 2015;1(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA)2012. [PubMed] [Google Scholar]
- 72. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA)2014. [Google Scholar]
- 73. Lenoir M, Starosciak AK, Ledon J, et al. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ahsan HM, de la Peña JB, Botanas CJ, Kim HJ, Yu GY, Cheong JH. Conditioned place preference and self-administration induced by nicotine in adolescent and adult rats. Biomol Ther (Seoul). 2014;22(5):460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de la Pena JB, Ahsan HM, Botanas CJ, et al. Adolescent nicotine or cigarette smoke exposure changes subsequent response to nicotine conditioned place preference and self-administration. Behav Brain Res. 2014;272:156–164. [DOI] [PubMed] [Google Scholar]
- 76. Levin ED, Johnson JE, Slade S, et al. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011;338(3):890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. [DOI] [PubMed] [Google Scholar]
- 78. Natividad LA, Torres OV, Friedman TC, O’Dell LE. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav Brain Res. 2013;257:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl). 2003;169(2):141–149. [DOI] [PubMed] [Google Scholar]
- 80. Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 82. Shram MJ, Li Z, Lê AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl). 2008;197(1):45–58. [DOI] [PubMed] [Google Scholar]
- 83. Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9(4):39–46(Eng); 39. [PubMed] [Google Scholar]
- 84. Adriani W, Spijker S, Deroche-Gamonet V, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23(11):4712–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2014;19(6):1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Smith PH, Mazure CM, McKee SA. Smoking and mental illness in the U.S. population. Tob Control. 2014;23(e2):e147–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Annamalai A, Singh N, O’Malley SS. Smoking Use and Cessation Among People with Serious Mental Illness. Yale J Biol Med. 2015;88(3):271–277. [PMC free article] [PubMed] [Google Scholar]
- 88. Smith RC, Infante M, Ali A, Nigam S, Kotsaftis A. Effects of Cigarette Smoking on Psychopathology Scores in Patients With Schizophrenia: An Experimental Study. Subst Abus. 2001;22(3):175–186. [DOI] [PubMed] [Google Scholar]
- 89. Tidey JW, Cassidy RN, Miller ME. Smoking Topography Characteristics of Very Low Nicotine Content Cigarettes, With and Without Nicotine Replacement, in Smokers With Schizophrenia and Controls. Nicotine Tob Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. ClinicalTrials.gov. Very Low Nicotine Cigarettes in Smokers with Schizophrenia Available: https://clinicaltrials.gov/show/NCT02019459 Accessed August 4, 2016.
- 92. Smith PH, Mazure CM, McKee SA. Smoking and mental illness in the U.S. population. Tob Control. 2014;23(e2):e147–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring). 2007;15(5):1311–1318. [DOI] [PubMed] [Google Scholar]
- 94. Hussaini AE, Nicholson LM, Shera D, Stettler N, Kinsman S. Adolescent obesity as a risk factor for high-level nicotine addiction in young women. J Adolesc Health. 2011;49(5):511–517. [DOI] [PubMed] [Google Scholar]
- 95. Blendy JA, Strasser A, Walters CL, et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology (Berl). 2005;180(2):306–315. [DOI] [PubMed] [Google Scholar]
- 96. Rupprecht LE, Smith TT, Donny EC, Sved AF. Self-administered nicotine differentially impacts body weight gain in obesity-prone and obesity-resistant rats. Physiol Behav. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90(1):164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gross J, Stitzer ML, Maldonado J. Nicotine replacement: effects of postcessation weight gain. J Consult Clin Psychol. 1989;57(1):87–92. [DOI] [PubMed] [Google Scholar]
- 99. Rupprecht LE, Koopmeiners JS, Dermody SS, et al. Reducing nicotine exposure results in weight gain in smokers randomised to very low nicotine content cigarettes. Tob Control. 2016;26(e1):e43–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rupprecht LE, Smith TT, Donny EC, Sved AF. Self-Administered Nicotine Suppresses Body Weight Gain Independent of Food Intake in Male Rats. Nicotine Tob Res. 2016;18(9):1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]