Abstract
Background
Nicotine replacement therapy (NRT) was licensed for harm reduction in the United Kingdom in 2005, and guidance to UK Stop-Smoking Services (SSS) to include long-term partial or complete substitution of cigarettes with NRT was issued in 2013. Yet, NRT prevalence data and data on changes in biomarkers associated with long-term NRT use among SSS clients are scarce.
Methods
SSS clients abstinent 4 weeks postquit date were followed up at 12 months. At baseline standard sociodemographic, smoking and SSS use characteristics were collected and of those eligible, 60.6% (1047/1728) provided data on smoking status and NRT use at follow-up. A subsample also provided saliva samples at baseline and of those eligible, 36.2% (258/712) provided follow-up samples. Saliva was analyzed for cotinine (a metabolite of nicotine) and alpha-amylase (a stress biomarker).
Results
Among those who had used NRT during their initial quit attempt (61.5%, 95% CI 58.4%–64.6%), 6.0% (95% CI 4.3%–8.3%) were still using NRT at 1 year, significantly more ex-smokers than relapsed smokers (9.5% vs. 3.7%; p = .005). In adjusted analysis, NRT use interacted with smoking status to determine change in cotinine, but not alpha-amylase, levels (Wald χ2 (1) = 13.0, p < .001): cotinine levels remained unchanged in relapsed smokers and ex-smokers with long-term NRT use but decreased in ex-smokers without long-term NRT use.
Conclusions
Long-term NRT use is uncommon in SSS clients, particularly among relapsed smokers. Its use is associated with continued high intake of nicotine among ex-smokers but does not increase nicotine intake in smokers. It does not appear to affect stress response.
Implications
Little is known about the long-term effects of NRT. Given an increasing shift towards harm reduction in tobacco control, reducing the harm from combustible products by partial or complete substitution with noncombustible products, more data on long-term use are needed. This study shows that in the context of SSS, clients rarely use products for up to a year and that NRT use does not affect users’ stress response. Ex-smokers using NRT long-term can completely replace nicotine from cigarettes with nicotine from NRT; long-term NRT use by continuing smokers does not increase nicotine intake. Long-term NRT appears to be a safe and effective way to reduce exposure to combustible nicotine.
Introduction
The main aim of the UK Stop-Smoking Services (SSS) is to support attempts to quit smoking. However, not all smokers either feel able to or want to stop smoking completely. For this reason, alternative approaches have been explored to reduce harm from smoking in this population. Harm reduction refers to the reduced psychological or physiological harm from substance use without complete cessation.1 For current smokers, harm reduction may refer to the partial substitution of cigarettes with noncombustible forms of nicotine delivery such as nicotine replacement therapy (NRT) to reduce cigarette consumption or for temporary abstinence. For ex-smokers, harm reduction constitutes the complete, long-term substitution of combustible tobacco products (eg, cigarettes) with less harmful noncombustible nicotine delivery devices.2 There is good evidence from both population studies and clinical trials that the provision of NRT to smokers who cut down their cigarette consumption results in more sustained decreases in cigarette consumption and improves their chances to stop smoking completely.3,4 It increases motivation to stop and improves quit rates1,3 but does not increase overall nicotine intake.5,6 Trials have also shown that extended use of NRT by ex-smokers may result in better long-term abstinence rates by reducing relapse.7,8 For these reasons, NRT has been licensed for harm reduction in the United Kingdom since 2005.9,10 Based on a previous report,11 guidance was also issued in 2013 requiring SSS to include partial or complete long-term substitution of cigarettes with NRT in tailored quit plans for smokers who have difficulty stopping smoking completely so as to help them reduce consumption with the eventual aim to stop smoking.12
The vast majority of the harm from smoking is caused by the burning of tobacco and not nicotine.13 Thus NRT as a substitute for cigarettes is important to study. Although the importance of e-cigarettes for harm reduction purposes cannot be doubted, NRT is likely to remain a major component of harm-reduction strategies, given its long history in tobacco control and continuing NRT product innovation14 and on-going resistance of some smokers to e-cigarettes.15 Despite being an established treatment, there is considerable worry among potential users16 and stop-smoking advisors17 regarding the safety of long-term NRT use, possibly due to misunderstandings about the role of nicotine separate from smoked tobacco.18 While studies which have looked at this issue find that long-term NRT use is safe and any associated health risks small,19 certainly compared with continued smoking,20,21 most data come from clinical trials, which have samples that tend to differ in important ways from general population samples, biasing outcomes.22 Given recent calls for further research in the area of harm reduction,12 more studies on real-world use are required.
A recent population-based study suggested that only a small percentage of ex-smokers continue to use NRT beyond the standard length of 3 months and that long-term use is associated with lower nicotine intake compared with smokers.6 However, in many industrialized countries most NRT is purchased over the counter,23 rather than coupled with specialist behavioral support, which is more effective.24 Therefore, existing findings may not generalize to smokers attending SSS, especially since in this context the NRT provided is either free or heavily subsidized. In light of the recent broadening in the provision of NRT in SSS, there remains a need to evaluate harm reduction with NRT in this context.
This study describes the impact of longer-term NRT use among smokers who made a quit attempt with SSS support and agreed to take part in the “Evaluating Long Term Outcomes of NHS Stop-Smoking Services” (ELONS) study conducted 2012–2014.25 Participants were followed up for 1 year and provided information on their NRT use. A subset also provided saliva samples which were analyzed for two biomarkers of interest: cotinine, the primary metabolite of nicotine as a biomarker of exposure; and alpha-amylase, a digestive enzyme and indicator of autonomic nervous system activation which correlates with acute and chronic stress, as a biomarker of risk/potential harm.26 We included this biomarker as animal research has shown that chronic nicotine self-administration can increase stress response in rodents.27,28 Specifically, this study aimed to answer the following research questions:
What is the prevalence of long-term NRT use among smokers and ex-smokers who had attempted to stop smoking using SSS?
What is the impact of long-term NRT use on biomarkers of nicotine exposure and stress among smokers and ex-smokers who had attempted to stop smoking using SSS?
Methods
Study Design and Participants
Given the aims of this study, we report only on those with baseline and follow-up data. Full details of the study design and sampling are provided elsewhere.25 Briefly, as part of the ELONS study, clients participating in English SSS who set a quit date were asked if they were interested in taking part in a long-term (12 months) evaluation of the services by advisors and informed consent was obtained from all participants, resulting in a baseline sample of 3045 clients. As per standard NHS SSS guidelines, smoking status was recorded at 4-week follow-up29 and only those who were abstinent at 4 weeks (56.7%; 1728/3045) were eligible for long-term follow-up. Of all eligible participants for 12 month follow-up, 60.6% (1047/1728) could be contacted by telephone to assess smoking status and NRT use, thus providing complete baseline and follow-up questionnaire data (see Table 1 for participant details). Of those contactable, 53.3% (558/1047) self-reported as abstinent and were eligible for a home visit to verify their smoking status, of whom 4.6% (26/558) failed carbon monoxide verification and were therefore reclassified as smokers for the purposes of this analysis. The 12-month follow-up started in April 2013 and finished in March 2014.
Table 1.
Baseline Characteristics
| Questionnaire data | Biomarker data | |||
|---|---|---|---|---|
| Available (N = 1047) | Lost to follow-up (N = 681) | Available (N = 258) | Lost to follow-up (N = 454) | |
| Sociodemographic/health characteristics | ||||
| Mean (SD) Age | 46.4 (14.0) | 41.1 (13.7)*** | 45.7 (13.4) | 42.2 (14.6)** |
| % (N) Female | 55.0 (576) | 53.6 (365) | 51.6 (133) | 48.7 (221) |
| % (N) White | 97.2 (1018) | 94.7 (645)** | 96.5 (249) | 93.8 (426) |
| % (N) Cohabiting | 53.4 (559) | 47.3 (322)* | 53.9 (139) | 44.9 (204)* |
| % (N) Routine/manual occupation | 30.9 (323) | 34.5 (235) | 25.2 (65) | 30.6 (139) |
| % (N) Degree or equivalent | 10.6 (111) | 10.4 (71) | 10.5 (27) | 9.3 (42) |
| % (N) Medical condition | 59.5 (622) | 52.9 (360)** | 57.4 (148) | 58.4 (265) |
| Smoking characteristics | ||||
| Mean (SD) Heaviness of smoking index | 3.28(1.45) | 3.22 (1.46) | 3.19 (1.54) | 3.51 (1.41)** |
| % (N) Smoking length < 10 years | 10.9 (114) | 17.4 (118)*** | 9.3 (24) | 14.3 (65) |
| % (N) Quit attempt last 12 months | 41.7 (434) | 41.0 (275) | 38.1 (98) | 38.4 (172) |
| NHS SSS treatment characteristics | ||||
| % (N) Intervention type* | ||||
| Closed group | 3.2 (34) | 2.9 (20) | 6.6 (17) | 4.6 (21) |
| Open (rolling) group | 20.8 (218) | 17.6 (120) | 21.3 (55) | 13.9 (63) |
| Drop-in clinic | 26.5 (277) | 27.2 (185) | 24.4 (63) | 30.4 (138) |
| One to one support | 49.2 (515) | 51.9 (353) | 47.7 (123) | 50.7 (230) |
| Other | 0.3 (3) | 0.3 (2) | 0 (0) | 0.4 (2) |
| % (N) Medication** | ||||
| Single NRT | 17.4 (182) | 17.9 (122) | 17.4 (45) | 15.2 (69) |
| Combination NRT | 12.2 (128) | 15.1 (103) | 16.3 (42) | 27.3 (124) |
| Varenicline | 50.2 (526) | 48.5 (330) | 48.4 (125) | 37.4 (170) |
| Othera | 19.0 (199) | 17.0 (116) | 16.3 (42) | 19.2 (87) |
| None | 1.1 (12) | 1.5 (10) | 1.6 (4) | 0.9 (4) |
NRT = nicotine replacement therapy; SSS = UK Stop-Smoking Services.
aBupropion and mixed medication (mainly NRT).
*p < .05; **p < .01; ***p < .001.
A subsample of participants also provided a saliva sample at baseline, before their target quit date (61.6%; 1875/3045). Of those who were eligible to provide a saliva sample at follow-up (ie, successful quitters at 4 weeks with a baseline saliva sample who self-reported abstinence at 12-month follow-up and therefore had a home visit), 52.8% (169/320) provided a sample. Because relapsers did not have a home visit (and therefore were not asked to provide a saliva sample), an additional random selection of participants with baseline saliva samples who had relapsed at 4-week follow-up were contacted at 12 months (83.4%, 392/470) to obtain follow-up saliva samples from smokers. Participants were sent a saliva kit through the post and asked to return samples directly to UCL. The saliva kit contained two Sarstedt Salivettes, a letter from the Principal Investigator asking for their help, detailed instructions on sample collection and a £10 shopping voucher. Of those approached, 22.6% (89/392) returned a saliva sample, resulting in an overall response rate from face-to-face or postal collection of 36.2% (258/712) with complete baseline and follow-up biomarker data (see Table 1 for participant details).
Measures
Questionnaire Items
In addition to standard questions on smoking and sociodemographic characteristics, a number of items were included in the baseline questionnaire to help evaluate SSS.25 Advisors recorded the types of pharmacotherapy and behavioral intervention used during the quit attempt. It should be noted that at the time of the study, e-cigarettes (another harm reduction tool) were only just becoming popular and client use was not routinely recorded by SSS. At 12-month follow-up, questions related to long-term NRT use were also assessed retrospectively: participants were asked to indicate whether they had used NRT for their initial quit attempt and, if so, how long they had used NRT for, and if they were still using NRT now. As the use of other nicotine-containing products (including e-cigarettes) was not assessed at baseline, this was assessed at follow-up only. In order to ascertain smoking status and use of NRT in those participants who provided a saliva sample through the post and did not receive a home visit, these respondents were asked to indicate on a tick box included on the salivettes whether they were currently smoking (yes/no) and used NRT or e-cigarettes (yes/no).
Biomarkers
Saliva samples were collected with Sarstedt Salivettes and stored in −20°C freezers, ready for analysis. Saliva was analyzed for cotinine by ABS laboratory using rapid liquid–gas chromatograpy30 and for alpha-amylase activity by Salimetrics laboratory using an established enzyme-kinetic methodology.31 Although alpha-amylase activity is largely independent of flow-rate,32 all participants were instructed to keep the salivettes in the mouth for the same amount of time (1–2 minutes) without chewing as per recommendation.33 In addition, all participants were asked to abstain from drinking or eating immediately before providing a sample. Whilst alpha-amylase exhibits a diurnal pattern, it remains relatively stable throughout the day following a rise in the first hours after waking.34 Participants were therefore instructed to provide two samples during waking hours, approximately 10 minutes apart to increase reliability of measurement (the average coefficient of variation in alpha-amylase activity at baseline was 1.7% and at follow-up 1.8%).
Analysis
Data were analyzed with IBM SPSS Statistics 20.0.0. Comparisons were made between those who did and did not have complete baseline and follow-up data for questionnaire items (to assess NRT prevalence) and those who did or did not have complete baseline and follow-up biomarker data (to assess impact). Differences were assessed with chi-square tests and independent t tests for categorical and continuous variables, respectively. In the prevalence analysis, descriptive statistics including 95% confidence intervals (95% CI) were calculated and, where applicable, groups compared using logistic regression. To correct for nonresponse all prevalence estimates are weighted.25
In the biomarker analysis, due to the typically positively skewed distribution of cotinine and alpha-amylase values and relatively small sample size, geometric means and interquartile ranges were calculated. The nonparametric Kruskal-Wallis and Wilcoxon tests were used to assess between-group differences and within-group differences (to look at change across time), respectively. In sensitivity analysis, findings were re-examined with generalized linear models for between- and within-group comparisons that used a gamma distribution with a log link (all zero values were replaced with 0.001) to account for the non-normal distribution and adjusted for potential confounders (age, sex, ethnicity, occupation, any medical condition and nicotine dependence). Statistical significance was set at the standard level (p < .05), and the Bonferroni correction was applied to account for multiple comparisons and Type I error rate. The study received ethical approval from the South East Scotland Research Ethics Committee (11/AL/0256) and was carried out in accordance with the ethical principles on human research, as set out in the Declaration of Helsinki.
Results
Prevalence of Long-term NRT Use Among Current Smokers and Ex-smokers
Information on long-term NRT use was provided by 1047 participants (34.4% of the total ELONS sample) who constitute the analytic sample for the prevalence analysis. Those who were lost at follow-up were younger, had smoked for a shorter period, were less likely to have a medical condition, to be white or cohabiting (Table 1). All prevalence estimates in this section are weighted.
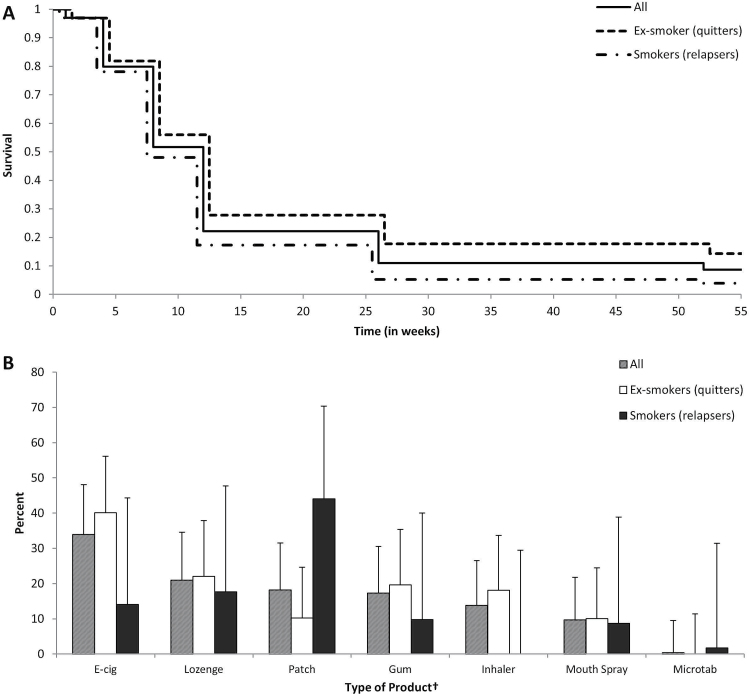
Of clients followed-up, 61.5% (95% CI 58.4%–64.6%, N = 583) reported using NRT during their initial quit attempt. This figure was somewhat higher than the recorded NRT use in SSS (around N = 500 when including the “Other” category in Table 1), suggesting that some participants had obtained additional NRT over the counter. Figure 1A provides a breakdown of clients in terms of the length of use of NRT and as a function of smoking status at follow-up. As can be seen, most clients who started on NRT used it for at least 8 weeks and more than one in five (21.5%, 95% CI 18.3%–25.0%, N = 137) for longer than the standard 12 weeks. However, long-term use was relatively rare with less than 1 in 10 participants still using noncombustible nicotine delivery devices at 12-month follow-up (8.4%, 95% CI 6.4%–11.0%, N = 50), including both NRT and e-cigarettes. In this sample, NRT use was twice as prevalent (6.0%, 95% CI 4.3%–8.3%, N = 35) as use of e-cigarettes at 12 months (2.9%, 95% CI 1.8%–4.7%, N = 18; some participants were dual product users).
Figure 1.
(A) Nicotine replacement therapy (NRT) use across follow-up period among those who had used NRT during initial quit attempt (N = 583)*; (B) Product type used among those with long-term NRT use at follow-up (N = 50). *Includes e-cigarettes (users of products at 12-month provide denominator for Figure 1B); †No use of nicotine nasal spray reported at 12-month follow-up; users could indicate multiple products; Error bars are 95% confidence intervals.
Generally, the pattern of NRT use across the study period was relatively similar for those who had remained abstinent and those who had relapsed by 12-month follow-up (Figure 1A). However, ex-smokers had higher rates of NRT use compared with relapsers at all time-points. At 12-month follow-up, long-term ex-smokers were over four-times more likely than relapsers to be still using noncombustible nicotine delivery devices (OR 4.25, 95% CI 2.15–8.40, p < .001): 14.0% (95% CI 10.3%–18.7%, N = 38) of ex-smokers were still using these compared with 3.7% (95% CI 2.0%–6.5%, N = 12) of relapsers. This difference, while being attenuated, remained significant when excluding those who used e-cigarettes only (OR 2.91, 95% CI 1.38–6.11, p = .005) with 9.5% (95% CI 6.4%–13.8%, N = 25) of ex-smokers and 3.5% (95% CI 1.9%–6.3%, N = 10) of relapsers still using NRT, respectively. Comparing the quitters and relapsers who were or were not using NRT at follow-up in terms of the characteristics presented in Table 1 showed that dependence was the only variable (other than medication use, as would be expected) that differed between groups (F(3, 1037) = 5.52, p < .001). Relapsers without NRT use had significantly higher dependence scores than quitters, irrespective of their NRT use.
When looking at individual nicotine-delivery devices still used at 12-month follow-up, e-cigarettes were the most popular, followed by the nicotine lozenge, patch, and gum (Figure 1B). No one used the nasal spray, possibly due to the higher cost of the nasal spray compared with other NRT products, and 16.8% were using multiple products. Due to the small numbers involved, there was insufficient power to detect meaningful differences between those who had remained abstinent and those who had relapsed.
Impact of Long-term NRT Use on Biomarkers of Nicotine Exposure and Stress Among Current Smokers and Ex-smokers
Baseline and follow-up saliva samples were provided by 258 participants (8.5% of the total sample) who constitute the analytic sample for the biomarker analysis. Those lost to follow-up were younger, less likely to be cohabiting and there were some differences in the treatments used; they were also more dependent (Table 1).
There were no differences in baseline cotinine levels between any of the groups (Table 2). This was confirmed in adjusted analysis controlling for potential confounders which showed that older age (Wald χ2 (1) = 6.6, p = .011) and greater dependence (Wald χ2 (1) = 26.7, p < .001) were the only significant predictors of baseline cotinine levels. Similarly, there were no group differences in baseline alpha-amylase levels, again confirmed in adjusted analysis (Table 2). This showed that older age (Wald χ2 (1) = 10.6, p = .001), being non-white (Wald χ2 (1) = 5.3, p = .022) and having any medical condition (Wald χ2 (1) = 9.8, p = .002) were associated with higher alpha-amylase activity at baseline.
Table 2.
Biomarker Results by Follow-up NRT Use and Follow-up Smoking Status
| Smokers (relapsers) | Ex-smokers (quitters) | |||
|---|---|---|---|---|
| NRT use (N = 18) | No NRT use (N = 73) | NRT use (N = 14) | No NRT use (N = 153) | |
| Baseline assessment | ||||
| Geometric mean (IQR/n) cotinine in ng/mL | 193.7 (323.1/17) | 241.1 (238.8/68) | 340.1 (163.9/13) | 197.6 (174.6/146) |
| Geometric mean (IQR/n) alpha-amylase in U/mL | 20.1 (59.1/12) | 21.8 (27.2/45) | 29.1 (14.2/11) | 23.6 (30.5/109) |
| Follow-up assessment | ||||
| Geometric mean (IQR/n) cotinine in ng/ml | 210.8 (240.0/16)a | 244.7 (198.7/69)a | 169.9 (449.6/10)a | 1.2 (21.6/149)b |
| Geometric mean (IQR/n) alpha-amylase in U/mL | 25.8 (69.6/13) | 26.7 (32.2/43) | 22.4 (49.7/10) | 27.6 (37.7/111) |
IQR = interquartile range; NRT = nicotine replacement therapy.
a,bDifferent letters indicate significant differences between groups(p < .05).
At follow-up, there was a clear difference between groups in cotinine levels (Kruskal Wallis H (3) = 130.2, p < .001). Ex-smokers using no NRT had significantly lower cotinine values at follow-up than all other groups (Table 2). Adjusted analysis confirmed these group differences (Wald χ2 (3) = 78.9, p < .001) and showed baseline nicotine dependence as the only additional significant predictor of follow-up cotinine levels (Wald χ2 (1) = 15.4, p < .001). There were no group differences in follow-up alpha-amylase levels which was confirmed in adjusted analysis (Table 2); only greater nicotine dependence at baseline was positively associated with follow-up alpha-amylase activity (Wald χ2 (1) = 8.1, p = .004).
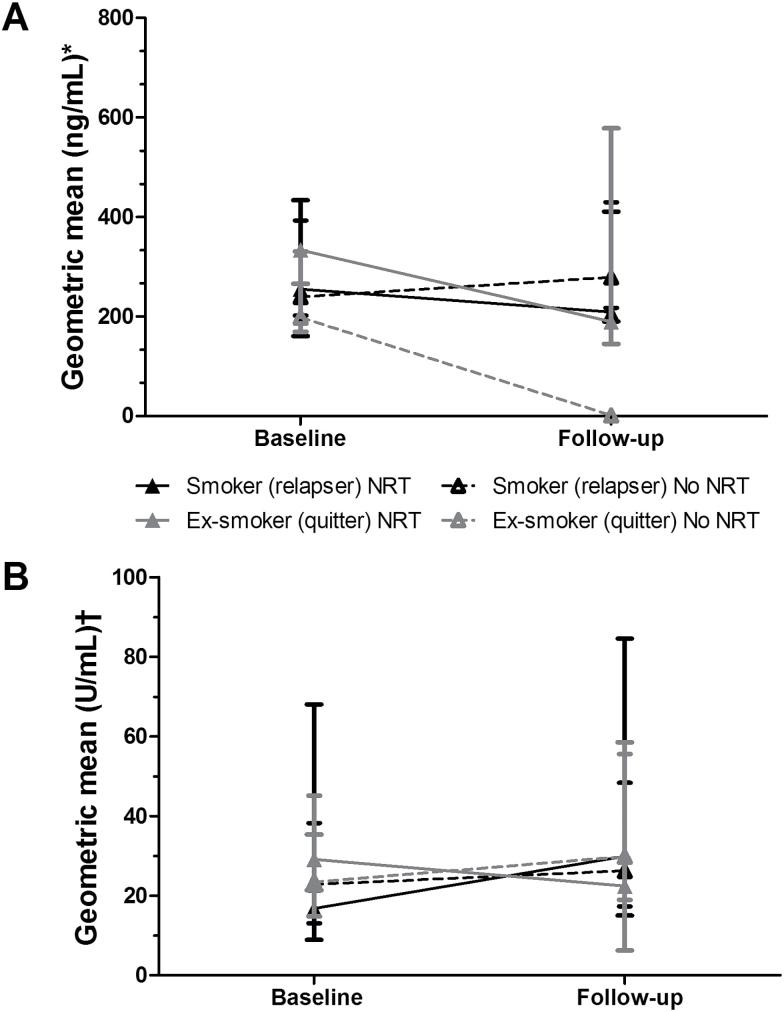
In addition to the cross-sectional analyses for baseline and follow-up data reported above, we also examined within-person changes from baseline to follow-up in longitudinal analysis (please note that this group is slightly smaller as not all participants who provided both baseline and follow-up saliva samples had provided either two samples at each time point or samples that were viable). As shown in Figure 2A, cotinine levels significantly reduced from baseline to follow-up only in ex-smokers not using NRT at follow-up (Standardized Z = −9.9, p < .001) and not in other groups. Adjusted analysis confirmed the significant NRT use by smoking status interaction for changes in cotinine levels (Wald χ2 (1) = 13.0, p < .001) and also showed that greater baseline age (Wald χ2 (1) = 4.3, p = .037) and dependence (Wald χ2 (1) = 44.8, p < .001) were associated with an increase in cotinine levels.
Figure 2.
Change in (A) cotinine levels (N = 232) and (B) alpha-amylase activity (N = 166) from baseline to follow-up as a function of nicotine replacement therapy (NRT) use and smoking status at follow-up. Data not available from N participants due to insufficient samples or contamination: *26 cases; †92 cases; Error bars are interquartile range.
While unadjusted analysis indicated that there was an increase in alpha-amylase activity from baseline to follow-up in ex-smokers not using NRT at follow-up (Standardized Z = 3.0, p = .003) and not in other groups (Figure 2B), this was not confirmed in adjusted analysis. Neither the NRT use by smoking status interaction for changes in alpha-amylase levels (Wald χ2 (1) = 2.1, p = .147) nor main effects for NRT use (Wald χ2 (1) = 0.9, p = .352) or smoking status (Wald χ2 (1) = 0.8, p = .373) were significant. However, greater baseline age (Wald χ2 (1) = 4.4, p < .036), dependence (Wald χ2 (1) = 6.0, p = .014) and reporting any medical condition at baseline (Wald χ2 (1) = 5.8, p = .016) were independently associated with an increase in alpha-amylase activity.
Discussion
Extended use of NRT among SSS clients was relatively prevalent, with over one in five who achieve short-term abstinence continuing to use it beyond the standard treatment length of 3 months, but continued long-term use of NRT by those who achieve long-term abstinence at one year is less common at just below 10%. Nonetheless, given that 1-year usage rates were estimated at around 5% among ex-smokers who attend SSS in 2002,35 this suggests that recent policy and licensing changes in favor of harm reduction9,10,12 may have had some impact on long-term NRT use among services users. This contrasts with a lack of change in NRT usage pattern observed in the general population following an earlier relaxation of NRT licensing in 2005.36 However, the low 4% prevalence of concurrent long-term use of NRT among SSS clients who had relapsed is similar to figures from the general population suggesting that longer-term NRT use among smokers is rare.37 Indeed, concurrent NRT use among smokers, either for temporary abstinence or cutting down, has remained relatively stable since 2002,38 with most smokers using NRT for less than 3 months.37
Interestingly, despite a steady increase in the prevalence of e-cigarette use among smokers and ex-smokers in the United Kingdom,39 the long-term use of e-cigarettes among past SSS clients in this study was surprisingly low at less than 3% compared with estimates of one in five smokers or recent ex-smokers using e-cigarettes in the general population.40 However, this may be due to the specificity of the sample selection and the timing of the study, being conducted around the time of increasing awareness of e-cigarettes in the United Kingdom but before use became widespread amongst smokers and recent quitters.41
This study provides some rare insights in the exposure to nicotine associated with long-term dual or single use of NRT, as well as its impact on a biological index of stress, alpha-amylase. Clinical trials suggest that permanent replacement of cigarettes with NRT among ex-smokers can result in 40% of baseline levels of nicotine being substituted by nicotine replacement products long-term.42,43 Our findings not only confirm substantial substitution of nicotine from cigarettes with nicotine from NRT but, given the lack of changes in ex-smokers using NRT from baseline to follow-up, suggest that virtually all baseline nicotine may be replaced by NRT among long-term ex-smokers. This increase in substitution levels compared with previous work may reflect differences in our sample or changes in the NRT products available. It is unlikely to be the result of other product use as all ex-smokers were carbon monoxide-verified and participants with concurrent use of other nicotine delivery devices, that is, e-cigarettes, were excluded.
Confirming previous research,5,44 the concurrent use of NRT among smokers did not appear to increase their nicotine intake. These findings are in agreement with the hypothesis that smokers are very adept at titrating nicotine levels, with some nicotine otherwise obtained from cigarettes being replaced by nicotine from NRT.45 However, our results indicate this also applies to ex-smokers, which is consistent with a strong genetic component in nicotine intake46 but at odds with clinical42 and general population studies6 showing that nicotine substitution from NRT tapers off over time. Behavioral support in SSS includes detailed instructions on the correct use of NRT47 which is not available in other settings and may explain the differential in both NRT effectiveness and associated nicotine intake when used with and without behavioral support.
Although it is unlikely that a substantially increased nicotine intake from NRT would be harmful,48,49 it clearly is a concern for some people and a potential barrier to effective use of nicotine products.16 Our results not only suggest that dual use with NRT does not increased nicotine intake compared with continued smoking, they also indicate that use of NRT (either with or without concurrent smoking) is not associated with an increase in a biomarker of stress response, alpha-amylase, used as a proxy here to signal potential harm. Given observed reductions in stress levels in smokers following cessation,50 it was surprising not to see any reductions of alpha-amylase levels in quitters. However, it should be noted that tobacco smoke has been shown to acutely inhibit alpha amylase activity,33 which means that the benefit of smoking cessation may have been masked by the impact of baseline smoking. Moreover, spot sampling may not be reliable enough to pick up true long-term changes. While there was an expected association of increased biological stress with older age and having a medical condition, the association of increased alpha-amylase activity with greater baseline nicotine dependence was not predicted and deserves further investigation as it suggests that the stress response is dependence-mediated (rather than nicotine-mediated). Altogether, these findings are consistent with the view that long-term NRT use is safe and not associated with increased health risks, certainly compared with continued smoking.21
This study has a number of limitations. Despite an initial large sample size, drop out across the study was inevitably substantial, resulting in relatively few clients with complete baseline and follow-up data on biomarkers. In addition, the baseline sample differed from the sample followed up. However, differences were relatively modest, and prevalence data were weighted to account for differential drop out. As clients self-selected into groups rather than being experimentally assigned, we cannot exclude potential reverse causation, for example, particular individuals who happen to have a high sensitivity to nicotine intake may use NRT for longer. Moreover, we were only able to assess current NRT use but not frequency of NRT use at follow-up which means that it is difficult to ascertain how comparable NRT use was across relapsers and quitters. However, this study reflects real-world use of NRT and the longitudinal within-group design reduced confounding by allowing participants to be their own control. Lastly, different methodologies were used to collect follow-up saliva samples which may have impacted results. However, the same clear instructions were provided to participants and researchers for postal and face-to-face collection, respectively. All assessments were carried out with established, ecologically valid measures and smoking status verified, but further research would benefit from measuring a wider array of biomarkers of smoking-related harm, including different biomarkers of chronic stress such as cortisol.
In conclusion, among former SSS clients long-term NRT use by ex-smokers is relatively rare but more common than use by smokers. Furthermore, long-term use seems to have increased since the introduction of harm reduction guidance in the United Kingdom. Long-term use of NRT does not appear to have a detrimental effect on chronic stress response among smokers or ex-smokers and does not increase overall nicotine intake in smokers but is associated with continued nicotine intake in ex-smokers, comparable to when they were smoking.
Funding
The ELONS study was funded by the NIHR HTA programme (09/161/01). This work received additional support from a grant by the former UK Centre for Tobacco Control Studies (UKCTS). Funding from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council and the National Institute for Health Research under the auspices of the UK Clinical Research Collaboration is gratefully acknowledged (RES-590-28-0004). All authors are also members of the UK Centre for Tobacco and Alcohol Studies (UKCTAS), funded under the auspices of the above UK Clinical Research Collaboration (MR/K023195/1).
Declaration of Interests
LS has received an honorarium for a talk, an unrestricted research grant and travel expenses to attend meetings and workshops from Pfizer, a pharmaceutical company that makes smoking cessation products, and has acted as paid reviewer for grant awarding bodies and as a paid consultant for health care companies. The other authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank the English SSS, the Primary Care Research Network, Susan Murray, Claire Mimnagh and Madiha Sajid for their help with data collection. LS, AM, and LB conceived the original idea for this study. LB obtained funding. LS, FD, and RH managed the day-to-day running of the study. LS undertook the data analyses and wrote the initial draft with further input from FD, RH, AM, and LB. LS is guarantor for this article. All authors read, reviewed, and approved the final version. All researchers listed as authors are independent from the funders and all final decisions about the research were taken without constraint by the investigators. LS had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1. Beard E, McNeill A, Aveyard P, Fidler J, Michie S, West R. Association between use of nicotine replacement therapy for harm reduction and smoking cessation: a prospective study of English smokers. Tob Control. 2013;22(2):118–122. [DOI] [PubMed] [Google Scholar]
- 2. Le Houezec J, McNeill A, Britton J. Tobacco, nicotine and harm reduction. Drug Alcohol Rev. 2011;30(2):119–123. [DOI] [PubMed] [Google Scholar]
- 3. Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess. 2008;12(2):iii–iv, ix. [DOI] [PubMed] [Google Scholar]
- 4. Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;338:b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beard E, Fidler J, West R. Is use of nicotine replacement therapy while continuing to smoke associated with increased nicotine intake? Evidence from a population sample. Psychopharmacology (Berl). 2011;218(3):609–610. [DOI] [PubMed] [Google Scholar]
- 6. Shahab L, Beard E, Brown J, West R. Prevalence of NRT use and associated nicotine intake in smokers, recent ex-smokers and longer-term ex-smokers. PLoS One. 2014;9(11):e113045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schnoll RA, Patterson F, Wileyto EP, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joseph AM, Fu SS, Lindgren B, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med. 2011;171(21):1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MHRA. MHRA Public Assessment Report. The use of nicotine replacement therapy to reduce harm in smokers 2010. www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON068572. Accessed October 22, 2010.
- 10. MHRA. Report of the Committee on Safety of Medicines Working Group on Nicotine Replacement therapy 2005. www.mhra.gov.uk/home/groups/pl-a/documents/websiteresources/con2023239.pdf. Accessed April 15, 2016.
- 11. Department of Health. Healthy Lives, Healthy People - A Tobacco Control Plan for England. London, UK: Department of Health; 2011. [Google Scholar]
- 12. NICE. Tobacco - Harm reduction 2013. http://publications.nice.org.uk/tobacco-harm-reduction-approaches-to-smoking-ph45. Accessed April 15, 2016.
- 13. Royal College of Physicians. Harm Reduction in Nicotine Addiction: Helping People Who Can’t Quit. A Report by the Tobacco Advisory Group of the Royal College of Physicians. London, UK: RCP; 2007. [Google Scholar]
- 14. Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27(12):1007–1019. [DOI] [PubMed] [Google Scholar]
- 15. Fairchild AL, Bayer R, Colgrove J. The renormalization of smoking? E-cigarettes and the tobacco “endgame”. N Engl J Med. 2014;370(4):293–295. [DOI] [PubMed] [Google Scholar]
- 16. Black A, Beard E, Brown J, Fidler J, West R. Beliefs about the harms of long-term use of nicotine replacement therapy: perceptions of smokers in England. Addiction. 2012;107(11):2037–2042. [DOI] [PubMed] [Google Scholar]
- 17. Beard E, McDermott M, McEwen A, West R. Beliefs of stop smoking practitioners in United Kingdom on the use of nicotine replacement therapy for smoking reduction. Nicotine Tob Res. 2012;14(6):639–647. [DOI] [PubMed] [Google Scholar]
- 18. Silla K, Beard E, Shahab L. Nicotine replacement therapy use among smokers and ex-smokers: associated attitudes and beliefs: a qualitative study. BMC Public Health. 2014;14:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94(5):878–881. [DOI] [PubMed] [Google Scholar]
- 20. Murray RP, Connett JE, Zapawa LM. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res. 2009;11(9):1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sims TH, Fiore MC. Pharmacotherapy for treating tobacco dependence: what is the ideal duration of therapy?CNS Drugs. 2002;16(10):653–662. [DOI] [PubMed] [Google Scholar]
- 22. Le Strat Y, Rehm J, Le Foll B. How generalisable to community samples are clinical trial results for treatment of nicotine dependence: a comparison of common eligibility criteria with respondents of a large representative general population survey. Tob Control. 2011;20(5):338–343. [DOI] [PubMed] [Google Scholar]
- 23. Shiffman S, Sweeney CT. Ten years after the Rx-to-OTC switch of nicotine replacement therapy: what have we learned about the benefits and risks of non-prescription availability?Health Policy. 2008;86(1):17–26. [DOI] [PubMed] [Google Scholar]
- 24. Kotz D, Brown J, West R. ‘Real-world’ effectiveness of smoking cessation treatments: a population study. Addiction. 2014;109(3):491–499. [DOI] [PubMed] [Google Scholar]
- 25. Dobbie F, Hiscock R, Leonardi-Bee J, et al. Evaluating Long-term Outcomes of NHS Stop Smoking Services (ELONS): a prospective cohort study. Health Technol Assess. 2015;19(95):1–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soo-Quee KD, Choon-Huat KG. The use of salivary biomarkers in occupational and environmental medicine. Occup Environ. Med. 2007;64(3):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33(4):721–730. [DOI] [PubMed] [Google Scholar]
- 28. Caggiula AR, Donny EC, Epstein LH, et al. The role of corticosteroids in nicotine’s physiological and behavioral effects. Psychoneuroendocrinology. 1998;23(2):143–159. [DOI] [PubMed] [Google Scholar]
- 29. Department of Health. Local Stop Smoking Services: Service Delivery and Monitoring Guidance - 2011/12. London, UK: Department of Health; 2011. [Google Scholar]
- 30. Feyerabend C, Russell MA. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol. 1990;42(6):450–452. [DOI] [PubMed] [Google Scholar]
- 31. Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Nieuw Amerongen AV. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med. 2003;65(2):245–258. [DOI] [PubMed] [Google Scholar]
- 32. Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43(6):645–652. [DOI] [PubMed] [Google Scholar]
- 33. Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. [DOI] [PubMed] [Google Scholar]
- 34. Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biol Psychol. 2008;78(1):20–28. [DOI] [PubMed] [Google Scholar]
- 35. Hajek P, McRobbie H, Gillison F. Dependence potential of nicotine replacement treatments: effects of product type, patient characteristics, and cost to user. Prev Med. 2007;44(3):230–234. [DOI] [PubMed] [Google Scholar]
- 36. Shahab L, Cummings KM, Hammond D, Borland R, West R, McNeill A. The impact of changing nicotine replacement therapy licensing laws in the United Kingdom: findings from the International Tobacco Control Four Country Survey. Addiction. 2009;104(8):1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silla K, Beard E, Shahab L. Characterization of long-term users of nicotine replacement therapy: evidence from a national survey. Nicotine Tob Res. 2014;16(8):1050–1055. [DOI] [PubMed] [Google Scholar]
- 38. West R, DiMarino ME, Gitchell J, McNeill A. Impact of UK policy initiatives on use of medicines to aid smoking cessation. Tob Control. 2005;14(3):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15(10):1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: findings from a general population survey of smokers. Addict Behav. 2014;39:1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown J, West R. Latest trends on smoking in England from the Smoking toolkit study. 2015. www.smokinginengland.info. Accessed April 15, 2016. [Google Scholar]
- 42. Tønnesen P, Paoletti P, Gustavsson G, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J. 1999;13(2):238–246. [DOI] [PubMed] [Google Scholar]
- 43. Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98(10):1395–1402. [DOI] [PubMed] [Google Scholar]
- 44. Fidler JA, Stapleton JA, West R. Variation in saliva cotinine as a function of self-reported attempts to reduce cigarette consumption. Psychopharmacology (Berl). 2011;217(4):587–593. [DOI] [PubMed] [Google Scholar]
- 45. Fagerström KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker?Tob Control. 1997;6(4):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77(3):145–158. [DOI] [PubMed] [Google Scholar]
- 47. McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- 48. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422–1431. [DOI] [PubMed] [Google Scholar]
- 49. Benowitz NL, Jacob P, III, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221(2):368–372. [PubMed] [Google Scholar]
- 50. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]