Abstract
Introduction
Characterization of aerosols generated by electronic cigarettes (e-cigarettes) is one method used to evaluate the safety of e-cigarettes. While some researchers have modified smoking machines for e-cigarette aerosol generation, these machines are either not readily available, not automated for e-cigarette testing or have not been adequately described. The objective of this study was to build an e-cigarette vaping machine that can be used to test, under standard conditions, e-liquid aerosolization and nicotine and toxicant delivery.
Methods
The vaping machine was assembled from commercially available parts, including a puff controller, vacuum pump, power supply, switch to control current flow to the atomizer, three-way value to direct air flow to the atomizer, and three gas dispersion tubes for aerosol trapping. To validate and illustrate its use, the variation in aerosol generation was assessed within and between KangerTech Mini ProTank 3 clearomizers, and the effect of voltage on aerosolization and toxic aldehyde generation were assessed.
Results
When using one ProTank 3 clearomizer and different e-liquid flavors, the coefficient of variation (CV) of aerosol generated ranged between 11.5% and 19.3%. The variation in aerosol generated between ProTank 3 clearomizers with different e-liquid flavors and voltage settings ranged between 8.3% and 16.3% CV. Aerosol generation increased linearly at 3–6V across e-liquids and clearomizer brands. Acetaldehyde, acrolein, and formaldehyde generation increased markedly at voltages at or above 5V.
Conclusion
The vaping machine that we describe reproducibly aerosolizes e-liquids from e-cigarette atomizers under controlled conditions and is useful for testing of nicotine and toxicant delivery.
Implications
This study describes an electronic cigarette vaping machine that was assembled from commercially available parts. The vaping machine can be replicated by researchers and used under standard conditions to generate e-cigarette aerosols and characterize nicotine and toxicant delivery.
Introduction
The popularity of electronic cigarettes (e-cigarettes) has increased dramatically since its introduction into the US market1,2 but there are safety concerns associated with their use.3 E-cigarettes are comprised of a battery, a heating element (atomizer), and a reservoir with e-liquid, which typically contains nicotine dissolved in vegetable glycerin (VG), propylene glycol (PG), or a combination, and flavorings. Some e-cigarettes are cigarette-like (cig-a-like) (first generation), some are eGo-type e-cigarettes with small tanks (second generation) which operate at higher power than cig-a-likes, and others are customizable advanced personal vaporizers with larger tanks and/or rebuildable atomizers (RBAs).4
The rapid growth in the number of brands of e-cigarettes, the evolving designs, and the large number of different e-liquids available are posing a significant challenge to safety assessment of these products.5 One approach to e-cigarette safety evaluation involves characterization of the chemical composition of the aerosol.6–11 Toxic substances such as carbonyls have been found in e-cigarette aerosols.6 Formaldehyde, a probable human carcinogen (US EPA Group B1), can be produced in high levels in e-cigarettes.12 Farsalinos et al.13 reported that high levels of formaldehyde are only produced during “dry puff” conditions but this has been questioned by other researchers.14
Characterization of e-cigarette aerosolization as well as aerosol composition is important for both clinical and nonclinical studies. For example, it may be useful for clinical investigators to know the nicotine yield and repeatability of e-cigarettes before use in human studies. Some researchers have adapted tobacco cigarette smoking machines for use with e-cigarettes, primarily cig-a-likes6,11 and adaptations can be made to allow testing of more advanced products. These machines can be prohibitively costly and their functionality may be beyond the scope of what many researchers need in their research programs. Further, to the best of our knowledge, the smoking/vaping machines on the market do not offer the ability to use external power supplies to power the e-cigarette; e-cigarette batteries can be unreliable as they discharge.
Thus, the objective of this study was to build an e-cigarette vaping machine from commercially available parts that can be used to test, under standard conditions, e-cigarette repeatability, e-liquid aerosolization/vaporization, nicotine delivery, toxicant delivery and/or formation, and toxicity assessments. Since most advanced e-cigarettes (tanks, RBAs) use interchangeable mouthpieces (drip-tips), and cig-a-likes can fit in the tubing used, the machine is adaptable to most e-cigarettes currently on the market. We validated the machine by testing the variability of aerosol generation by one device (intra-device variability) and several devices of the same brand and model (inter-device variability), which were immediately applicable to our ongoing pharmacokinetic assessments of e-cigarettes.15 We also used the machine to demonstrate the effects of voltage/power on aerosol generation and toxic aldehyde emissions.
Methods
Materials
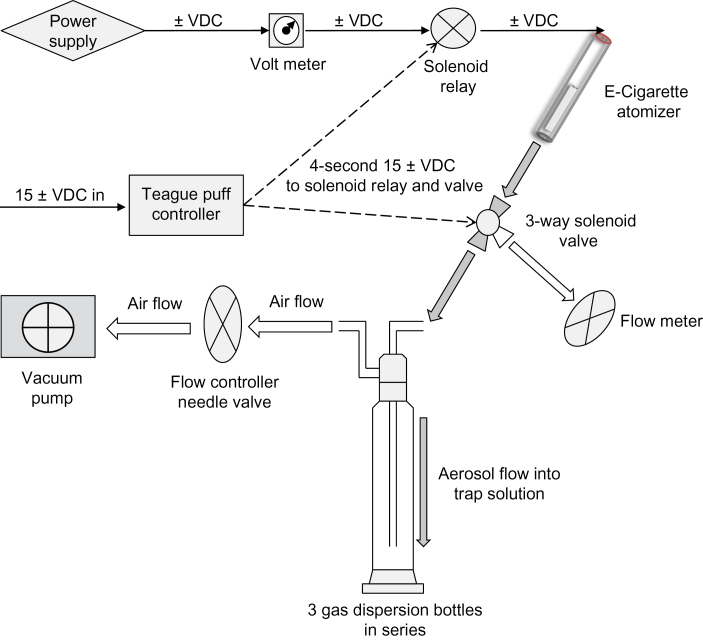
The component parts include a puff controller which gives a 3- or 4-s 15-V pulse every 30s, a vacuum pump (which we used at flow rate of 1–4 liters per minute [Lpm]), and a solenoid driven e-cigarette battery activator designed to fit button-activated eGo-type batteries (not included in Figure 1 but shown in Supplementary Figure 1, H), all purchased from Teague Enterprises (Woodland, CA). A 12-V, 10-amp solenoid relay was purchased locally and a direct current (DC) power supply (Dr. Meter, 30V, 5 amps) was purchased online. A three-way 12-V actuated solenoid valve (Ingersoll Rand, Davidson, NC) was supplied by Teague Enterprises. Other parts included three 125mL (extra coarse frit) gas dispersion bottles (Wilmad Lab Glass, Vineland, NJ) and a 510-thread compatible connection to power supply. RM series gas flowmeters (0–2.5 Lpm), with and without needle valve control, were used to measure and adjust flow rates (Dwyer, Michigan City, IN).
Figure 1.
Schematic of the electronic cigarette vaping machine.
Vaping Machine Setup
The components are assembled as shown in Figure 1 and Supplementary Figure 1, A–G. A 15-V 4-second pulse from the Teague puff controller is directed simultaneously to the three-way solenoid valve and the solenoid relay; the functioning of the solenoid relay and valve are synchronized. The three-way valve directs the vacuum to the e-cigarette atomizer. Simultaneously, the circuit in the solenoid relay closes so that the current from the power supply is directed to the atomizer. The cycle time is 30s. A solenoid actuated button pusher can substitute for the relay for the testing of 510-thread batteries instead of the power supply (Supplementary Figure 1, H). The measured power (product of voltage and current) delivered to the atomizer is lower under load than what the power supply is set to. To apply the desired power to the atomizer, all voltages were measured using a volt-ohm-meter (VOM meter) connected to the atomizer while power was being delivered to the atomizer. The power supply was adjusted until the VOM read the desired voltage. All of the voltages tested and presented in this manuscript are the measured voltages under load. Three gas dispersion bottles were filled with 40 or 50mL of 0.02N hydrochloric acid (HCl) (Fisher ACS reagent grade) when trapping nicotine, VG, and PG or 2,4-dinitrophenylhydrazine (DNPH) when trapping aldehydes (Sigma–Aldrich) and connected to the mouthpiece of the e-cigarette at one end and the vacuum pump at the other. The vacuum pump can be set to a range of flow rates using the adjustable flowmeter. We set the flow rate to 1.2 Lpm (measured at the three-way valve) for the machine validation experiments performed in this study. The vaping machine produces a square puff profile as shown in Supplementary Figure 2, which was measured using a MEMS (MicroElectroMechanical System) flow sensor (Quantified Vapor, Oakland, CA). The time from zero to maximum pressure is 0.2s (when tested at 20mL/s [1.2 Lpm]).
E-cigarettes
The primary e-cigarettes were KangerTech Mini ProTank 3 clearomizers (1.5 ohms) and KangerTech 3.7V batteries purchased directly from Kangertech.com (clearomizers refer to clear e-cigarette tanks) (Supplementary Figure 1, I). This pen-style e-cigarette is typical in design of many of the commonly used small eGo-type tanks. We have also observed from our clinical studies and participant screenings that KangerTech is among the most popular brands of e-cigarettes. Other e-cigarettes used included a Joytech Delta 23 (1.5 ohms) and a Smokio Smart Vaping (1.5 ohm) which were obtained from research collaborators at another university, and Kanger EVOD2, V2 Cigs, Kanger T3D, Vulcan, Kanger Aerotank V2, K101, Blu e-cigarette, Nimbus, Vapor4life, and Kanger Protank II, which were the usual brands of e-cigarettes of participants enrolled in a clinical study on e-cigarette pharmacology.16 The participants’ usual e-cigarettes were used in the initial validation of the gas traps, described below. Unflavored, strawberry, and tobacco flavored e-liquids containing 18mg/mL nicotine in 50:50 VG and PG were purchased from BulkeJuice.com. The water content of the strawberry and tobacco e-liquids was measured at Galbraith Laboratories and was found to be 0.1%. The KangerTech Mini ProTank 3 clearomizers and tobacco and strawberry flavored e-liquids are the study e-cigarette and e-liquids being used in a clinical study.15
Gas Trap Validation
We have described the validation of the gas traps in a previous publication, in which the trapping efficiency of only nicotine was assessed.16 On average, 86.2% ± 5.6% (mean ± SD) (range 76%–92%) of the machine-vaped nicotine was recovered. We performed a test of the trapping efficiency of nicotine, VG, and PG by the gas traps using the usual brand of e-cigarette for each subject (n = 13) enrolled in a clinical study on e-cigarette pharmacology.16 Each e-cigarette was manually vaped (ie, without the puff controller) with the participants’ usual e-liquids by placing the e-cigarette at the mouthpiece connected to the gas trap, 1 puff every 30s for a total of 15 puffs. The puff duration ranged from 2 to 6s and was based on the subjects’ puff duration during the standardized session of the clinical study. The aerosol was collected at 2 Lpm. E-cigarettes were weighed before and after vaping using a microbalance and the nicotine, VG, and PG concentrations of the e-liquids and gas trap solution after vaping were measured. Individual and average recoveries of nicotine, VG, and PG from the gas traps are presented in Table 1. Average recoveries were as follows: nicotine, 86.8% ± 9.6% (range 75.0%–100%); VG, 93.0% ± 10.3% (range 73.3%–100%); and PG, 86.7% ± 10.0% (range 72.7%–100%). Further, average recovery of nicotine ranged from 87% to 90% at flow rates of 1–4 Lpm.
Table 1.
Recoveries of Nicotine, Vegetable Glycerin, and Propylene Glycol in the Gas Traps After 15 Puffs Using Different Electronic Cigarettes
| E-cigarette | E-liquid vaped (mg) | Nicotine in e-liquid (µg/mg) | Nicotine delivered (mg) | Nicotine recovered (mg) | % Nicotine recovered | VG delivered (mg) | PG delivered (mg) | VG recovered (mg) | PG recovered (mg) | % VG recovered | % PG recovered |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44.0 | 15.3 | 0.67 | 0.68 | 100 | 35.5 | 3.2 | 30.4 | 3.1 | 85.5 | 96.8 |
| 2 | 90.6 | 13.9 | 1.26 | 1.16 | 92.5 | 18.9 | 57.4 | 19.1 | 53.9 | 100 | 93.8 |
| 3 | 180.5 | 11.4 | 2.05 | 1.73 | 84.1 | 106.8 | 43.3 | 121.7 | 35.9 | 100 | 82.9 |
| 4 | 488.0 | 5.7 | 2.78 | 2.37 | 85.3 | 227.7 | 191.7 | 171.2 | 161.6 | 75.2 | 84.3 |
| 5 | 93.0 | 15.3 | 1.42 | 1.19 | 83.4 | 60.7 | 23.5 | 61.8 | 20.3 | 100 | 86.4 |
| 6 | 56.3 | 8.6 | 0.49 | 0.51 | 100 | 18.5 | 31.6 | 21.3 | 28.3 | 100 | 89.5 |
| 7 | 83.9 | 12.1 | 1.02 | 0.76 | 75.0 | 54.9 | 16.9 | 60.1 | 12.3 | 100 | 72.7 |
| 8 | 470.0 | 5.0 | 2.34 | 1.79 | 76.4 | 270.0 | 112.3 | 197.9 | 85.9 | 73.3 | 76.5 |
| 9 | 70.6 | 12.6 | 0.89 | 0.71 | 79.8 | 56.3 | 0.0 | 46.3 | 0.0 | 82.1 | n/a |
| 10 | 115.1 | 5.6 | 0.65 | 0.58 | 89.5 | 72.6 | 27.2 | 76.3 | 24.0 | 100 | 88.4 |
| 11 | 251.0 | 5.0 | 1.27 | 1.23 | 96.9 | 158.5 | 72.8 | 147.9 | 72.5 | 93.4 | 99.5 |
| 12 | 70.1 | 5.9 | 0.41 | 0.29 | 71.3 | 29.6 | 39.5 | 34.3 | 27.6 | 100 | 69.8 |
| 13 | 182.0 | 5.7 | 1.04 | 0.99 | 94.6 | 56.7 | 99.7 | 69.3 | 118.5 | 100 | 100 |
| Average | 168.9 | 9.4 | 1.25 | 1.07 | 86.8 | 89.8 | 55.3 | 81.3 | 49.5 | 93.0 | 86.7 |
| SD | 149.8 | 4.1 | 0.73 | 0.59 | 9.6 | 80.5 | 53.4 | 59.3 | 48.1 | 10.3 | 10.0 |
E-cigarette brands tested are as follows: 1, Kanger EVOD2 (tank); 2, V2 Cigs Red 18 (cig-a-like); 3, Kanger T3D (tank); 4, Vulcan (RBA); 5, Kanger T3D (tank); 6, Kanger EVOD (tank); 7, Kanger Aerotank V2 (tank); 8, K101 (RBA); 9, Blu E-cigarette (cig-a-like); 10, Kanger EVOD (tank); 11, Nimbus (RBA); 12, Vapor4Life (tank); 13, Kanger Protank II (tank). Each e-cigarette was tested once.
E-cigarette Vaping Machine Validation
To assess the e-cigarette vaping machine repeatability, we performed two experiments. The first experiment assessed the within-e-cigarette repeatability of aerosol generation by three KangerTech Mini ProTank 3 clearomizers. The three clearomizers were filled with either unflavored, strawberry, or tobacco-flavored e-liquid and machine-vaped using the power supply set to 3.7V, flow rate of 1.2 Lpm, 40mL of HCl in each trap, and using the puffing regimen of one 4-s puff every 30s for a total of 15 puffs. Vaping topography studies have shown that the average puff duration is about 4s,17–20 hence our use of a 4-s puff. The clearomizers were weighed before and after the 15 puffs to assess the amount of e-liquid aerosolized. The clearomizers were refilled to the same level as before vaping and the session was repeated. Each clearomizer was machine-vaped a total of eight times using only one e-liquid, with 30min resting period between each session. We computed the average amount of e-liquid aerosolized, standard deviation, and the within-device coefficient of variation (CV) for each of the three clearomizers/e-liquids.
The second experiment assessed the repeatability of e-liquid aerosolization within the same brand and model using the vaping machine at different voltage settings. Six new KangerTech Mini ProTank 3 clearomizers (all 1.5 ohm atomizer resistance) were filled with strawberry e-liquid and machine-vaped as before (15 4-s puffs at 1.2 Lpm), at voltages of 3.7 and 5.0V under load using the power supply and fully charged KangerTech batteries at 3.7V under load. Clearomizers were weighed before and after each session to determine the amount of e-liquid aerosolized. After each session, the clearomizers were refilled to the same level as before vaping and the session was repeated at the other voltage. The average amount of e-liquid aerosolized for each voltage setting was computed and the between-device CVs were calculated. We used ANOVA to test the differences in amount of e-liquid aerosolized at the different voltages, including comparisons between the 3.7-V setting of the power supply and the 3.7-V KangerTech battery.
E-cigarette Vaping Machine Application
We performed five experiments to demonstrate the application of the vaping machine. First, we demonstrate how the vaping machine can be used to assess the effect of flavors on amount of e-liquid aerosolized. The e-liquids used were the unflavored, strawberry, and tobacco flavored e-liquids, which have been described before (all 50/50 VG/PG and 18mg/mL nicotine, 0.1% water). We used one KangerTech Mini ProTank 3 clearomizer (tank) per e-liquid but after each session of 15 puffs we replaced the used atomizer with new replacement KangerTech Mini ProTank 3 atomizers purchased in bulk from KangerTech (all 1.5 ohms) (Supplementary Figure 1, I). The atomizer is the replaceable heating element, which contains the coil(s) and wick. Twelve atomizers were tested per flavor (36 in total) using the puffing protocol of 15 puffs, 1 puff every 30s, 4-s puff duration, 1.2 Lpm, and the power supply set to 3.7V under load. The clearomizers were refilled to the same level after each session and allowed to seat for 30min before the next session began. We used ANOVA to test the effect of flavors on amount of e-liquid aerosolized. We controlled for electrical power in the ANOVA model because power delivered to the atomizer varied (CV 1.7%–2.3%) due to discrepancies between the labeled and measured atomizer resistance. This is the only experiment which involved replacement atomizers.
Second, we confirmed, based on prior publication,21 that e-liquid aerosolization was independent of flow rate (puff velocity). Two KangerTech Mini ProTank 3 clearomizers were filled with either tobacco or strawberry e-liquids and the power supply was set to 3.7V. E-cigarettes were individually machine-vaped at flow rates of 0.8 Lpm (13mL/s), 1.2 Lpm (20mL/s), 2 Lpm (33mL/s), and 3 Lpm (50mL/s) using the same 15-puff regimen. E-cigarettes were weighed before and after each 15-puff session.
In the third experiment, we demonstrate the utility of the vaping machine to assess the effect of voltage on aerosolization over range of 3–5.9V. Three KangerTech Mini ProTank 3 clearomizers were filled with unflavored, strawberry, or tobacco-flavored e-liquid and machine-vaped as before using the power supply at 3.0V (6.0W), 3.5V (8.2W), 4.0V (10.7W), 5.0V (16.7W), and 5.9V (23.2W). Clearomizers were weighed before and after each voltage setting.
Fourth, we demonstrate the utility of the vaping machine to compare performance of different brands of e-cigarettes. A KangerTech Mini ProTank 3, Joytech Delta 23 and Smokio, all with 1.5 ohm atomizers, were filled with unflavored e-liquid and machine-vaped with the power supply at 3.0V (6.0W), 3.5V (8.2W), 4.0V (10.7W), 5.0V (16.7W), and 5.9V (23.2W) using the 15-puff regimen and flow rate of 1.2 Lpm.
Finally, we illustrate the use of the vaping machine to examine the effect of voltage/power on toxic aldehyde emissions from e-cigarettes. We machine-vaped a KangerTech Mini ProTank 3 with unflavored e-liquid at 3.0V (6.0W), 3.5V (8.2W), 4.0V (10.7W), 5.0V (16.7W), and 5.9V (23.2W) following the CORESTA recommended method for carbonyl determination in mainstream cigarette smoke.22 DNPH was used as the aldehyde trapping solution (40mL in each gas dispersion bottle).
All statistical analyses were carried out using SAS v. 9.4 (SAS Institute, Inc. Cary, NC). Statistical tests were considered significant at α < 0.05.
Analytical Chemistry
Nicotine was measured in the 0.02N HCl trap solution from each trap separately (and then summed to give total recovered amount) and in e-liquids after dilution in 0.02N HCl by LC-MS/MS using a method modified from a previous publication23 and described in detail elsewhere.16 The limit of quantitation (LOQ) was 0.5ng/mL. VG and PG were quantified as the benzoate esters using PG-d6 and VG-d5 as internal standards using a modified method based on a previous publication,24 as has also been described previously.16 The LOQ was 5 µg/mL for both PG and VG. Acrolein (LOQ 0.078 µg/mL), acetaldehyde (LOQ 0.153 µg/mL), and formaldehyde (LOQ 0.113 µg/mL) were analyzed as described previously using the ultraviolet (UV) DNPH CORRESTA method developed for tobacco cigarettes.22
Results
E-cigarette Vaping Machine Validation
Within Device Repeatability
The average amount of e-liquid aerosolized within each KangerTech Mini ProTank 3 clearomizer from 15 4-s puffs is as follows: clearomizers with unflavored e-liquid, 136.2±15.6mg (mean ± SD) and had an intra-device CV of 11.5%; clearomizers with strawberry e-liquid, 102.4±19.8mg (CV 19.3%); clearomizers with tobacco e-liquid, 99.4±15.1mg (CV 15.2%). (Note: we are not comparing aerosol delivery from the various flavors here because we tested only three clearomizers and one flavor per clearomizer).
Between Device Repeatability
Six KangerTech Mini ProTank 3 clearomizers were machine-vaped with strawberry e-liquid using the 3.7-V KangerTech battery and the power supply at 3.7 and 5.0V. The average amount of e-liquid aerosolized when using the 3.7-V battery was 86.1±11.2 (within-device CV, 13.1%). When using the power supply at 3.7V, the average amount aerosolized was 85.2±10.8mg (CV, 12.7%) and at 5.0V, 226.1±28.6mg (CV, 12.6%). The amount of e-liquid aerosolized from 15 puffs was significantly higher at 5.0V of the power supply compare to 3.7V of the battery or power supply (adjusted P-values < .001) while the amount aerosolized at 3.7V of the battery and power supply were not significantly different (adjusted P-value = 1.0).
In another experiment where the effect of voltage was assessed, the amount of e-liquid aerosolized increased linearly with voltage for the three e-liquids using the KangerTech Mini ProTank 3 clearomizer (R2 = 0.99 for all curves) (Supplementary Figure 3, A).
E-cigarette Vaping Machine Application
Assessing Effect of Flavors on e-liquid Aerosolization
Table 2 presents the effect of flavors on the amount of e-liquid aerosolized from KangerTech mini Protank 3 clearomizers using the external power supply set to deliver 3.75V and power of ~8.0W to the atomizer. The unadjusted average amount of e-liquid aerosolized was not significantly different by flavor (P = .47). After adjusting for power delivered to the atomizer (given discrepancies between measured resistance and labeled resistance of the replacement atomizers), the flavor effect was still not significant (P = .14) but differences in amount of e-liquid aerosolized between unflavored versus strawberry (P = .08) and tobacco and strawberry (P = .09) approached significance. The least square means with 95% confidence intervals of amount of e-liquid aerosolized were as follows: unflavored, 94.0 (85.8–102) mg; strawberry, 83.6 (75.3–91.9) mg; and, tobacco, 93.9 (85.5–102.2) mg. Power had a significant effect on amount of e-liquid aerosolized (F = 10.4, P = .003).
Table 2.
Assessment of the Effect of Flavors on Amount of E-liquid Aerosolized
| Atomizer per flavor | Unflavored | Strawberry | Tobacco | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Voltage (V) | Power (W) | Amount aerosolized (mg) | Voltage (V) | Power (W) | Amount aerosolized (mg) | Voltage (V) | Power (W) | Amount aerosolized (mg) | |
| 1 | 3.75 | 7.9 | 90.4 | 3.74 | 8.0 | 91.0 | 3.75 | 7.8 | 89.2 |
| 2 | 3.74 | 8.0 | 82.4 | 3.76 | 7.7 | 65.1 | 3.76 | 7.8 | 86.4 |
| 3 | 3.73 | 8.1 | 109.2 | 3.74 | 8.1 | 100.5 | 3.75 | 8.0 | 99.3 |
| 4 | 3.74 | 8.0 | 86.8 | 3.76 | 7.8 | 86.1 | 3.75 | 8.0 | 95.1 |
| 5 | 3.75 | 8.1 | 68.2 | 3.74 | 8.4 | 126.8 | 3.75 | 7.8 | 90.8 |
| 6 | 3.76 | 8.0 | 88.5 | 3.77 | 7.8 | 79.5 | 3.74 | 8.0 | 94.7 |
| 7 | 3.77 | 7.7 | 71.5 | 3.75 | 8.2 | 97.6 | 3.75 | 7.7 | 81.5 |
| 8 | 3.76 | 8.0 | 93.8 | 3.75 | 8.1 | 102.2 | 3.74 | 8.2 | 86.1 |
| 9 | 3.76 | 7.8 | 122.4 | 3.76 | 7.9 | 56.4 | 3.76 | 7.9 | 78.8 |
| 10 | 3.75 | 8.0 | 114.5 | 3.75 | 8.0 | 77.4 | 3.75 | 8.1 | 110.1 |
| 11 | 3.76 | 7.7 | 97.9 | 3.75 | 8.0 | 88.6 | 3.77 | 7.6 | 82.7 |
| 12 | 3.75 | 7.9 | 102.8 | 3.75 | 7.9 | 62.6 | 3.76 | 7.7 | 99.9 |
| Average | 3.75 | 7.9 | 94.0 | 3.75 | 8.0 | 86.1 | 3.75 | 7.9 | 91.2 |
| SD | 0.01 | 0.1 | 16.4 | 0.01 | 0.2 | 19.7 | 0.01 | 0.2 | 9.0 |
| CV (%) | 0.3 | 1.7 | 17.4 | 0.2 | 2.3 | 22.9 | 0.2 | 2.2 | 9.9 |
Unflavored, strawberry, and tobacco e-liquids were all 50/50 vegetable glycerin/propylene glycol (VG/PG) and 18mg/mL nicotine; one KangerTech mini Protank 3 clearomizer was used per flavor and 12 different replacement atomizers were used for each clearomizer. Each e-cigarette was vaped using a standardized puffing protocol: 15 puffs, 1 puff every 30s, 4-s puff duration.
Flow Rate Effect on Amount of E-liquid Aerosolized
The amount of e-liquid aerosolized did not increase with increasing flow rate (puff velocity) for both tobacco and strawberry study e-liquids (we observed a flat relationship, data not shown), an observation which has been previously reported.21
Assessing E-liquid Aerosolization From Different E-cigarettes
We also illustrate the utility of the e-cigarette vaping machine to assess aerosolization of different e-cigarettes. The KangerTech Mini ProTank 3 (R2 = 0.96), Joytech Delta 23 (R2 = 0.99), and Smokio (R2 = 0.96) showed a linear relationship between voltage and amount of e-liquid aerosolized (Supplementary Figure 3, B).
Assessing Toxicant Emissions
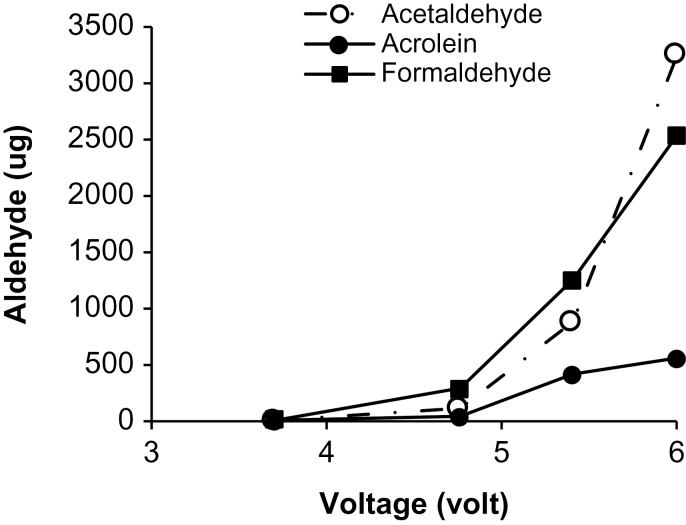
Figure 2 demonstrates the utility of the e-cigarette vaping machine to assess the emissions of toxic aldehydes from e-cigarettes. Acetaldehyde, acrolein, and formaldehyde increased markedly at voltages at or above 5V.
Figure 2.
Relationship between voltage and toxic aldehyde formation by the KangerTech Mini ProTank 3. Aldehyde emissions were tested at 3.0V (6.0W), 3.5V (8.2W), 4.0V (10.7W), 5.0V (16.7W), and 5.9V (23.2W).
Discussion
We describe an inexpensive e-cigarette vaping machine built from commercially available parts that can be used to test e-cigarette aerosolization/vaporization, nicotine delivery, and toxicant delivery and/or formation under standard conditions. We demonstrate that the 0.02N HCl solution used in the gas dispersion bottles efficiently traps nicotine (87%), VG (93%), and PG (87%). The intra-device variability of the amount of e-liquid aerosolized by the KangerTech Mini ProTank 3 ranged between 11.5% and 19.3% CV. The inter-device variability of aerosol generated by the same e-cigarette brand and model ranged between 12.6% and 13.1% CV. In demonstrating the capabilities and applications of the machine, we showed that aerosol formation increased with voltage, consistent with prior reports,21 and that significant amounts of aldehydes can be emitted by e-cigarettes in high voltage conditions.12 There seems to be a threshold voltage for high aldehyde formation, also consistent with prior reports.13
We observed some intra- and inter-device variability when testing e-liquid aerosolization from the KangerTech Mini ProTank 3 with the vaping machine, although these levels of variation are acceptable for various analytical methods. Since the voltage, power, velocity, and puff frequency are under stringent control, the variation would appear to be due to differences in the individual atomizer coils, the rate of absorption of e-liquid into the atomizer wick, and/or the temperature of the coils when activated. To minimize variability, it is important to run each test with the atomizers filled to the same level and at the same temperature. Larger amounts of e-liquid in the tank and higher atomizer temperatures would facilitate the entry of e-liquid into the coil chamber. Higher volumes of e-liquid would exert more pressure, and higher temperatures would reduce the viscosity of the VG and PG, both facilitating the entry of e-liquid into the coil chamber.
The amount of e-liquid aerosolized by the power supply set at 3.7V was not significantly different from when the 3.7-V KangerTech battery was used. This confirms that the power supply is able to deliver equivalent power to the e-cigarette as the eGo battery when set to the same voltage.
We did not see a significant effect of flavor on e-liquid aerosolization from the KangerTech Mini Protank 3. We used replacement atomizers purchased in bulk, which had discrepancies between the measured and labeled resistance. The power delivered to the coils varied slightly, CV ranging between 1.7% and 2.3% (Table 2). Despite the small variation in power supplied, power was a significant covariate in a model with flavor as the main predictor. This indicates the sensitivity of e-cigarettes to changes in power and highlights the need for careful control of power when performing these experiments. When we controlled for power statistically, differences between unflavored and strawberry and tobacco and strawberry e-liquids approached statistical significance. Our initial assessment of the flavor effect on e-liquid aerosolization was a within-device comparison and included a sonication (washing) step between flavors. While we saw a significant flavor effect, the sonication step may alter the wick in the atomizer and confound the findings. We caution against using a sonication step in these experiments.
As previously reported, we saw a strong relationship between voltage and e-liquid aerosolization in the e-cigarettes tested.21 Use of a power supply with control over voltage and current input, and thus power supplied to the atomizer allows us to replicate a range of realistic conditions at which e-cigarettes are used in the population.
We demonstrate the utility of our machine to assess toxicant emissions from e-cigarettes. We showed that significant amounts of aldehydes are emitted at voltages higher than 5V (~16.7W). Others have suggested that high levels of aldehydes are primarily produced during dry puff conditions13 but the voltage, and by extension power, may not be the only factor affecting aldehyde formation. The same researchers showed that minimal amounts of aldehydes were released even when high power levels were used and concluded that aldehyde release was associated with the efficiency of the atomizer design to accommodate the high power levels through effective liquid supply to the wick.13 Our machine will allow for comparison of toxicant emissions by a variety of e-cigarette designs.
Limitations and Future Improvements
Our vaping machine has some limitations. Although our vaping machine is compatible with most e-cigarettes, we have not tested the machine on devices that are not cig-a-likes or second and third generation devices that do not have standard mouthpieces (drip-tips), such as e-cigars and e-hookahs. Further, we did not adjust flow rate (and by extension, puff volume) for the effects of possible deadspace of the gas dispersion bottles (gas traps). As a result, the true flow rates through the system may be lower than the reported flow rates.
During the course of our clinical studies, we have observed that some e-cigarette users push the button on the battery for 1–2s before puffing thereby preheating the coil before vaping. We are working with Teague Enterprises on a new controller which will allow for independent actuation of the voltage to the controller and three-way valve so that this user behavior can be replicated by the machine. We have also observed that there is some condensation of the aerosol in the three-way valve. The recovery of e-liquid using the fully automated machine with the three-way valve was 90% in the traps and 10% in the valve at 3.7V. We are exploring ways to reduce aerosol condensation in the valve. Although e-liquid aerosol condensation in the value does not affect characterization of amount of e-liquid aerosolized by e-cigarettes, we recommend a thorough valve wash, as done for the gas traps and tubing, to be included in any aerosol collection protocol, such as that used in toxicology studies or aerosol chemical characterization.
Conclusions
Characterization of e-cigarette aerosol is an important aspect of the safety evaluation of e-cigarettes. We describe an e-cigarette vaping machine that reproducibly aerosolizes e-liquids from e-cigarette atomizers under controlled conditions and is useful for testing of nicotine and toxicant formation and delivery. The machine allows fine control of electrical parameters such as voltage and current, determinants of the power supplied to e-cigarettes, and can be used to assess nicotine delivery and toxicant emissions along a range of these characteristics. Machine testing of e-cigarettes and e-liquids can be useful not only for regulatory purposes but also when designing clinical studies of e-cigarettes.
Supplementary Material
Supplementary Figures 1–3 can be found online at http://www.ntr.oxfordjournals.org
Funding
Research reported in this publication was supported from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products (1P50CA180890 and P50CA180890-02S1) and the National Institute on Drug Abuse (P30DA012393). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration (FDA).
Declaration of Interests
Dr Neal Benowitz serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
Supplementary Material
References
- 1. Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011–2014. MMWR. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 2. King BA, Patel R, Nguyen K, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–227. doi:10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(suppl 2):ii36–ii40. doi:10.1136/tobaccocontrol-2013–051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grana R, Benowitz N, Glantz SA. E-cigarettes a scientific review. Circulation. 2014;129(19):1972–1986. doi:10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu S-H, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2014–051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi:10.1136/tobaccocontrol-2012–050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628–637. doi:10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors—effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. doi:10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2, 4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 2013;29(12):1219–1222. doi:10.2116/analsci.29.1219. [DOI] [PubMed] [Google Scholar]
- 10. Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418(2015):192–199. doi:10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 11. El-Hellani A, El-Hage R, Baalbaki R, et al. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem Res Toxicol. 2015;28(8):1532–1537. doi:10.1021/acs.chemrestox.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. doi:10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 13. Farsalinos KE, Voudris V, Poulas K. E‐cigarettes generate high levels of aldehydes only in ‘dry puff’conditions. Addiction. 2015;110(8): 1352–1356. doi:10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- 14. Shihadeh A, Talih S, Eissenberg T. Commentary on Farsalinos et al. (2015): e-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction. 2015;110:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. St Helen G, Havel C, Dempsey D, Jacob P, III, Benowitz N. Impact of flavors on nicotine intake, retention, and effects among electronic cigarette users. Symposium presented at Society for Research on Nicotine and Tobacco; March 4, 2016; Chicago, IL.
- 16. St.Helen G, Havel C, Dempsey D, Jacob P, III, Benowitz NL. Nicotine delivery, retention, and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–544. doi:10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norton KJ, June KM, O’Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. doi:10.1186/1617-9625-12-17. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Pub Health. 2013;10(6):2500–2514. doi:10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22(2):103–106. doi:10.1136/tobaccocontrol-2011–050226. [DOI] [PubMed] [Google Scholar]
- 20. Robinson R, Hensel E, Morabito P, Roundtree K. Electronic cigarette topography in the natural environment. PLoS One. 2015;10(6):e0129296. doi:10.1371/journal.pone.0129296. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17(2):150–157. doi:10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CORESTA recommended method N° 74. Determination of selected carbonyls in mainstream cigarette smoke by HPLC. http://www.coresta.org/Recommended_Methods/CRM_74-update(July14).pdf. Accessed October 16, 2015. [Google Scholar]
- 23. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chrom Relat Tech. 2011;34(14):1442–1458. doi:10.1080/10826076.2011.572213. [Google Scholar]
- 24. Frieler RA, Mitteness DJ, Golovko MY, Gienger HM, Rosenberger TA. Quantitative determination of free glycerol and myo-inositol from plasma and tissue by high-performance liquid chromatography. J Chromatogr B. 2009;877(29):3667–3672. doi:10.1016/j.jchromb.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.